Adaptive Responses of Biofortified Common Bean Lines to Acidic Soil and High Temperatures in the Colombian Amazon Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Meteorological Conditions
2.2. Plant Material and Experimental Design
2.3. Canopy Biomass, Dry Matter Partitioning Indices, Grain Yield, and Yield Components
2.4. Phenological Behavior and Pollen Viability
2.5. Energy Use Efficiency and Leaf Cooling Capacity under High Temperature Conditions
2.6. Determination of Fe and Zn in Seeds
2.7. Data Analysis
3. Results
3.1. Phenotypic Differences in Agronomic Performance
3.2. Phenotypic Differences in Phenology and Pollen Viability
3.3. Phenotypic Differences in Energy Use, Leaf Cooling, and Linear Electron Flow
3.4. Correlations between Physiological and Agronomic Traits
4. Discussion
4.1. Differences in Crop Development and Photosynthate Remobilization Affect Agronomic Performance
4.2. Combined Stress Affects Phenology and Pollen Viability
4.3. Capacity of Photosynthetic Apparatus for Coping with Soil Acidity and High Temperature
4.4. Differences in Physiological Response between Common Bean Lines Reflect Possible Mechanisms in the Translocation of Assimilates That Favor Better Yields
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia Jefe, W.; Péan, C.; De Operaciones, J.; et al. Resumen Para Responsables de Políticas. Calentamiento global de 1.5 °C Informe Especial del IPCC Sobre los Impactos del Calentamiento Global de 1.5 °C Con Respecto a los Niveles Preindustriales y las Trayectorias Correspondientes que Deberían Seguir las e; IPCC: Rome, Italy, 2019; ISBN 9789291693511. Available online: www.ipcc.ch (accessed on 1 March 2023).
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; García, R.; Rivera, M. Evidence for genotypic differences among elite lines of common bean in the ability to remobilize photosynthate to increase yield under drought. J. Agric. Sci. 2017, 155, 857–875. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Pourkheirandish, M.; Golicz, A.A.; Bhalla, P.L.; Singh, M.B. Global Role of Crop Genomics in the Face of Climate Change. Front. Plant Sci. 2020, 11, 922. [Google Scholar] [PubMed]
- Porch, T.G.; Beaver, J.S.; Brick, M.A. Registration of Tepary Germplasm with Multiple-Stress Tolerance, TARS-Tep 22 and TARS-Tep 32. J. Plant Regist. 2013, 7, 358–364. [Google Scholar] [CrossRef]
- Raggi, L.; Caproni, L.; Carboni, A.; Negri, V. Genome-Wide Association Study Reveals Candidate Genes for Flowering Time Variation in Common Bean (Phaseolus vulgaris L.). Front. Plant Sci. 2019, 10, 962. [Google Scholar] [CrossRef]
- Lizana, C.; Wentworth, M.; Martinez, J.P.; Villegas, D.; Meneses, R.; Murchie, E.H.; Pastenes, C.; Lercari, B.; Vernieri, P.; Horton, P.; et al. Differential adaptation of two varieties of common bean to abiotic stress: I. Effects of drought on yield and photosynthesis. J. Exp. Bot. 2006, 57, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M. Digging deep into defining physiological responses to environmental stresses in the tropics: The case of common bean and Brachiaria forage grasses. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1099–1140. [Google Scholar]
- Buitrago-Bitar, M.A.; Cortés, A.J.; López-Hernández, F.; Londoño-Caicedo, J.M.; Muñoz-Florez, J.E.; Carmenza Muñoz, L.; Blair, M.W. Allelic diversity at abiotic stress responsive genes in relationship to ecological drought indices for cultivated tepary bean, phaseolus acutifolius a. Gray, and its wild relatives. Genes 2021, 12, 556. [Google Scholar] [CrossRef]
- Ligarreto, G. Componentes de variancia en variables de crecimiento y fotosíntesis en fríjol común (Phaseolus vulgaris L.). Rev. U.D.C.A Actual. Divulg. Científica 2013, 16, 87–96. [Google Scholar]
- Assefa, T.; Rao, I.M.; Cannon, S.B.; Wu, J.; Gutema, Z.; Blair, M.; Otyama, P.; Alemayehu, F.; Dagne, B. Improving adaptation to drought stress in white pea bean (Phaseolus vulgaris L.): Genotypic effects on grain yield, yield components and pod harvest index. Plant Breed. 2017, 136, 548–561. [Google Scholar] [CrossRef]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C.; et al. A reference genome for common bean and genome-wide analysis of dual domestications. Nat. Genet. 2014, 46, 707–713. [Google Scholar] [CrossRef]
- Beebe, S.; Rao, I.M.; Blair, M.; Acosta, J. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S. Common bean breeding in the tropics. Plant Breed. Rev. 2012, 36, 357–426. [Google Scholar]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective use of water and increased dry matter partitioned to grain contribute to yield of common bean improved for drought resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M. Advances in Improving Adaptation of Common Bean and Brachiaria Forage Grasses to Abiotic Stresses in the Tropics. In Handbook of Plant and Crop Physiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 847–889. ISBN 9781466553293. [Google Scholar]
- Jarvis, A.; Ramirez, J.; Bonilla-Findji, O.; Zapata, E. Impacts of climate change on crop production in Latin America. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley: Chichester, UK, 2011; pp. 44–56. [Google Scholar]
- Zapata-Caldas, E.; Hyman, G.; Pachón, H.; Monserrate, F.A.; Varela, L.V. Identifying candidate sites for crop biofortification in Latin America: Case studies in Colombia, Nicaragua and Bolivia. Int. J. Health Geogr. 2009, 8, 1–18. [Google Scholar] [CrossRef]
- ICBF (Instituto Colombiano de Bienestar Familiar). Encuesta Nacional de la Situación Nutricional en Colombia (ENSIN); ICBF: Bogotá, Colombia, 2010.
- Diaz, S.; Polania, J.; Ariza-Suarez, D.; Cajiao, C.; Grajales, M.; Raatz, B. Genetic correlation between Fe and Zn biofortification and yield components in a common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2022, 12, 739033. [Google Scholar] [CrossRef]
- Gonzalez, A.; Lynch, J.; Tohme, J.M.; Beebe, S.E.; Macchiavelli, R.E. Characters related to leaf photosynthesis in wild populations and landraces of common bean. Crop Sci. 1995, 35, 1468–1476. [Google Scholar] [CrossRef]
- Amongi, W.; Nkalubo, S.T.; Ochwo-Ssemakula, M.; Badji, A.; Dramadri, I.O.; Odongo, T.L.; Nuwamanya, E.; Tukamuhabwe, P.; Izquierdo, P.; Cichy, K.; et al. Phenotype based clustering, and diversity of common bean genotypes in seed iron concentration and cooking time. PLoS ONE 2023, 18, e0284976. [Google Scholar] [CrossRef]
- Huertas, R.; Karpinska, B.; Ngala, S.; Mkandawire, B.; Maling’a, J.; Wajenkeche, E.; Kimani, P.M.; Boesch, C.; Stewart, D.; Hancock, R.D.; et al. Biofortification of common bean (Phaseolus vulgaris L.) with iron and zinc: Achievements and challenges. Food Energy Secur. 2023, 12, e406. [Google Scholar] [CrossRef]
- Lamptey, M.; Adu-Dapaah, H.; Osei Amoako-Andoh, F.; Butare, L.; Bediako, K.A.; Amoah, R.A.; Tawiah, I.; Yeboah, S.; Asibuo, J.Y. Genetic studies on iron and zinc concentrations in common bean (Phaseolus vulgaris L.) in Ghana. Heliyon 2023, 9, e17303. [Google Scholar] [CrossRef]
- Beebe, S. Biofortification of common bean for higher iron concentration. Front. Sustain. Food Syst. 2020, 4, 573449. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 301899. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Ariza-Suarez, D.; Izquierdo, P.; Lobaton, J.D.; de la Hoz, J.F.; Acevedo, F.; Duitama, J.; Guerrero, A.F.; Cajiao, C.; Mayor, V.; et al. Genetic mapping for agronomic traits in a MAGIC population of common bean (Phaseolus vulgaris L.) under drought conditions. BMC Genom. 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Kimani, P.M.; Warsame, A.H.M.E.D. Breeding second-generation biofortified bean varieties for Africa. Food Energy Secur. 2019, 8, e00173. [Google Scholar] [CrossRef]
- CIAT. Breeding Micronutrient Dense Bean Varieties in Eastern Africa. Bean Improvement for the Tropics, Annual Report I-P1 Report; CIAT: Cali, Colombia, 2008; pp. 15–25. [Google Scholar]
- Blair, M.W.; Izquierdo, P. Use of the advanced backcross-QTL method to transfer seed mineral accumulation nutrition traits from wild to Andean cultivated common beans. Theor. Appl. Genet. 2012, 125, 1015–1031. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Graham, R. QTL analyses for seed iron and zinc concentrations in an intra-genepool population of Andean common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 122, 511–521. [Google Scholar] [CrossRef]
- Blair, M.W.; González, L.F.; Kimani, P.M.; Butare, L. Genetic diversity, inter-gene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from Central Africa. Theor. Appl. Genet. 2010, 121, 237–248. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Grusak, M.A.; Graham, R.; Beebe, S.E. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- Cichy, K.A.; Caldas, G.V.; Snapp, S.S.; Blair, M.W. QTL analysis of seed Iron, Zinc, and Phosphorus levels in an andean bean population. Crop Sci. 2009, 49, 1742–1750. [Google Scholar]
- Pereira, H.; Del Peloso, M.; Bassinello, P.; Guimarães, C.; Melo, L.; Faria, L. Genetic variability for iron and zinc content in common bean lines and interaction with water availability. Genet. Mol. Res. 2014, 13, 6773–6785. [Google Scholar] [CrossRef]
- Cichy, K.; Chiu, C.; Isaacs, K.; Glahn, R. Dry Bean Biofortification with Iron and Zinc. In Biofortification of Staple Crops; Springer: Singapore, 2022; pp. 225–270. [Google Scholar]
- Saradadevi, R.; Mukankusi, C.; Li, L.; Amongi, W.; Mbiu, J.P.; Raatz, B.; Ariza, D.; Beebe, S.; Varshney, R.K.; Huttner, E.; et al. Multivariate genomic analysis and optimal contributions selection predicts high genetic gains in cooking time, iron, zinc, and grain yield in common beans in East Africa. Plant Genome 2021, 14, e20156. [Google Scholar] [CrossRef]
- Philipo, M.; Ndakidemi, P.A.; Mbega, E.R. Environmental and genotypes influence on seed iron and zinc levels of landraces and improved varieties of common bean (Phaseolus vulgaris L.) in Tanzania. Ecol. Genet. Genomics 2020, 15, 100056. [Google Scholar] [CrossRef]
- Porch, T.G.; Jahn, M. Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant. Cell Environ. 2001, 24, 723–731. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsukaguchi, T.; Takeda, H.; Egawa, Y. Decrease of pollen stainability of green bean at high temperatures and relationship to heat tolerance. J. Am. Soc. Hortic. Sci. 2001, 126, 571–574. [Google Scholar] [CrossRef]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open photosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ostendorf, E.; Kohzuma, K.; Hoh, D.; Strand, D.D.; Sato-Cruz, M.; Savage, L.; Cruz, J.A.; Fisher, N.; Froehlich, J.E.; et al. Chloroplast ATP synthase modulation of the thylakoid proton motive force: Implications for photosystem I and photosystem II photoprotection. Front. Plant Sci. 2017, 8, 264832. [Google Scholar] [CrossRef]
- Sacksteder, C.A.; Kramer, D.M. Dark-interval relaxation kinetics (DIRK) of absorbance changes as a quantitative probe of steady-state electron transfer. Photosynth. Res. 2000, 66, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chovancek, E.; Zivcak, M.; Brestic, M.; Hussain, S.; Allakhverdiev, S.I. The different patterns of post-heat stress responses in wheat genotypes: The role of the transthylakoid proton gradient in efficient recovery of leaf photosynthetic capacity. Photosynth. Res. 2021, 150, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ(T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant. Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Suárez, J.C.; Contreras, A.T. BD Exp. C17d y F5.9_Sco. PROJECT ID: 11013, 4169. [Dataset]. 2023. Available online: https://www.photosynq.org/projects/bd-exp-c17d-y-f5-9_sco (accessed on 5 December 2023).
- Di Rienzo, J.A.; Macchiavelli, R.; Casanoves, F. Mixed Models in Infostat. Electronic Version. 2012. 1994p. Available online: http://www.infostat.com.ar (accessed on 5 December 2023).
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of Digestion Methods Using Atomic Absorption Spectrometry for the Determination of Metal Levels in Plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- R Development Core Team. “Vigorous Calisthenics” Copyright (C) 2024 The R Foundation for Statistical Computing Platform: x86_64-apple-darwin17.0 (64-bit), R version 4.2.0; R Development Core Team: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 5 December 2023).
- Nguyen, N.; Drakou, E.G. Farmers intention to adopt sustainable agriculture hinges on climate awareness: The case of Vietnamese coffee. J. Clean. Prod. 2021, 303, 126828. [Google Scholar] [CrossRef]
- Rodriguez, D.F.C.; Urban, M.O.; Santaella, M.; Gereda, J.M.; Contreras, A.D.; Wenzl, P. Using phenomics to identify and integrate traits of interest for better-performing common beans: A validation study on an interspecific hybrid and its Acutifolii parents. Front. Plant Sci. 2022, 13, 1008666. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 85, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Poschenrieder, C.; Rao, I.M.; Beebe, S. Estimation of phenotypic variability in symbiotic nitrogen fixation ability of common bean under drought stress using 15N natural abundance in grain. Eur. J. Agron. 2016, 79, 66–73. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Kazai, P.; Noulas, C.; Khah, E.; Vlachostergios, D. Yield and seed quality parameters of common bean cultivars grown under water and heat stress field conditions. AIMS Agric. Food 2019, 4, 285–302. [Google Scholar] [CrossRef]
- Rao, I.M.; Beebe, S.E.; Polanía, J.; Grajales, M.; Cajiao, C.; García, R.; Ricaurte, J.; Rivera, M. Physiological basis of improved drought resistance in common bean: The contribution of photosynthate mobilization to grain. In Proceedings of the Paper presented at Interdrought III: The 3rd International Conference on Integrated Approaches to Improve Crop Production Under Dro, Shanghai, China, 11–16 October 2009. [Google Scholar]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Assefa, T.; Beebe, S.E.; Rao, I.M.; Cuasquer, J.B.; Duque, M.C.; Rivera, M.; Battisti, A.; Lucchin, M. Pod harvest index as a selection criterion to improve drought resistance in white pea bean. F Crop. Res. 2013, 148, 24–33. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.; Butare, L. Breeding for abiotic stress tolerance in common bean: Present and future challenges. In Proceedings of the 14th Australian Plant Breeding & 11th SABRAO Conference, Brisbane, Australia, 10–14 August 2009. [Google Scholar]
- Assefa, T.; Sperling, L.; Dagne, B.; Argaw, W.; Tessema, D.; Beebe, S. Participatory plant breeding with traders and farmers for white pea bean in Ethiopia. J. Agric. Educ. Ext. 2014, 20, 497–512. [Google Scholar] [CrossRef]
- Klaedtke, S.M.; Cajiao, C.; Grajales, M.; Polanía, J.; Borrero, G.; Guerrero, A.; Rivera, M.; Rao, I.; Beebe, S.E.; Léon, J. Photosynthate remobilization capacity from drought-adapted common bean (Phaseolus vulgaris L.) lines can improve yield potential of interspecific populations within the secondary gene pool. J. Plant Breed. Crop Sci. 2012, 4, 49–61. [Google Scholar]
- Rao, I.M.; Beebe, S.; Polania, J.; Ricaurte, J.; Cajiao, C.; Garcia, R.; Rivera, M. Can tepary bean be a model for improvement of drought resistance in common bean? Afr. Crop Sci. J. 2013, 21, 265–281. [Google Scholar]
- Hall, A.E. Comparative ecophysiology of cowpea, common bean, and peanut. In Physiology and Biotechnology Integration for Plant Breeding; CRC Press: Boca Raton, FL, USA, 2004; pp. 243–287. [Google Scholar]
- Rosales-Serna, R.; Kohashi-Shibata, J.; Acosta-Gallegos, J.A.; Trejo-López, C.; Ortiz-Cereceres, J.; Kelly, J.D. Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. Field Crop. Res. 2004, 85, 203–211. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S. Physiological traits associated with drought resistance in Andean and Mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Omae, H.; Kumar, A.; Shono, M. Adaptation to high temperature and water deficit in the common bean (Phaseolus vulgaris L.) during the reproductive period. J. Bot. 2012, 2012, 803413. [Google Scholar]
- Peláez, D.; Aguilar, P.A.; Mercado, M.; López-Hernández, F.; Guzmán, M.; Burbano-Erazo, E.; Denning-James, K.; Medina, C.I.; Blair, M.W.; De Vega, J.J.; et al. Genotype selection, and seed uniformity and multiplication to ensure common bean (Phaseolus vulgaris L.) var. Liborino. Agronomy 2022, 12, 2285. [Google Scholar] [CrossRef]
- Bellucci, E.; Benazzo, A.; Xu, C.; Bitocchi, E.; Rodriguez, M.; Alseekh, S.; Di Vittori, V.; Gioia, T.; Neumann, K.; Cortinovis, G.; et al. Selection and adaptive introgression guided the complex evolutionary history of the European common bean. Nat. Commun. 2023, 14, 1908. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Sattari Vayghan, H.; Tavalaei, S.; Grillon, A.; Meyer, L.; Ballabani, G.; Glauser, G.; Longoni, P. Growth temperature influence on lipids and photosynthesis in Lepidium sativum. Front. Plant Sci. 2020, 11, 515048. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Bhanu, D.; Sarkar, B.; Yadav, S.K.; Jyothilakshmi, N.; Maheswari, M. Infra red thermography reveals transpirational cooling in pearl millet (Pennisetum glaucum) plants under heat stress. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shanker, A.K.; Amirineni, S.; Bhanu, D.; Yadav, S.K.; Jyothilakshmi, N.; Vanaja, M.; Singh, J.; Sarkar, B.; Maheswari, M.; Singh, V.K. High-resolution dissection of photosystem II electron transport reveals differential response to water deficit and heat stress in isolation and combination in pearl millet [Pennisetum glaucum (L.) R. Br.]. Front. Plant Sci. 2022, 13, 892676. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Noriega, J.E.; Deva, C.; Beebe, S.E.; Polanía, J.A.; Casanoves, F.; Rao, I.M. Water use, leaf cooling and carbon assimilation efficiency of heat resistant common beans evaluated in Western Amazonia. Front. Plant Sci. 2021, 12, 644010. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Fernández-Calleja, M.; Monteagudo, A.; Casas, A.M.; Boutin, C.; Pin, P.A.; Morales, F.; Igartua, E. Rapid on-site phenotyping via field fluorimeter detects differences in photosynthetic performance in a hybrid—Parent barley germplasm set. Sensors 2020, 20, 1486. [Google Scholar] [CrossRef]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.J.; Zhang, S.B.; Zhang, J.L.; Cao, K.F. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 2012, 235, 819–828. [Google Scholar] [CrossRef]
- Rott, M.; Martins, N.F.; Thiele, W.; Lein, W.; Bock, R.; Kramer, D.M.; Schöttlera, M.A. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 2011, 23, 304–321. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Gracia-Romero, A.; López-Cristoffanini, C.; Vicente, R.; Kthiri, Z.; Kefauver, S.C.; López-Carbonell, M.; Serret, M.D.; Araus, J.L.; Hamada, W. The promising MultispeQ device for tracing the effect of seed coating with biostimulants on growth promotion, photosynthetic state and water–nutrient stress tolerance in durum wheat. Euro-Mediterr. J. Environ. Integr. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M. Role of physiology in improving crop adaptation to abiotic stresses in the tropics: The case of common bean and tropical forages. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2001; pp. 605–636. [Google Scholar]
- Çiçek, N.; Pekcan, V.; Arslan, Ö.; Çulha Erdal, Ş.; Balkan Nalçaiyi, A.S.; Çil, A.N.; Şahin, V.; Kaya, Y.; Ekmekçi, Y. Assessing drought tolerance in field-grown sunflower hybrids by chlorophyll fluorescence kinetics. Rev. Bras. Bot. 2019, 42, 249–260. [Google Scholar] [CrossRef]
- Che, X.; Zhang, T.; Li, H.; Zhang, L.; Liu, J. Effect of hypoxia on photosystem II of tropical seagrass enhalus acoroides in the dark. Photochem. Photobiol. 2022, 98, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Giorio, P.; Sellami, M.H. Polyphasic okjip chlorophyll a fluorescence transient in a landrace and a commercial cultivar of sweet pepper (Capsicum annuum, L.) under long-term salt stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Janeeshma, E.; Kalaji, H.M.; Puthur, J.T. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol. Plant. 2021, 43, 1–16. [Google Scholar] [CrossRef]
- Nsiri, K.; Krouma, A. The Key Physiological and Biochemical Traits Underlying Common Bean (Phaseolus vulgaris L.) Response to Iron Deficiency, and Related Interrelationships. Agronomy 2023, 13, 2148. [Google Scholar] [CrossRef]
- Mari, S.; Bailly, C.; Thomine, S. Handing off iron to the next generation: How does it get into seeds and what for? Biochem. J. 2020, 477, 259–274. [Google Scholar] [CrossRef]

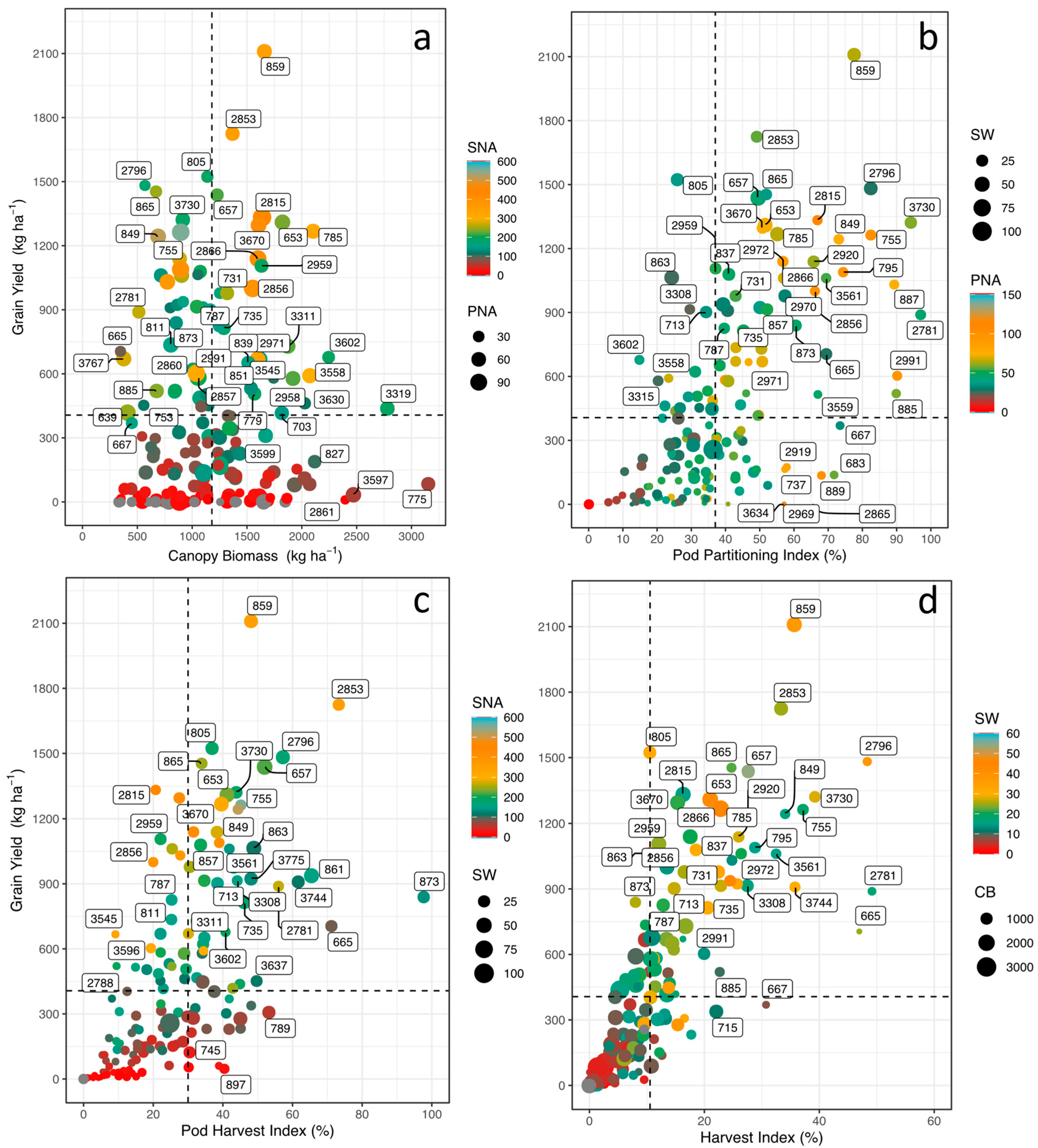
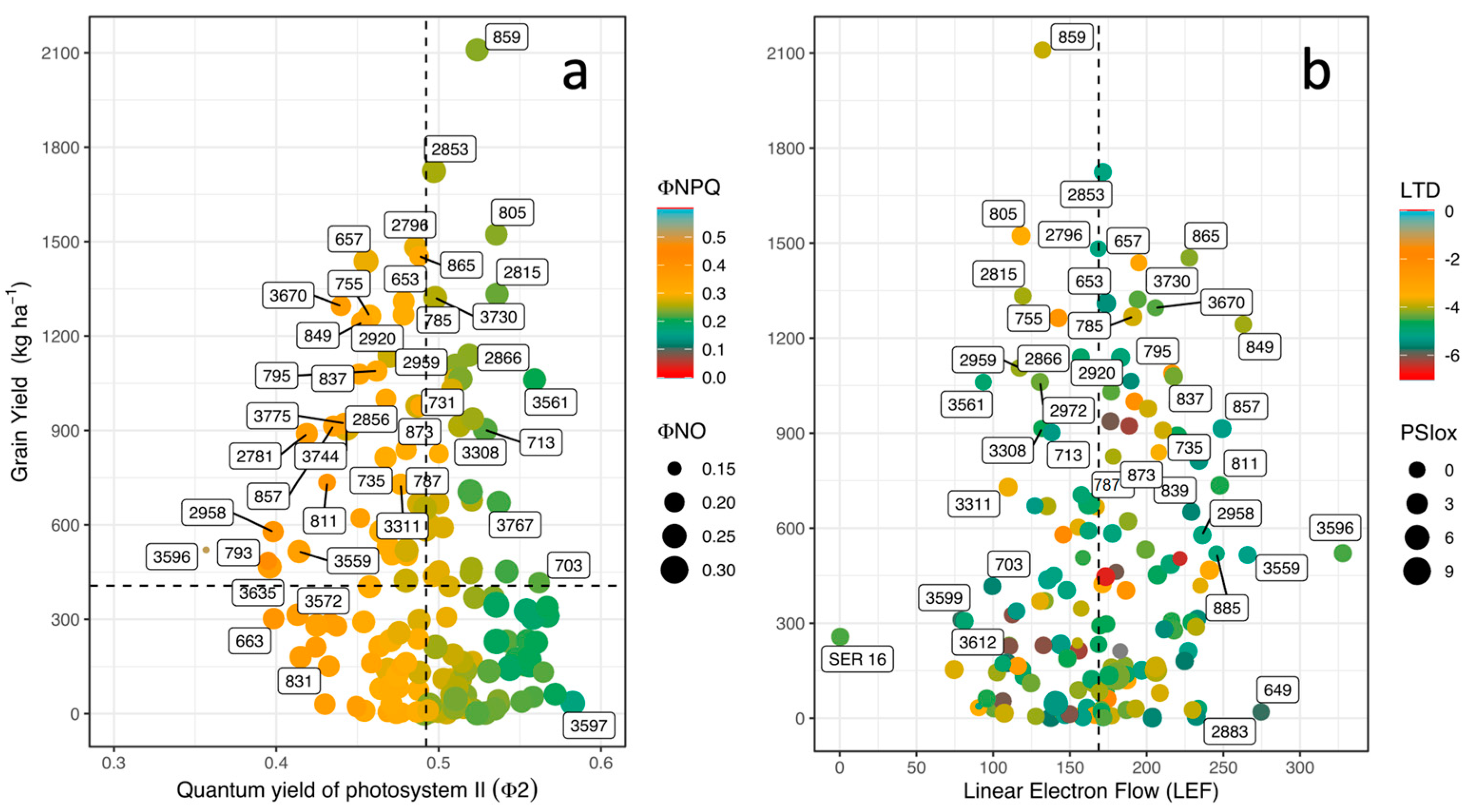
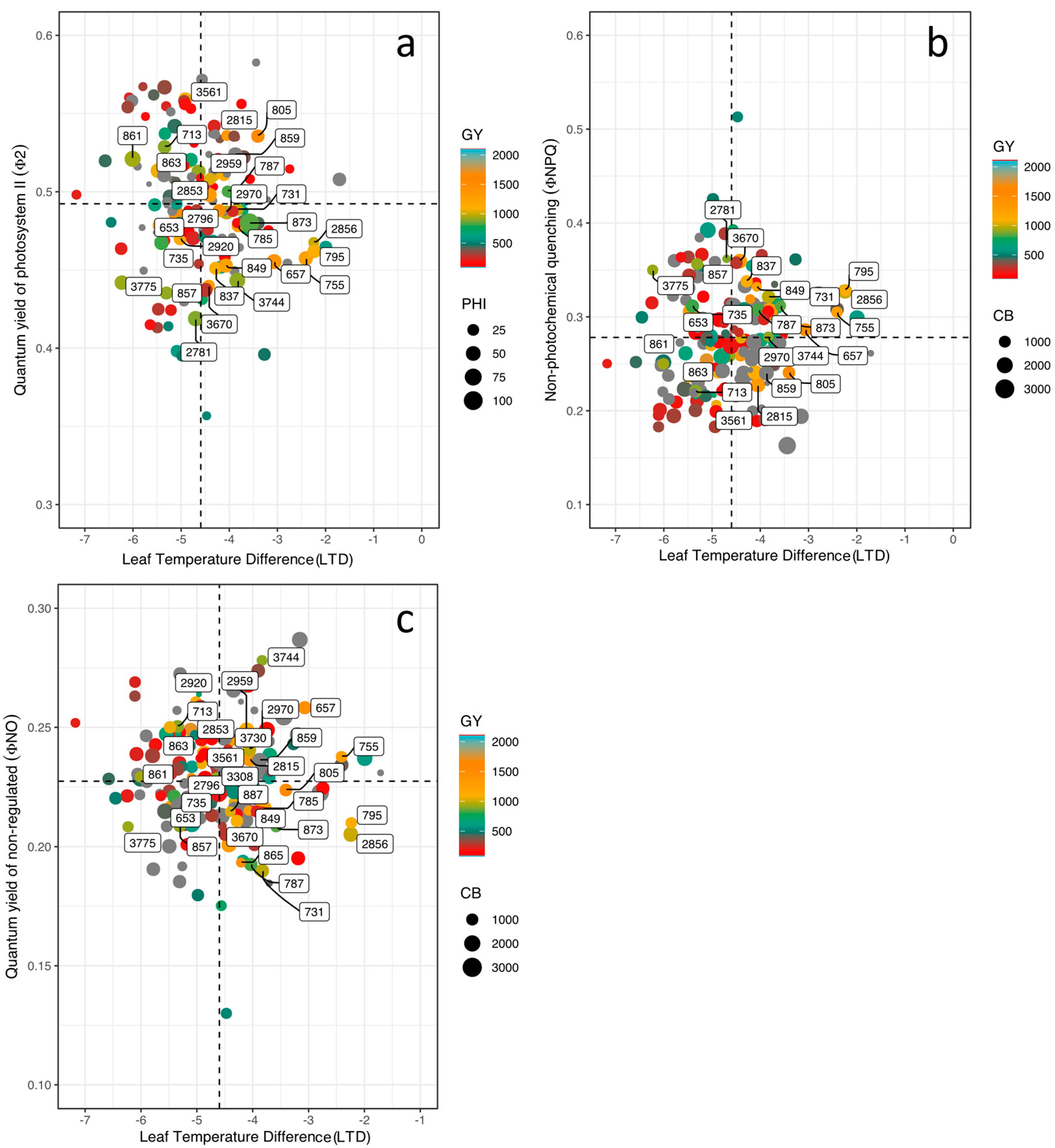
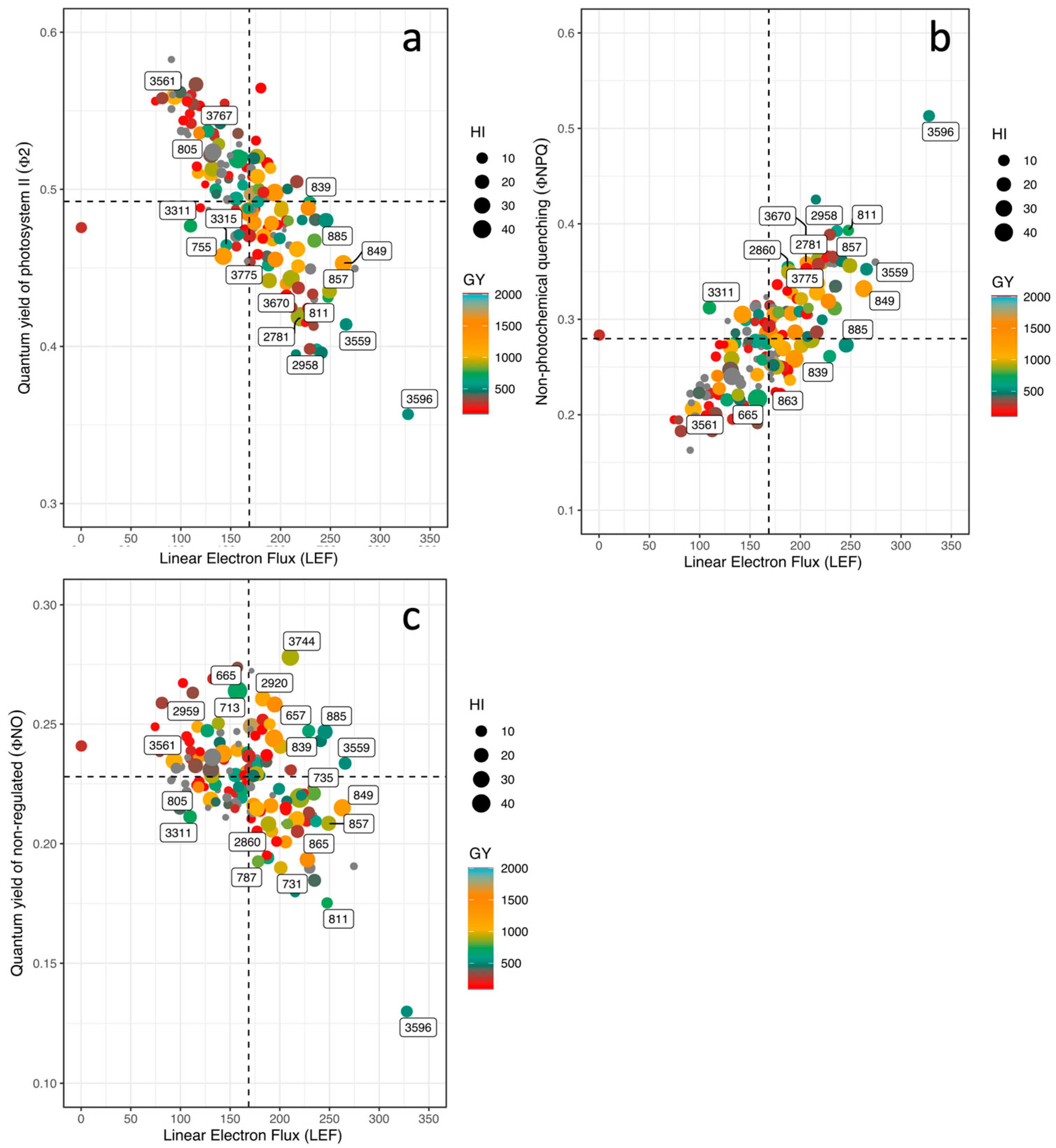
| Variables | GY | HI | PHI | DF | DPM | LTD | ΦII | LEF | Seed Fe | Seed Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Grain yield (GY) | 0.8 *** | 0.68 *** | 0.11 * | −0.17 * | ||||||
| Canopy biomass (CB) | 0.36 *** | −0.23 *** | 0.13 * | |||||||
| Pod partitioning index (PPI) | 0.6 *** | 0.73 *** | 0.42 *** | −0.15 * | 0.15 * | 0.18 * | ||||
| Pod harvest index (PHI) | 0.68 *** | 0.75 *** | −0.25 *** | −0.16 | 0.2 ** | −0.17 * | ||||
| Harvest index (HI) | 0.8 *** | 0.75 *** | −0.18 * | −0.16 | 0.19 ** | −0.14 * | ||||
| Days to flowering (DF) | −0.25 *** | −0.19 ** | 0.21 * | |||||||
| Days to physiological maturity (DPM) | 0.2 ** | −0.13 | ||||||||
| Ambient humidity (AH) | 0.68 *** | 0.21 *** | −0.31 *** | |||||||
| Ambient temperature (AT) | 0.15 * | 0.19 ** | −0.27 *** | −0.55 *** | −0.23 *** | 0.46 *** | −0.39 * | |||
| Amplitude of the electrochromic band shift signal (ECSt mAU) | −0.16 ** | −0.21 ** | ||||||||
| Activity of ATP synthase (gH+) | −0.28 *** | 0.24 *** | −0.38 * | −0.29 * | ||||||
| Leaf angle (LA) | 0.27 *** | 0.19 * | 0.11 * | −0.15 * | ||||||
| Leaf temperature difference (LTD) | 0.2 ** | −0.11 * | ||||||||
| Linear electron flow (LEF) | 0.11 * | 0.19 ** | 0.2 ** | −0.19 ** | −0.78 *** | −0.18 * | ||||
| Photosynthetically active radiation (PAR) | 0.19 ** | 0.19 ** | −0.15 * | −0.9 *** | 0.96 *** | −0.14 * | ||||
| Energy dissipated as heat total (NPQt) | −0.13 * | −0.76 *** | 0.67 *** | |||||||
| Fractions of energy received by photosystem II (ΦII) | −0.16 * | −0.16 * | −0.11 * | −0.78 *** | ||||||
| Fractions of energy not dissipated (ΦNO) | 0.01 * | 0.16 * | 0.35 *** | −0.41 *** | ||||||
| Fractions of energy dissipated as heat (ΦNPQ) | −0.93 *** | 0.78 *** | ||||||||
| Total active PSI centers (PSIact) | 0.13 * | 0.17 * | 0.13 * | 0.14 * | ||||||
| Over-reduced PSI (PSIor) | 0.14 * | |||||||||
| Fraction of an oxidized PSI center (PSIox) | −0.03 * | |||||||||
| Relative chlorophyll content (RChl) | 0.2 ** | 0.22 *** | −0.22 *** | −0.16 ** | 0.25 *** | −0.21 * | ||||
| Leaf thickness (T) | −0.1 | −0.15 * | 0.17 ** | 0.16 * | ||||||
| Proton flux (vH+) | 0.15 * | 0.16 | −0.18 * | −0.87 *** | 0.92 *** | 0.17 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, J.C.; Contreras, A.T.; Urban, M.O.; Grajales, M.A.; Beebe, S.E.; Rao, I.M. Adaptive Responses of Biofortified Common Bean Lines to Acidic Soil and High Temperatures in the Colombian Amazon Region. Agronomy 2024, 14, 154. https://doi.org/10.3390/agronomy14010154
Suárez JC, Contreras AT, Urban MO, Grajales MA, Beebe SE, Rao IM. Adaptive Responses of Biofortified Common Bean Lines to Acidic Soil and High Temperatures in the Colombian Amazon Region. Agronomy. 2024; 14(1):154. https://doi.org/10.3390/agronomy14010154
Chicago/Turabian StyleSuárez, Juan Carlos, Amara T. Contreras, Milan O. Urban, Miguel A. Grajales, Stephen E. Beebe, and Idupulapati M. Rao. 2024. "Adaptive Responses of Biofortified Common Bean Lines to Acidic Soil and High Temperatures in the Colombian Amazon Region" Agronomy 14, no. 1: 154. https://doi.org/10.3390/agronomy14010154
APA StyleSuárez, J. C., Contreras, A. T., Urban, M. O., Grajales, M. A., Beebe, S. E., & Rao, I. M. (2024). Adaptive Responses of Biofortified Common Bean Lines to Acidic Soil and High Temperatures in the Colombian Amazon Region. Agronomy, 14(1), 154. https://doi.org/10.3390/agronomy14010154






