Enhancing Soil Aggregation and Organic Carbon Retention in Greenhouse Vegetable Production through Reductive Soil Disinfestation with Straw and Fertiliser: A Comprehensive Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Area
2.2. Test Materials
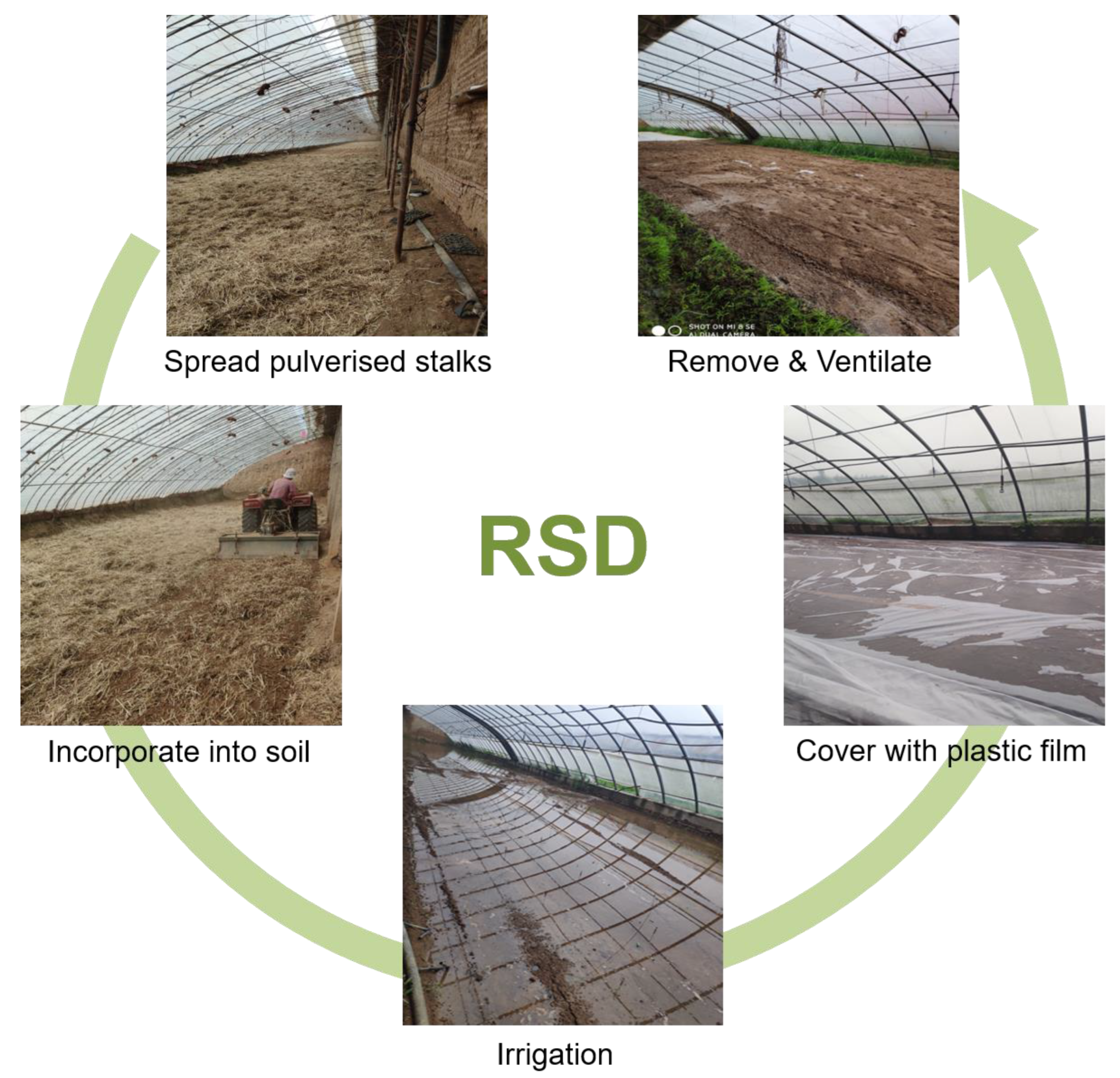
2.3. Experimental Design
2.4. Collection and Measurement of Infrared Spectrum
2.5. Related Calculation
× percentage composition of aggregates (%)/SOC] × 100.
2.6. Statistical Analysis of Data
3. Results and Analysis
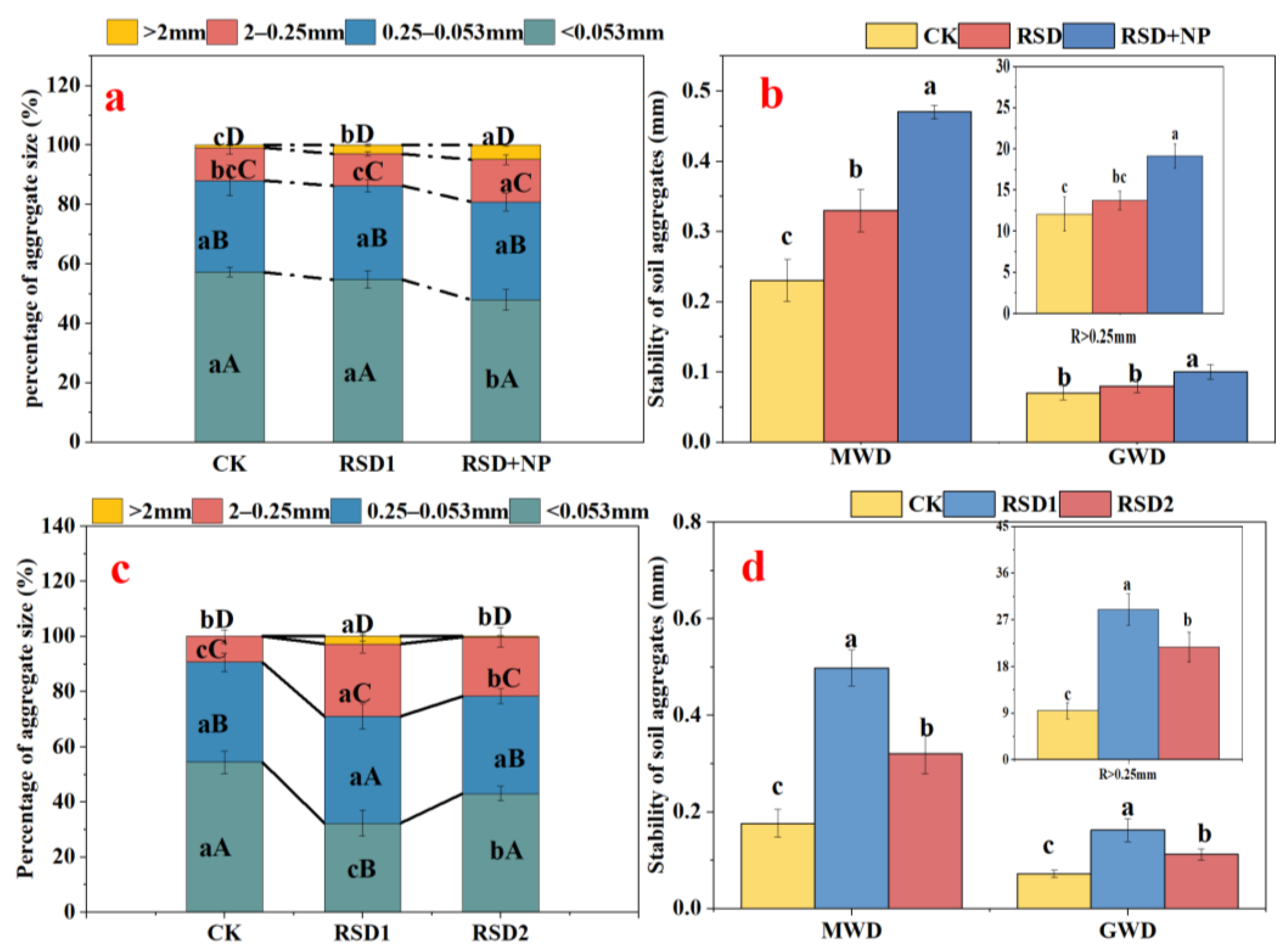
3.1. Structural Characteristics of Soil Aggregates
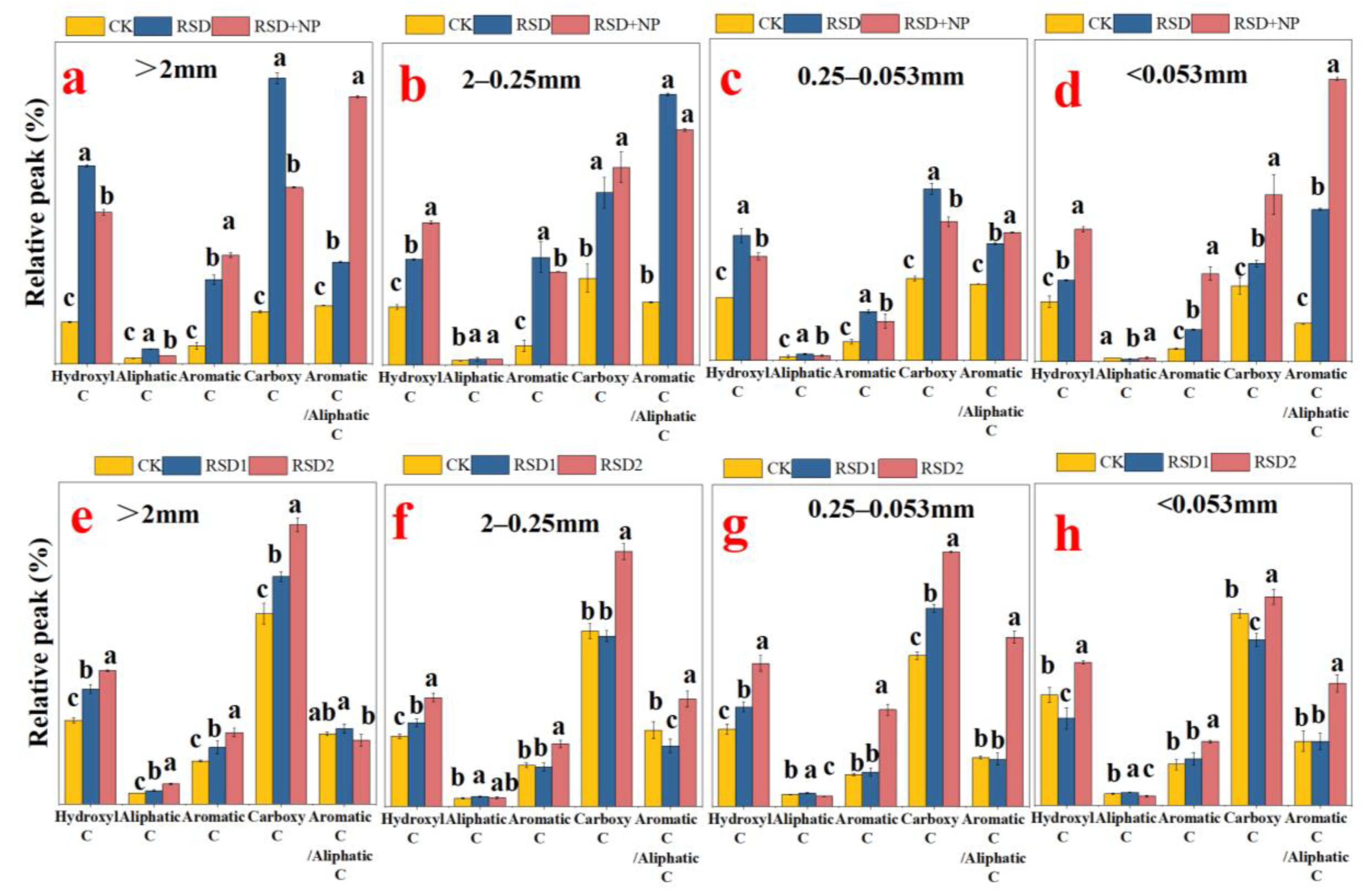
3.2. Relative Contribution Rate of Organic Carbon in Soil Aggregate Components
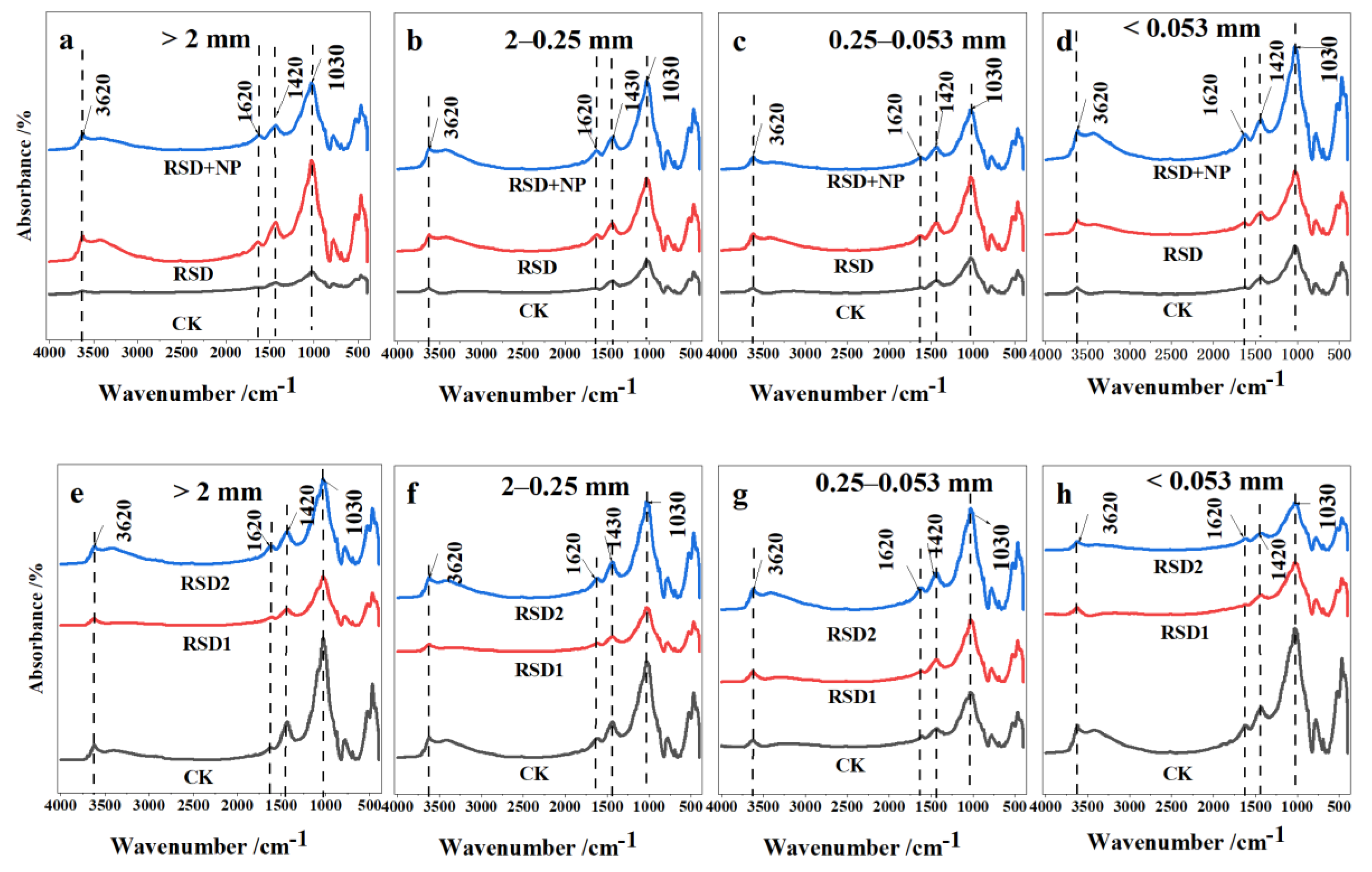
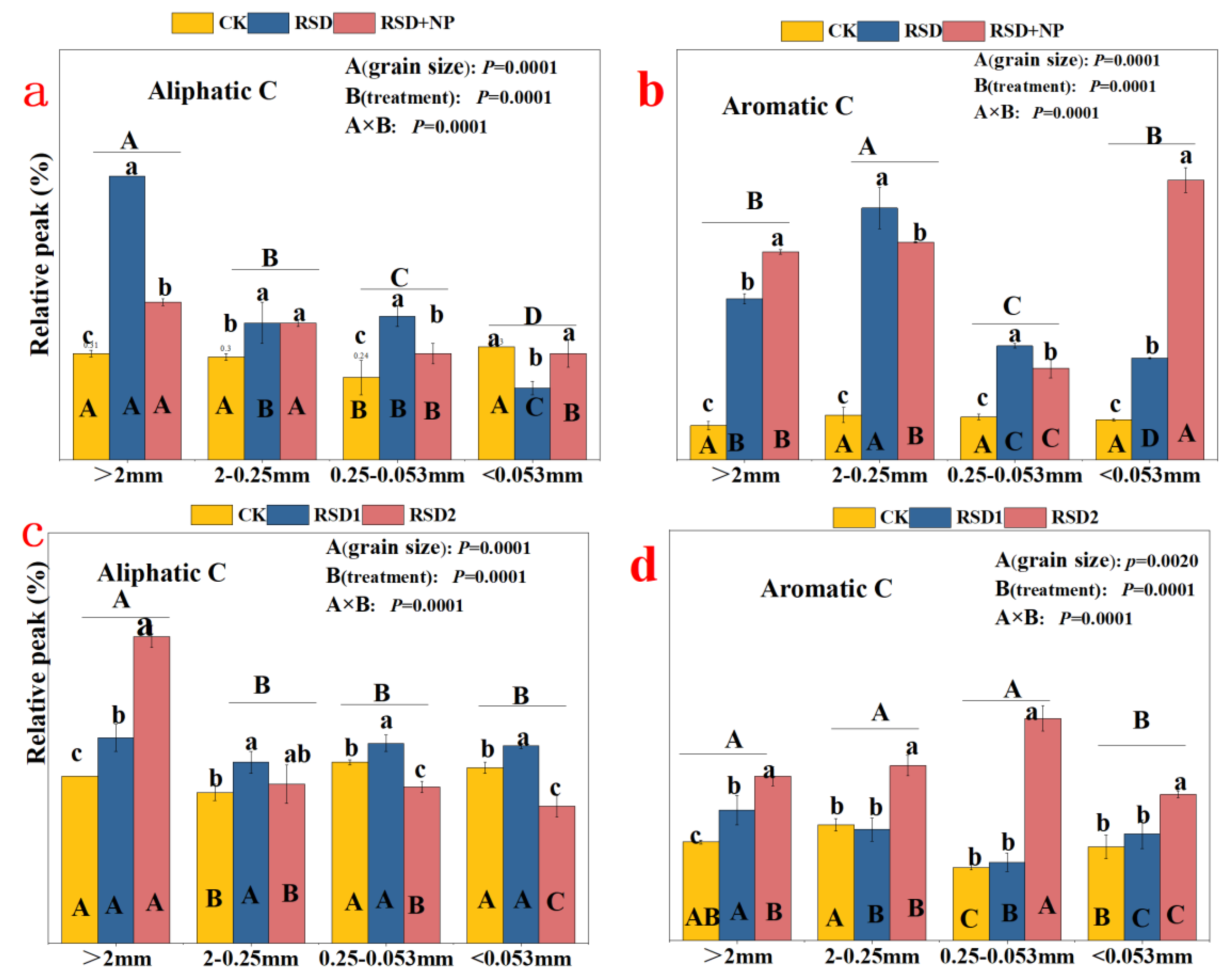
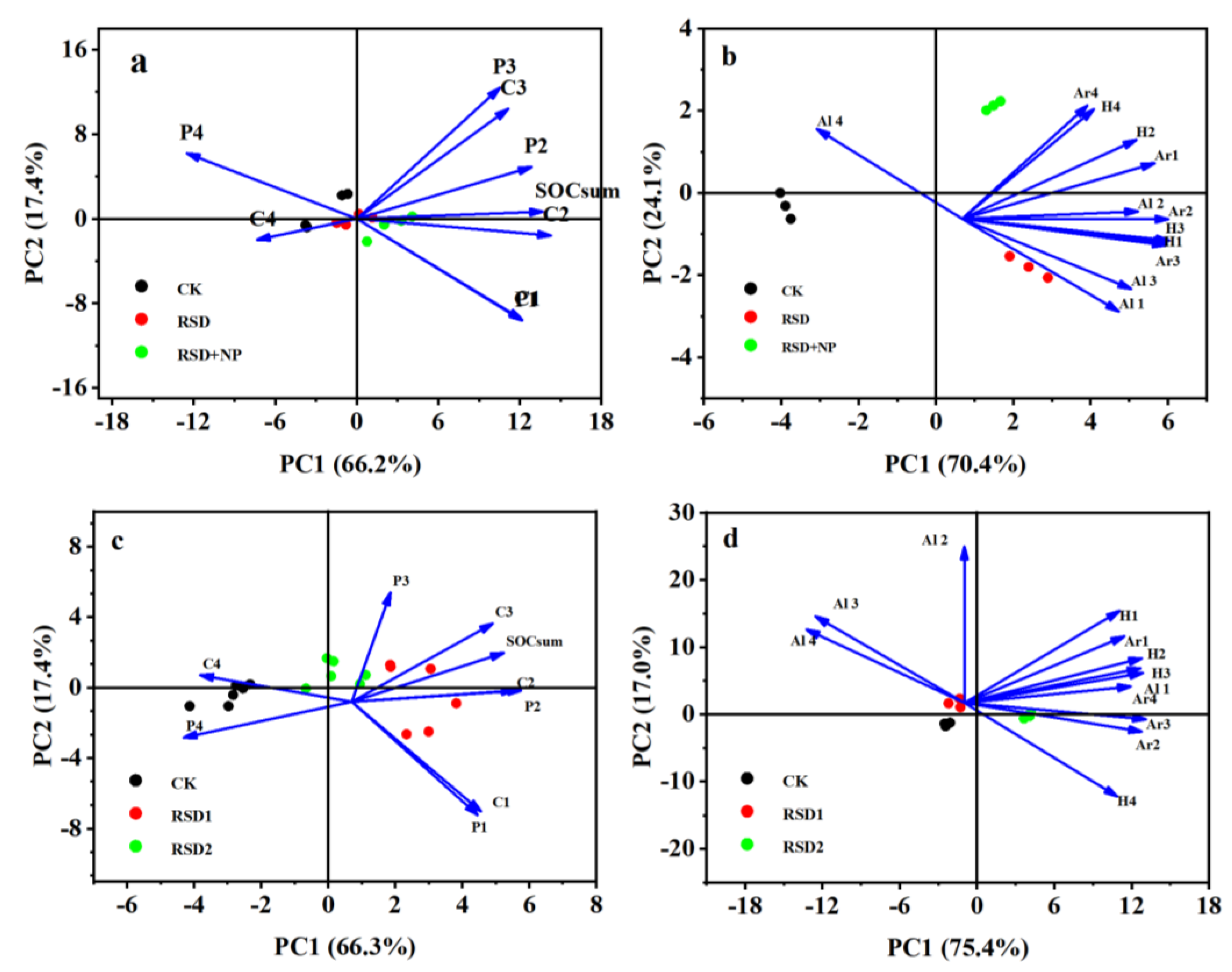
3.3. Functional Groups of Organic Carbon in Soil Aggregates
4. Discussion
4.1. Effects of RSD on Soil Aggregate Organic Carbon Content and Retention
4.2. Effects of RSD on the Chemical Structure of Soil Aggregate Organic Carbon
5. Conclusions
6. Shortcomings and Prospects of the Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Liu, L.; Long, S.; Deng, B.; Kuang, J.; Wen, K.; Li, T.; Bai, Z.; Shao, Q. Effects of Plastic Shed Cultivation System on the Properties of Red Paddy Soil and Its Management by Reductive Soil Disinfestation. Horticulturae 2022, 8, 279. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of Soilborne Plant Pathogens by Incorporating Fresh Organic Amendments Followed by Tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Shen, Q.; Cai, Z. Reductive Soil Disinfestations Combined or Not with Trichoderma for the Treatment of a Degraded and Rhizoctonia Solani Infested Greenhouse Soil. Sci. Hortic. 2016, 206, 51–61. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Zhao, J.; Zhang, J.; Cai, Z. The Families Ruminococcaceae, Lachnospiraceae, and Clostridiaceae Are the Dominant Bacterial Groups during Reductive Soil Disinfestation with Incorporated Plant Residues. Appl. Soil Ecol. 2019, 135, 65–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Kang, Y.; Han, C.; Zhu, T.; Deng, H.; Xie, Z.; Zhong, W. Biochar Amendment in Reductive Soil Disinfestation Process Improved Remediation Effect and Reduced N 2 O Emission in a Nitrate-Riched Degraded Soil. Arch. Agron. Soil Sci. 2020, 66, 983–991. [Google Scholar] [CrossRef]
- Momma, N. Biological Soil Disinfestation (BSD) of Soilborne Pathogens and Its Possible Mechanisms. JARQ 2008, 42, 7–12. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Li, C.; Xu, R.; Pei, Z.; Li, F.; Wu, Y.; Chen, F.; Liang, Y.; Li, Z.; et al. Linking Soil Organic Carbon Dynamics to Microbial Community and Enzyme Activities in Degraded Soil Remediation by Reductive Soil Disinfestation. Appl. Soil Ecol. 2023, 189, 104931. [Google Scholar] [CrossRef]
- Adesodun, J.K.; Mbagwu, J.S.C.; Oti, N. Distribution of Carbon, Nitrogen and Phosphorus in Water-Stable Aggregates of an Organic Waste Amended Ultisol in Southern Nigeria. Bioresour. Technol. 2005, 96, 509–516. [Google Scholar] [CrossRef]
- Wu, M.; Pang, D.; Chen, L.; Li, X.; Liu, L.; Liu, B.; Li, J.; Wang, J.; Ma, L. Chemical Composition of Soil Organic Carbon and Aggregate Stability along an Elevation Gradient in Helan Mountains, Northwest China. Ecol. Indic. 2021, 131, 108228. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M. Soil Aggregate Stabilization and Carbon Sequestration: Feedbacks through Organomineral Associations. In Office of Scientific & Technical Information Technical Reports; Taylor & Francis Group: Abingdon, UK, 1998. [Google Scholar]
- Xiong, S.; Zhu, J.; Yang, J.; Guo, Y.; Meng, X.; Ren, S.; Du, J.; Wen, S.; Jiang, Y.; Shi, J.; et al. Straw Return plus Zinc Fertilization Increased the Accumulations and Changed the Chemical Compositions of Mineral-Associated Soil Organic Carbon. Agric. Ecosyst. Environ. 2023, 357, 108699. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, B.; Jiao, Y.; Liu, C.; Li, X.; Meng, X.; Shi, J.; Tian, X. Effect of the Combined Addition of Mineral Nitrogen and Crop Residue on Soil Respiration, Organic Carbon Sequestration, and Exogenous Nitrogen in Stable Organic Matter. Appl. Soil Ecol. 2022, 171, 104324. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil Macroaggregate Turnover and Microaggregate Formation: A Mechanism for C Sequestration under No-Tillage Agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Le Guillou, C.; Angers, D.A.; Leterme, P.; Menasseri-Aubry, S. Differential and Successive Effects of Residue Quality and Soil Mineral N on Water-Stable Aggregation during Crop Residue Decomposition. Soil Biol. Biochem. 2011, 43, 1955–1960. [Google Scholar] [CrossRef]
- Sall, S.N.; Masse, D.; Diallo, N.H.; Sow, T.M.B.; Hien, E.; Guisse, A. Effects of residue quality and soil mineral n on microbial activities and soil aggregation in a tropical sandy soil in senegal. Eur. J. Soil Biol. 2016, 75, 62–69. [Google Scholar]
- Zhu, Z.; Angers, D.A.; Field, D.J.; Minasny, B. Using Ultrasonic Energy to Elucidate the Effects of Decomposing Plant Residues on Soil Aggregation. Soil Tillage Res. 2017, 167, 1–8. [Google Scholar] [CrossRef]
- Rahman, M.T.; Liu, S.; Guo, Z.C.; Zhang, Z.B.; Peng, X.H. Impacts of Residue Quality and N Input on Aggregate Turnover Using the Combined 13C Natural Abundance and Rare Earth Oxides as Tracers. Soil Tillage Res. 2019, 189, 110–122. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Cadisch, G. Use of Specific Peaks Obtained by Diffuse Reflectance Fourier Transform Mid-Infrared Spectroscopy to Study the Composition of Organic Matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schütt, M.; Smirnova, N.; Schulz, E.; Cadisch, G. Combining a Coupled FTIR-EGA System and in Situ DRIFTS for Studying Soil Organic Matter in Arable Soils. Biogeosciences 2013, 10, 2897–2913. [Google Scholar] [CrossRef]
- Fultz, L.M.; Jennifer, M.K.; Francisco, C.; Veronica, A.-M. Using Fourier-Transform Mid-Infrared Spectroscopy to Distinguish Soil Organic Matter Composition Dynamics in Aggregate Fractions of Two Agroecosystems. Soil Sci. Soc. Am. J. 2014, 78, 1940–1948. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; Leboeuf, E.J.; Pan, H.; Dai, S. Spectroscopic Characterization of the Structural and Functional Properties of Natural Organic Matter Fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Brown, K.H.; Bach, E.M.; Drijber, R.A.; Hofmockel, K.S.; Jeske, E.S.; Sawyer, J.E.; Castellano, M.J. Long-Term Nitrogen Fertilizer Gradient Has Little Effect on Soil Organic Matter in a High-Intensity Maize Production System. Glob. Chang. Biol. 2014, 20, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhi-Fu, P.; Mei, H.; An, X.; Yue-Xian, Z.; Xin, W.; Hui-Xin, Z.; Qin-Guo, S. Chemical Structure Characteristics of Organic Carbon in Saline Soil Aggregates under Straw Returning Condition. Chin. J. Appl. Ecol. 2021, 32, 4401–4410. [Google Scholar]
- Stewart, C.E.; Follett, R.F.; Wallace, J.; Pruessner, E.G. Impact of Biosolids and Tillage on Soil Organic Matter Fractions: Implications of Carbon Saturation for Conservation Management in the Virginia Coastal Plain. Soil Sci. Soc. Am. J. 2012, 76, 1257–1267. [Google Scholar] [CrossRef]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; Asard, H.; AbdElgawad, H.; Shen, W.; Janssens, I.A. High Clay Content Accelerates the Decomposition of Fresh Organic Matter in Artificial Soils. Soil Biol. Biochem. 2014, 77, 100–108. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-Nutrient Stoichiometry to Increase Soil Carbon Sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627. [Google Scholar] [CrossRef]
- Kang, S.Z.; Liu, X.M.; Gao, X.K.; Xiong, Y.Z. Computer Simulation of Water Transport in Soil-Plant-Atmosphere Continuum. J. Hydraul. Eng. 1992, 3, 1–12. [Google Scholar]
- Matamala, R.; Jastrow, J.D.; Calderón, F.J.; Liang, C.; Fan, Z.; Michaelson, G.J.; Ping, C.L. Predicting the Decomposability of Arctic Tundra Soil Organic Matter with Mid Infrared Spectroscopy. Soil Biol. Biochem. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Bucka, F.B.; Klbl, A.; Uteau, D.; Peth, S.; Kgel-Knabner, I. Organic Matter Input Determines Structure Development and Aggregate Formation in Artificial Soils. Geoderma 2019, 354, 113881. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Huang, S.; Rui, W.; Peng, X.; Huang, Q.; Zhang, W. Organic Carbon Fractions Affected by Long-Term Fertilization in a Subtropical Paddy Soil. Nutr. Cycl. Agroecosyst. 2010, 86, 153–160. [Google Scholar] [CrossRef]
- Cosentino, D.; Chenu, C.; Bissonnais, Y.L. Aggregate Stability and Microbial Community Dynamics under Drying-Wetting Cycles in a Silt Loam Soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Jin, X.; An, T.; Gall, A.R.; Li, S.; Filley, T.; Wang, J. Enhanced Conversion of Newly-Added Maize Straw to Soil Microbial Biomass C under Plastic Film Mulching and Organic Manure Management. Geoderma 2018, 313, 154–162. [Google Scholar] [CrossRef]
- Xu, X.; An, T.; Zhang, J.; Sun, Z.; Schaeffer, S.; Wang, J. Transformation and Stabilization of Straw Residue Carbon in Soil Affected by Soil Types, Maize Straw Addition and Fertilized Levels of Soil. Geoderma 2019, 337, 622–629. [Google Scholar] [CrossRef]
- Guan, S.; Dou, S.; Chen, G.; Wang, G.; Zhuang, J. Isotopic Characterization of Sequestration and Transformation of Plant Residue Carbon in Relation to Soil Aggregation Dynamics. Appl. Soil Ecol. 2015, 96, 18–24. [Google Scholar] [CrossRef]
- Bravo-Garza, M.R.; Voroney, P.; Bryan, R.B. Particulate Organic Matter in Water Stable Aggregates Formed after the Addition of (14)C-Labeled Maize Residues and Wetting and Drying Cycles in Vertisols. Soil Biol. Biochem. 2010, 42, 953–959. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A History of Research on the Link between (Micro)Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Hu, R.; Li, Y. Aggregate Stability and Size Distribution of Red Soils under Different Land Uses Integrally Regulated by Soil Organic Matter, and Iron and Aluminum Oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef]
- Oliveira, M.; Barre, P.; Trindade, H.; Virto, I. Different Efficiencies of Grain Legumes in Crop Rotations to Improve Soil Aggregation and Organic Carbon in the Short-Term in a Sandy Cambisol. Soil Tillage Res. 2019, 186, 23–35. [Google Scholar] [CrossRef]
- Zhao, H.; Ning, P.; Chen, Y.; Liu, J.; Ghaffar, S.A.; Xiaohong, T.; Shi, J. Effect of Straw Amendment Modes on Soil Organic Carbon, Nitrogen Sequestration and Crop Yield on the North-Central Plain of China. Soil Use Manag. 2019, 35, 511–525. [Google Scholar] [CrossRef]
- Bearden, B.N.; Petersen, L. Influence of Arbuscular Mycorrhizal Fungi on Soil Structure and Aggregate Stability of a Vertisol. Plant Soil 2000, 218, 173–183. [Google Scholar] [CrossRef]
- Gosling, P.; Parsons, N.; Bending, G.D. What Are the Primary Factors Controlling the Light Fraction and Particulate Soil Organic Matter Content of Agricultural Soils? Biol. Fertil. Soils 2013, 49, 1001–1014. [Google Scholar] [CrossRef]
- Du, Z.-L.; Zhao, J.-K.; Wang, Y.-D.; Zhang, Q.-Z. Biochar Addition Drives Soil Aggregation and Carbon Sequestration in Aggregate Fractions from an Intensive Agricultural System. J. Soils Sediments 2017, 17, 581–589. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.K.; Saha, S.; Mani, P.K.; Mandal, B. Effect of Organic Inputs on Aggregate Associated Organic Carbon Concentration under Long-Term Rice–Wheat Cropping System. Geoderma 2010, 154, 379–386. [Google Scholar] [CrossRef]
- Caesar-TonThat, T.; Sainju, U.M.; Wright, S.F.; Shelver, W.L.; Kolberg, R.L.; West, M. Long-Term Tillage and Cropping Effects on Microbiological Properties Associated with Aggregation in a Semi-Arid Soil. Biol. Fertil. Soils 2011, 47, 157–165. [Google Scholar] [CrossRef]
- Gao, Y.; Song, X.; Liu, K.; Li, T.; Zheng, W.; Wang, Y.; Liu, Z.; Zhang, M.; Chen, Q.; Li, Z.; et al. Mixture of Controlled-Release and Conventional Urea Fertilizer Application Changed Soil Aggregate Stability, Humic Acid Molecular Composition, and Maize Nitrogen Uptake. Sci. Total Environ. 2021, 789, 147778. [Google Scholar] [CrossRef]
- Shenzhong, T.; Yu, W.; Yufeng, Z.; Wenfan, B.; Liang, D.; Jiafa, L.; Honghai, G. Residue Returning with Subsoiling Replacing Rotary Tillage Improving Aggregate and Associated Carbon. Trans. Chin. Soc. Agric. Eng. 2017, 33, 133–140. [Google Scholar]
- Hsiao-Tieh, H.; Corey, L.; Matthew, W.; John, B.; Katharine, M. A Molecular Investigation of Soil Organic Carbon Composition across a Subalpine Catchment. Soil Syst. 2018, 2, 6. [Google Scholar] [CrossRef]
- Kubar, K.A.; Huang, L.; Xue, B.; Li, X.; Lu, J. Straw Management Stabilizes the Chemical Composition of Soil Organic Carbon (SOC): The Relationship with Aggregate-associated C in a Rice-rape Cropping System. Land Degrad. Dev. 2021, 32, 851–866. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Gillespie, A.; Peak, D.; Van Rees, K.C.J. Spectroscopic Investigation of Soil Organic Matter Composition for Shelterbelt Agroforestry Systems. Geoderma 2017, 298, 1–13. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Kubar, K.A.; Lu, J. Straw Management Influences the Stabilization of Organic Carbon by Fe (Oxyhydr)Oxides in Soil Aggregates. Geoderma 2019, 358, 113987. [Google Scholar] [CrossRef]
- Chung, H.; Ngo, K.J.; Plante, A.; Six, J. Evidence for Carbon Saturation in a Highly Structured and Organic-Matter-Rich Soil. Soil Sci. Soc. Am. J. 2010, 74, 130–138. [Google Scholar] [CrossRef]
- De Gryze, S.; Six, J.; Brits, C.; Merckx, R. A Quantification of Short-Term Macroaggregate Dynamics: Influences of Wheat Residue Input and Texture. Soil Biol. Biochem. 2005, 37, 55–66. [Google Scholar] [CrossRef]


| Materials | TC (%) | TN (g/kg) | TP (g/kg) | TK (g/kg) | SOC (g/kg) | DOC (mg/kg) | MBC (mg/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) | pH | EC (us/cm) | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 1.59 | 1.1 | 0.78 | 20.1 | 8.62 | 119.40 | 161.24 | 44.19 | 7.27 | 8.48 | 367.67 | 14.5 |
| Wheat straw | 41.1 | 7.4 | 5.2 | 29.3 | - | - | - | - | - | - | - | 55.5 |
| Kiwifruit branches | 56.5 | 5.1 | 10.2 | 16.2 | - | - | - | - | - | - | - | 110.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Hao, J.; Wang, J.; Xiong, S.; Jiang, Y.; Zhu, Y.; Jiao, Y.; Yang, J.; Zhu, J.; Tian, X. Enhancing Soil Aggregation and Organic Carbon Retention in Greenhouse Vegetable Production through Reductive Soil Disinfestation with Straw and Fertiliser: A Comprehensive Study. Agronomy 2024, 14, 179. https://doi.org/10.3390/agronomy14010179
Wen S, Hao J, Wang J, Xiong S, Jiang Y, Zhu Y, Jiao Y, Yang J, Zhu J, Tian X. Enhancing Soil Aggregation and Organic Carbon Retention in Greenhouse Vegetable Production through Reductive Soil Disinfestation with Straw and Fertiliser: A Comprehensive Study. Agronomy. 2024; 14(1):179. https://doi.org/10.3390/agronomy14010179
Chicago/Turabian StyleWen, Shanju, Jiaqi Hao, Jiangyuzhuo Wang, Shijuan Xiong, Yuhan Jiang, Yihui Zhu, Yapeng Jiao, Jinglin Yang, Jinli Zhu, and Xiaohong Tian. 2024. "Enhancing Soil Aggregation and Organic Carbon Retention in Greenhouse Vegetable Production through Reductive Soil Disinfestation with Straw and Fertiliser: A Comprehensive Study" Agronomy 14, no. 1: 179. https://doi.org/10.3390/agronomy14010179





