Abstract
Pre-harvest sprouting (PHS) is a condition triggered by environmental factors, particularly prevalent in humid conditions, leading to substantial yield losses in black gram. While the potential for genotypic PHS tolerance exists, it has not been thoroughly assessed in black gram. Hence, the present study aimed to delve into the genetic variation for PHS tolerance in diverse black gram (Vigna mungo (L.) Hepper) germplasm, and also to comprehend the impact of various physical and physiological traits on PHS. A diverse set of 112 black gram accessions collected across the phytogeographical zones of India were examined for their seed and pod characteristics. Water absorption by pods and seeds and fresh-seed germination was calculated by following the standard procedure given by the International Seed Testing Association. The alpha-amylase activity was measured on dry seeds (0 h), 24 h, 48 h, and 72 h after germination of each accession, using a UV-VIS spectrophotometer, and hard-seededness was measured using a texture analyzer machine. The results showed a wide range in PHS tolerance and FSG, and 13 accessions were found to be PHS-tolerant (PHS value < 10%). An indicator of PHS, seed germination in a pod, ranged from 2.75% in IC485641 (highly tolerant to PHS) to 95.85% in IC530501 (highly susceptible to PHS). Correlation and multivariate analysis revealed that PHS was positively correlated with water imbibition by pod and seed, fresh-seed germination and alpha-amylase activity. PHS-tolerant accessions showed a slow increase in alpha-amylase activity, in contrast to PHS-susceptible accessions. The utilization of alpha-amylase activity as a biochemical marker has the potential for evaluating PHS tolerance across various black gram accessions. The identified PHS-tolerant accessions can be used as donors in crop improvement programs aimed at developing PHS-tolerant black gram varieties.
1. Introduction
India is the world’s largest producer of black gram (Vigna mungo (L.) Hepper), with a cultivation area of 4.63 million ha with a production output of 2.78 million tons (Indiastat.com). However, the productivity of black gram in India faces significant challenges, due to both biotic factors like mung bean yellow mosaic disease (MYMV), powdery mildew, (Erysiphe polygoni), bruchids (Callosobruchus maculates and C. chinensis [1]), and abiotic factors including pre-harvest sprouting, drought, and salinity. The traditional breeding approaches for black gram have scarcely tapped into the potential of germplasm resources, resulting in a notably narrow genetic pool in the cultivated species. Consequently, efforts directed at the genetic enhancement of black gram have faced challenges in overcoming the fundamental restrictions on crop output. Throughout the process of domestication, humans intentionally selected crop plants for their desirable characteristics that facilitated cultivation. As a result, these cultivated varieties evolved over time, differentiating them genetically from their wild ancestors. Some noteworthy examples include less shattering of seed, change in the mechanisms of seed dispersal, early maturity, a decrease in seed phenol or tannin content, the development of hard seed coats, seed size variations, and alternation in seed coat color [2]. However, reduced dormancy in cultivated seeds has led to challenges like pre-harvest sprouting [3].
PHS is a phenomenon of germination of physiologically mature grains in the ear or panicle or pod, usually under wet conditions shortly before harvest, leading to undesirable degradation of seed starch [4]. This procedure typically takes place when mature seeds are exposed to protracted periods of rainy or humid environments before mature seeds are harvested. Farmers may experience difficulties with PHS and suffer huge financial losses. Under typical circumstances, seeds do not germinate unless they are exposed to the favorable environmental aspects, such as moisture, temperature, and light. However, moisture can penetrate the seed coat and trigger the germination process early if there is a lengthy period of rain or high humidity before harvest. This has been reported in many crops, including black gram [5] green gram [6] peanut [7] and soybean [8]. Sprouting before harvest can harm crops in a variety of ways. First of all, germination renders seeds unfit for processing or eating because they lose their nutritional content. The energy reserves of the seed are used up during sprouting, resulting in decreased grain yield and quality. Additionally, during harvest and handling, the seedlings may be more vulnerable to microbial attack. Moreover, sprouted grains can have a worse baking quality, which will lower the crops’ overall worth. Black gram is suitable for growing all year round because of its short crop cycle and low input needs [9], and has the potential to grow and thrive in a variety of ecological circumstances [10]. But, during the wet harvest season, PHS has been identified as one of the main factors limiting crop productivity and quality [5,11]. PHS-related losses in black gram have been widely reported in different regions, often attributed to unpredictable climatic conditions such as untimely rainfall during maturity. Hence, there is a growing need to identify black gram accession with PHS tolerance and gain insights into the underlying mechanisms. Therefore, this current study was designed to evaluate diverse black gram germplasm for PHS tolerance, focusing on morpho–physiological characteristics of pods and seeds, as well as the evaluation of alpha-amylase activity associated with PHS tolerance in black gram.
2. Material and Method
2.1. Field Trial
Black gram germplasm (112 accessions) procured from the National Gene Bank, was grown at ICAR-NBPGR Pusa Farm during rainy season of 2022. A geo-referenced map representing the collection site of black gram accessions is shown in Figure 1. The research farm is situated at a latitude of 28.38° N and a longitude of 77.10° E; the altitude was 228.61 m above mean sea level. The soil at this site is sandy loam, offering an ideal pH balance for plant growth. Black gram accessions were planted under natural field conditions, following the recommended cultivation practices for this crop. The experimental design employed was an augmented block design [12]. The accessions were sown in paired rows, where each row measured two meters in length, with a distance of 30 cm between rows. To assess PHS tolerance, we implemented germination tests on harvest-ready pods and seeds under regulated conditions, following methodologies from studies [3,13,14,15,16,17]. In the field, black gram pods were collected at their physiological maturity. To maintain grain dormancy, these pods were stored at a temperature of −20 °C until the completion of the experiment [18]. During both the harvesting and subsequent handling, precautions were taken to make sure the pod wall remained undamaged and intact.
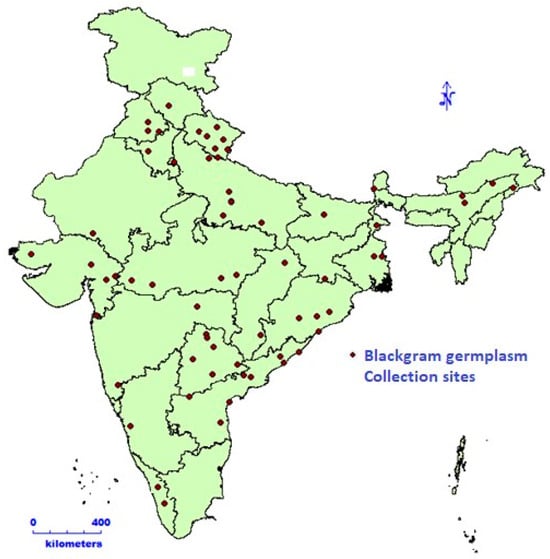
Figure 1.
Geo-referenced map representing collection site of black gram accessions.
2.2. Physiological Traits of Pods and Seeds
2.2.1. Water Imbibition in Pod (WIP)
Special care was taken to harvest undamaged and intact pods at physiological maturity, which were used to determine the water imbibition by pod. Ten pods of each accession after weighing on balance (Citizon, CX 200; d: 0.0001 g), each with three replications, were inserted in rolled paper towels by using the between-paper method of germination [19]. Rolls were kept in the incubator (MKSI, Instrument, with timer, temperature and light controller) maintained at a constant temperature of 25 ± 1 °C for a period of 24 h upright, and wrapped in plastic bags to preserve moisture in the pods. The pods were taken out after 24 h, dried off, weighed and then put back onto the germination paper. By calculating the weight gain, the amount of water imbibed by the pods was determined.
2.2.2. Seed Germination in a Pod (Indicates PHS Value)
After observing water imbibition in pods, the replication of the pods was kept for 4 days in the incubator at 25 ± 1 °C. After 4 days of incubation, germination of the seed in a pod (%) was recorded by following [3,16,18]. The seeds were estimated to have germinated only when the radical reached roughly 2 mm in length. The germination rate for each pod was calculated by comparing the number of sprouted seeds to the total seed count in that pod. The seed germination in the pods indicates the PHS value.
2.2.3. Water Imbibition by Seeds (WIS)
Following the top-of-paper method, 20 seeds from the replication of 3 were kept on top of Whatman No. 1 filter paper [19]. Then, the Petri dishes, covered with a lid, were kept upright at 25 ± 1 °C in the incubator. After 6 h, the seeds were taken off the germination paper, blotted dry, and weighed once more. The weight gain was used to calculate the water imbibition by seeds, which is expressed in (%).
2.2.4. Fresh-Seed Germination (FSG)
After recording water imbibition by seeds, the replication of the seeds was kept for 4 days in the incubator at 25 ± 1 °C. After 4 days of incubation, the percentage of seeds that germinated was noted, and fresh-seed germination was calculated and expressed in percentage.
2.2.5. Physical Traits of Pod and Seed
At maturity, 30 fully ripe pods were selected and harvested. These pods were then analyzed for pod width, pod length and thickness of pod wall, using an electronic Vernier Caliper. Similarly, 30 randomly selected seeds were used to measure seed length and seed width, using the electronic Vernier caliper (Fisher Scientific, Hampton, NH, USA, S/N:101860790; Stainless Hardened).
2.3. Seed Hardness (SH)
After harvesting the seeds of 112 accessions, they were dried to 9–11% moisture content. The force needed to break a seed was measured with a texture analyzer machine (model-TA. HD plus C texture analyzer) and texture expert software. A cylindrical probe of 30 mm was used for measuring the texture. The force in gm was used to measure compression (g). The instrument was calibrated with a 50 kg load cell and a strain of 20%. The test speed was 1 mm/s with pre-speed and post-speed of 1 mm/s and 10 mm/s, respectively. The probe was allowed to compress, with a trigger force of 5 g, into the sample. Fifteen replicates were tested for each accession, and the recorded values from the resulting one-compression cycle for each test were used. From the force–displacement curve, the maximum force required to break the sample was taken as hardness. Hardness data were extracted using software (Exponent Connect Lite, Stable Micro Systems, Surrey GU7 1YL, UK) provided by the instrument manufacturer [20].
2.3.1. Alpha-Amylase Activity
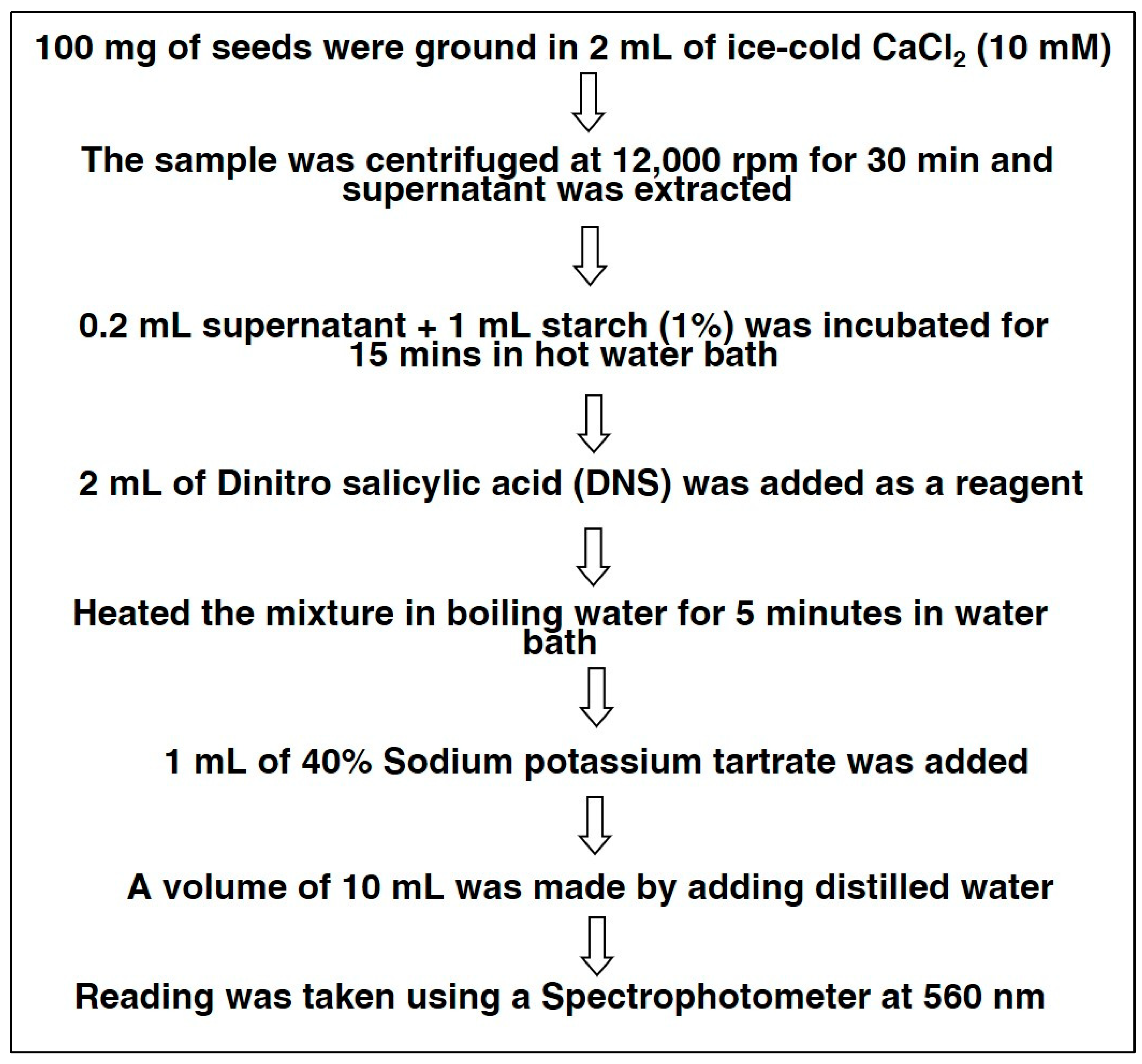
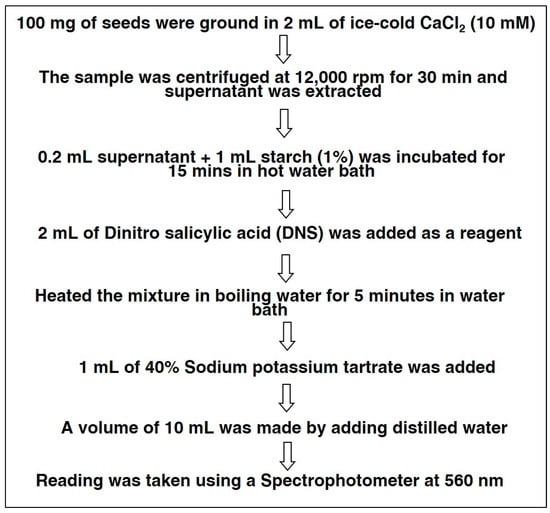
The activity of alpha-amylase was assessed on dry seeds, (0 h), 24 h, 48 h, and 72 h after germination of each accession. The methodology of alpha-amylase activity is presented in Scheme 1. For the estimation of alpha-amylase, 100 mg seeds of black gram were extracted in the 10 mL of cold 10 mM CaCl2 and were centrifuged at 12,000 rpm for 30 min. Then, the supernatant was separated, and 1 mL starch solution was added to 0.2 mL of supernatant. This mixture was incubated for 15 min at 27 °C in a hot water bath. Then, 2 mL dinitro-salicylic acid was added as a reagent. The reaction mix was heated for 5 min in a hot water bath, and 1 mL sodium potassium tartrate (40%) was added; then, distilled water was used to make a final volume of 10 mL. A reading was taken at 560 nm, using a UV-VIS spectrophotometer [21].
Scheme 1.
Methodology for Alpha-Amylase Estimation.
2.3.2. Statistical Analysis of the Data
All data were subjected to an analysis of variance (ANOVA), using completely randomized design (CRD), and a comparison of treatment means was performed using Duncan’s multiple range test (DMRT) at p < 0.05, using IBM SPSS Statistics software, Version 20. The mean values of individual genotypes for each trait were subsequently used for analyzing summary statistics and graphs, using MS Excel software, Version 2311. The homogeneity of variance was tested using Levene’s test. The histogram, correlation, regression, and hierarchical cluster analysis among traits were analyzed using SAS JMP Statistics software Version 17. Based on the data, accessions will be categorized into categories, viz., tolerant (<10% germination of seeds in the pod); moderately tolerant (10–30% germination of seeds in the pod); moderately susceptible (30–70% germination of seeds in the pod); and highly susceptible (>70% germination of seeds in the pod).
3. Results
3.1. Genotypic Variation for Morpho–Physiological Traits
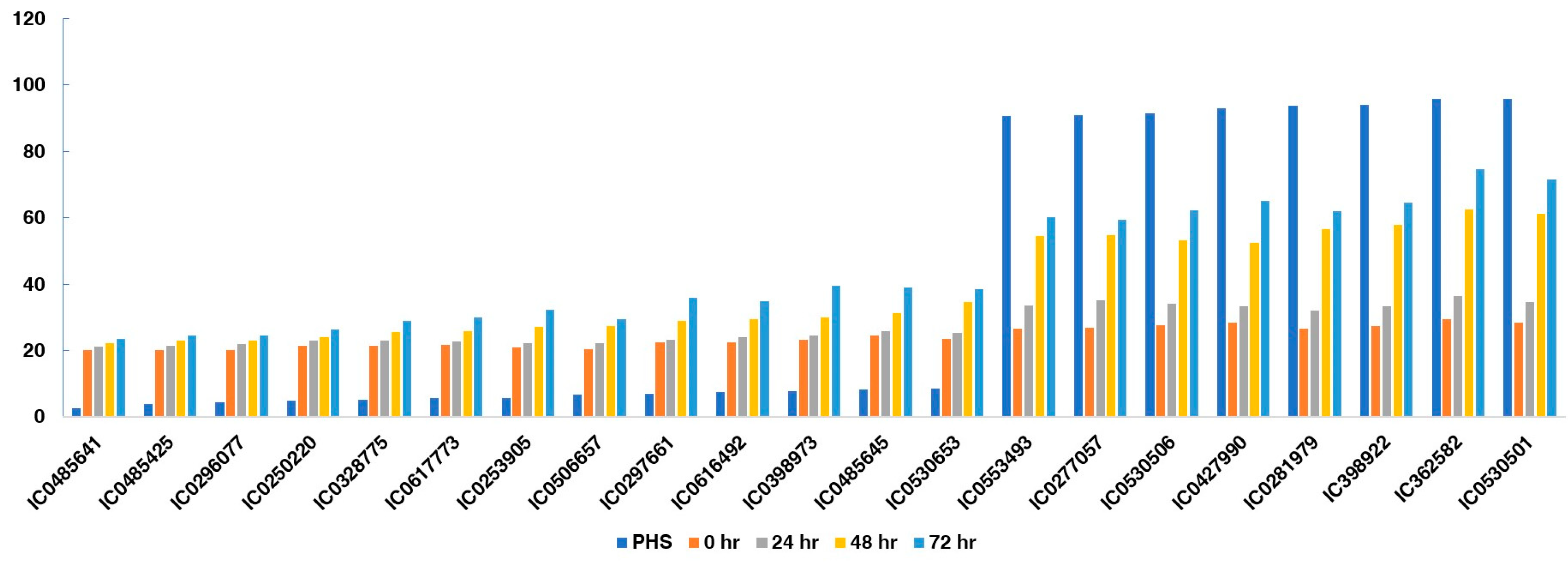
The combined analysis of variance (ANOVA) revealed significant variation for all the accessions for the studied traits, at a one-per-cent level of significance (Table 1). The descriptive statistics for 14 morpho–physiological traits of 112 black gram accessions are presented in Table 2. Pod length varied from 3.70 cm to 5.23 cm (mean 4.33 cm) and pod width from 3.31 mm to 5.00 mm (mean 4.29 mm). Seed length and width ranged from 3.67 mm to 5.23 mm and 2.47 mm and 3.73 mm, with an average of 4.39 mm and 3.19 mm, respectively. The mean pod-wall thickness was 0.39 mm, with a minimum of 0.28 mm and a maximum of 0.63 mm. Seed hardness varied from 4218.71 g to 8868.52 g, with a mean of 6378.33 g. The WIP varied greatly, from 4.59% to 90.76%, with a mean of 43.16%. WIS ranged from 1.63% to 66.68%, with a mean of 26.16%. FSG ranged from 10.76% to 100.00%, with an average of 54.58%. The accessions such as IC485425, IC485641, IC617773, and IC485645 showed minimum FSG (>15%), whereas accessions with a maximum FSG (100%) were IC530501, IC277057, and Mash 479. The PHS was found to range from 2.75% to 95.85%, with a mean of 44.69%. The accessions showing a minimum of PHS (<10%) were IC485641, IC485425, IC296077, IC250220, IC328775, IC617773, IC253905, IC506657, IC297661, IC616492, IC398973, IC485645, and IC530653, and those with maximum PHS (>90%) were IC553493, IC277057, IC530506, IC427990, IC281979, IC398922, IC362582, and IC530501 (Table 3).

Table 1.
Analysis of variance for morpho–physiological traits of black gram accessions.

Table 2.
Descriptive statistics of morpho–physiological traits of pods and seeds of black gram accessions.

Table 3.
Categorization of black gram accessions based on the tolerance to PHS.
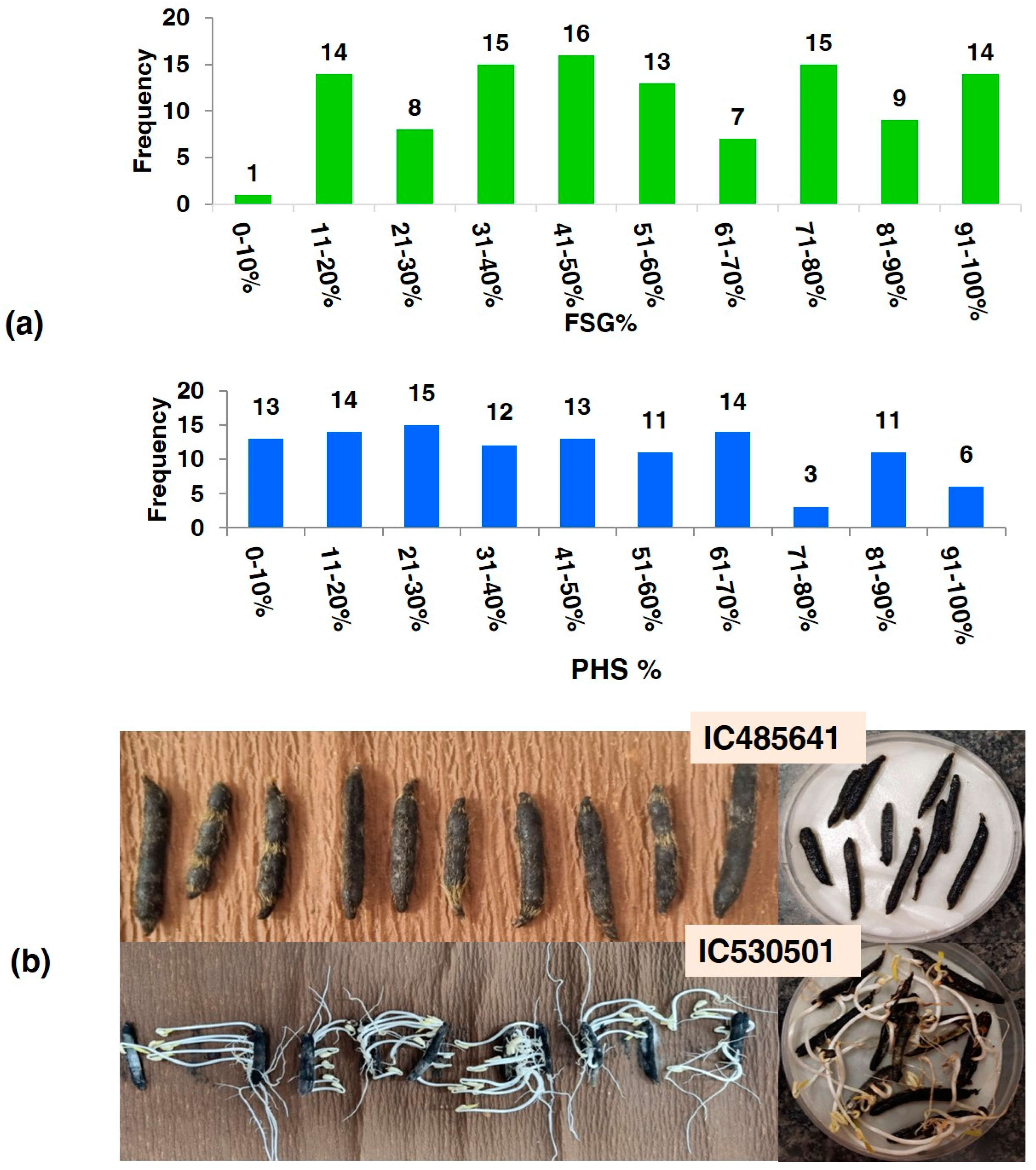
The activity of alpha-amylase was measured on dry seeds, (0 h), 24 h, 48 h, and 72 h after germination of all 112 accessions. The alpha-amylase activity at 0 h (20.12 to 30.25), at 24 h (21.12 to 36.54), at 48 h (22.13 to 62.45) and at 72 h (23.45 to 74.56) was measured for mg maltose hydrolyzed per min for all accessions. The greatest genotypic variations, as assessed by the coefficient of variation (CV), were noted for WIS (62.73), followed by PHS (60.82). The frequency of accessions based on PHS value and FSG- and PHS-tolerant accession (IC485641) showing no germination, and susceptible genotypes (IC530501) showing profuse germination of seeds inside pods, are depicted in Figure 2.
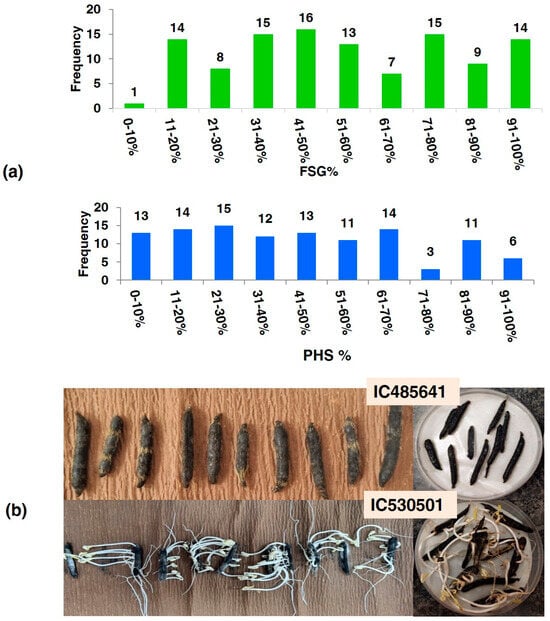
Figure 2.
(a) Frequency of accessions on the basis of PHS value and FSG; (b) PHS-tolerant accession (IC485641) showing no germination, and susceptible genotypes (IC530501) showing profuse germination of seeds inside pods.
3.2. Correlation and Regression Analysis
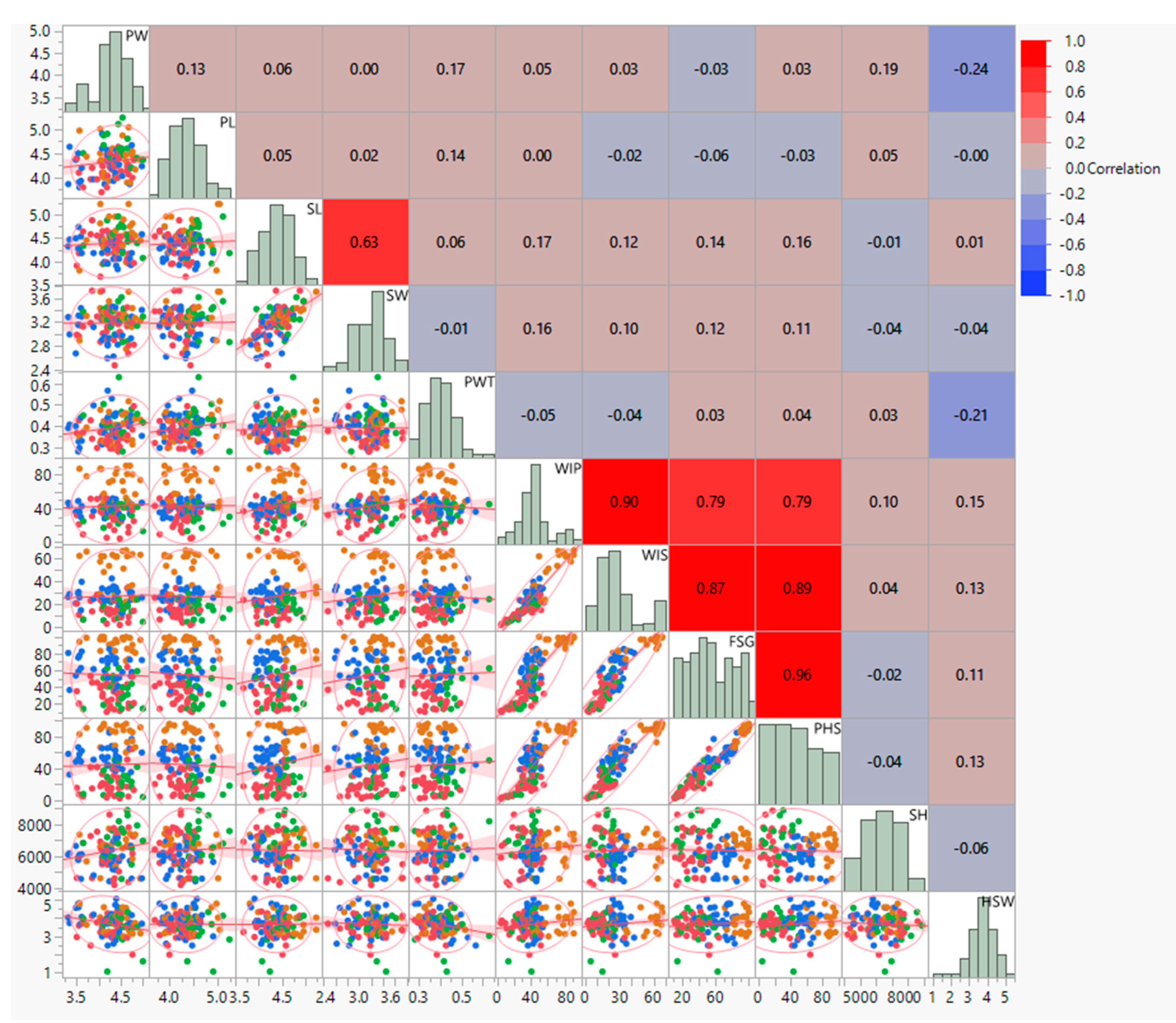
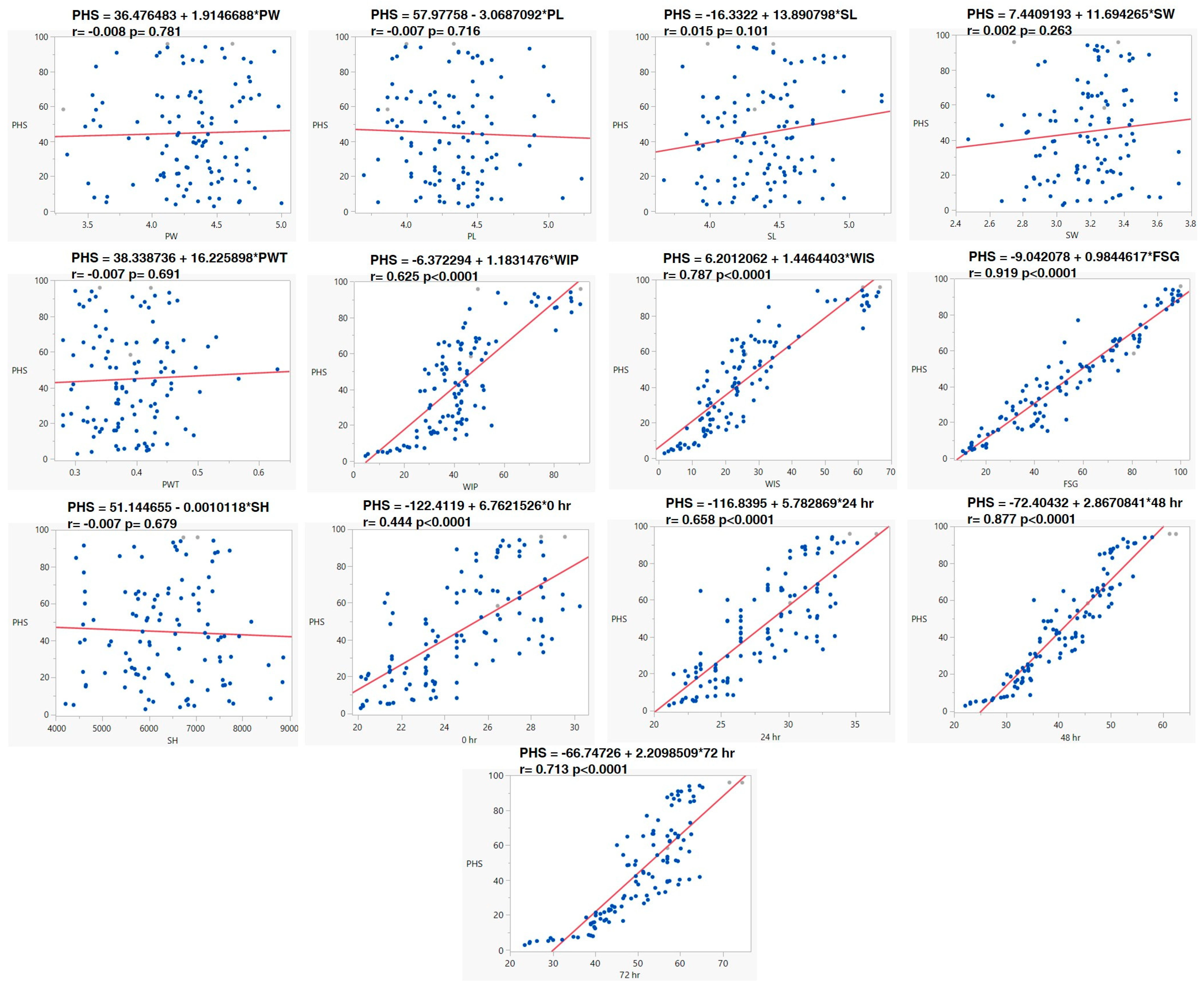
Correlation studies showed significant positive associations between PHS and fresh-seed germination (r. = +0.959 **), water imbibition by pods (r. = +0.793 **), and water imbibition by seeds (r. = +0.888 **). In contrast, there was a non-significant relation between pod length (r. = −0.035) and width (r. = +0.027), and seed length (r. = + 0.156) and seed width (r. = + 0.106). Seed hardness has a negative association with fresh-seed germination (r. = −0.023) and pre-harvest sprouting (r. = −0.040). Pod-wall thickness (PWT) is negatively correlated with water imbibition by pod (r. = −0.053) and water imbibition by seeds (r. = −0.038). In the correlation study of PHS with alpha-amylase activity, PHS showed a positive correlation with alpha-amylase activity at 0 h (r. = 0.670 **), 24 h (r. = 0.813 **), 48 h (r. = 0.937 **) and 72 h (r. = 0.846 **) of seed germination (Figure 3).
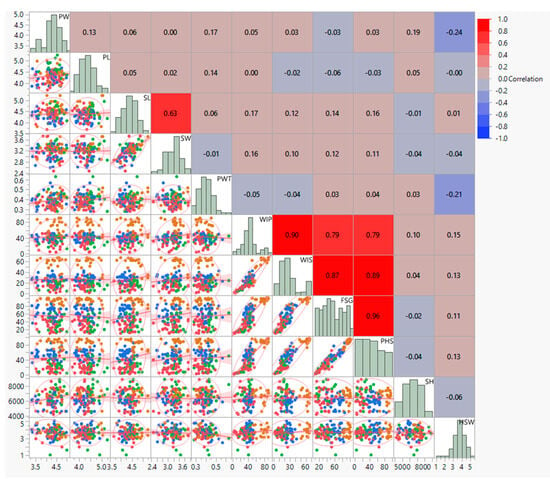
Figure 3.
Scatter−plot matrix and correlation coefficient morpho–physiological traits of black gram accessions. PW: pod width (mm), PL: pod length (cm), SL: seed length (mm), SW: seed width (mm), PWT: pod-wall thickness (mm), WIP: water imbibition by pod (%), WIS: water imbibition by seed (%), FSG: fresh-seed germination (%), PHS: pre-harvest sprouting (%), SH: seed hardness (g).
Likewise, the relationship association was also supported by regression analysis. Regression analysis revealed that PHS had a positive association with WIP (r. = 0.625, p < 0.001), WIS (r. = 0.787, p < 0.0001) and FSG (r. = 0.919, p < 0.0001). A regression analysis study was carried out between PHS and increases in alpha-amylase at 0, 24, 48, and 72 h. It revealed that PHS had a positive association with an increase in alpha-amylase at 0 h (r. = 0.444, p < 0.0001), 24 h (r. = 0.658, p < 0.0001), 48 h (r. = 0.877, p < 0.0001), and 72 h (r. = 0.713, p < 0.0001) of germination (Figure 4).
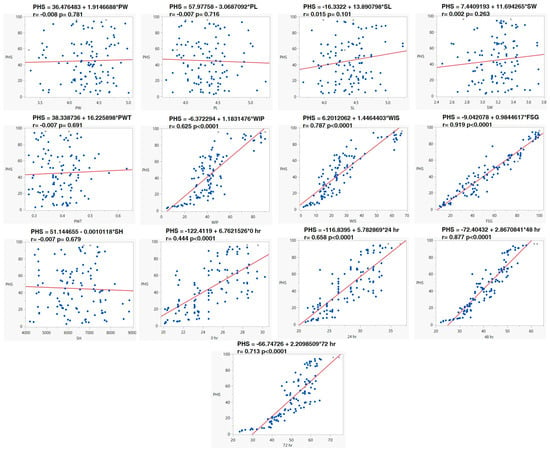
Figure 4.
Linear relationship of PHS with other morpho–physiological and biochemical traits of pods and seeds. Pod width (PW), Pod length (PL), Seed length (SL), Seed width (SW), Pod−wall thickness (PWT), Water imbibition by pod (WIP), Water imbibition by seed (WIS), Fresh-seed germination (FSG), Seed hardness (SH) and α−amylase activity at 0, 24, 48, and 72 h of germination.
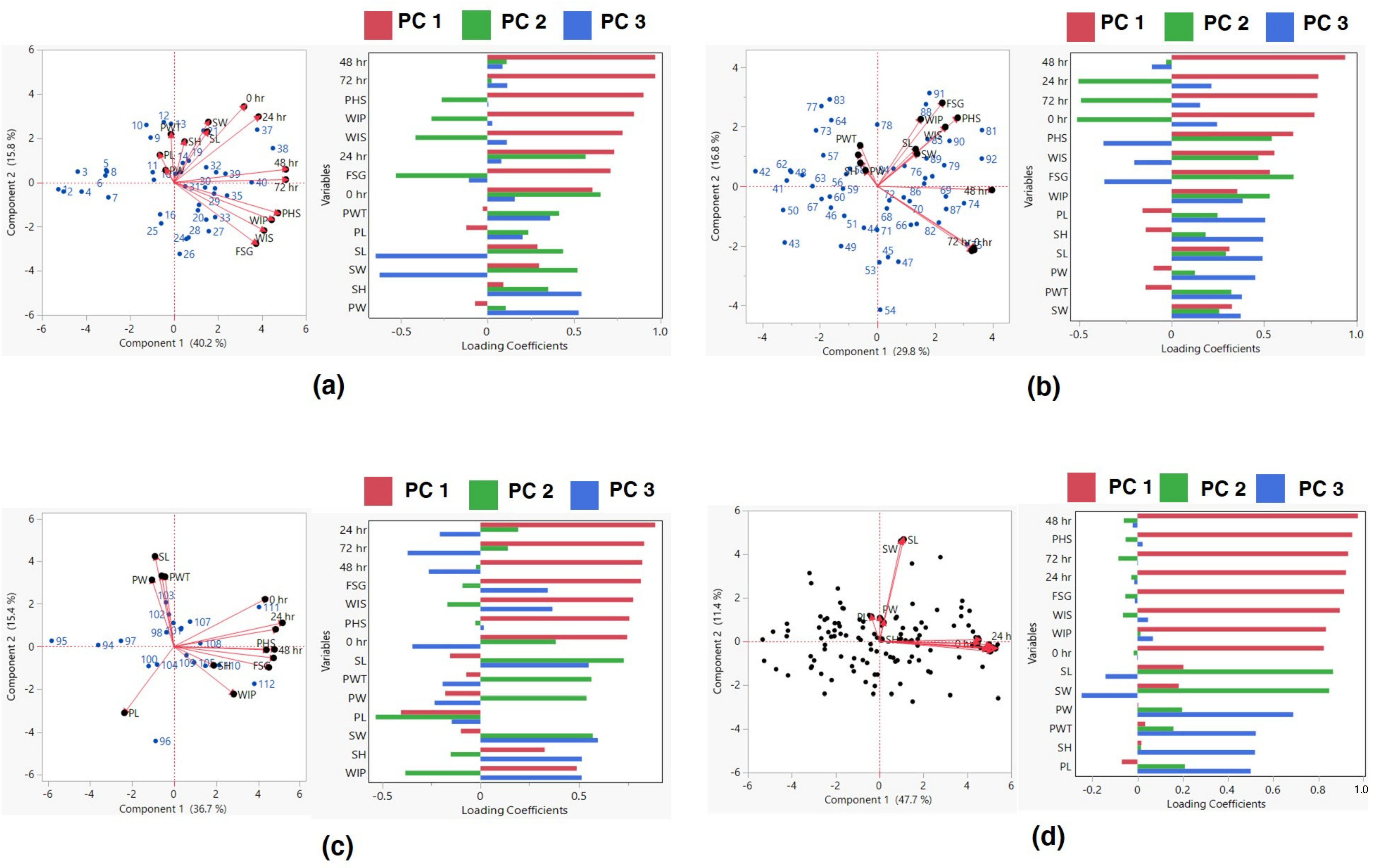
Further, the linear regression models for three groups, viz., Group I with PHS value < 10%, Group II with PHS value 30–70%, and Group III with PHS value > 70%, were made. Linear regression indicated that PHS had a significant positive correlation with WIP, WIS and FSG, and alpha-amylase activity at 24, 48 and 72 h for Group I (<30% PHS). Similarly, there were significant positive associations between PHS and WIP, and FSG and alpha-amylase activity at 48 h for the accessions belonging to Group II (30–70%PHS). In the accessions with >70% PHS, i.e., Group III, a positive correlation was observed among PHS, FSG, and the activity of alpha-amylase at 48 and 72 h (Table 4). To gain a comprehensive understanding of the interrelationships between various traits and their roles in the variability of black gram accessions, a biplot was conducted. The first three principal components (PCs) effectively summarized the data, with eigenvalues exceeding 1. Together, they accounted for approximately 68% of the total variation across the 112 black gram accessions, specifically for pod and seed traits (Table 5). PC1 explained around 48% of this variation, highlighting traits such as PHS, FSG, WIP, WIS, and alpha-amylase activity at intervals of 0, 24, 48, and 72 h PC2, accounting for approximately 11.43% of the variation, mainly emphasized seed length and seed width. However, WIS, FSG, PHS, and alpha-amylase activity at 0, 24, 48, and 72 h were negatively associated. Meanwhile, PC3, which covered about 10% of the variation, was primarily influenced by attributes like pod width, pod length, and pod-wall thickness. On the other hand, traits like seed length, seed width, FSG, and alpha-amylase activity at 24 and 48 h exhibited a negative association (Figure 5).

Table 4.
Linear regression models explaining associations of pre-harvest sprouting with seed and pod traits of black gram accessions.

Table 5.
Covariance matrix of principal components of morpho–physiological traits of black gram accessions.
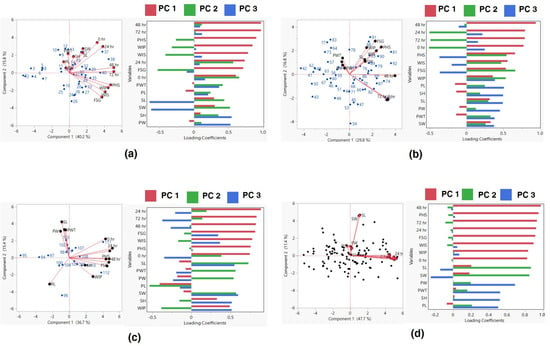
Figure 5.
Scatter plot and loading values of group I (a), group II (b) group III (c), and all accessions (d) based on PCA. PW: pod width (mm), PL: pod length (cm), SL: seed length (mm), SW: seed width (mm), PWT: pod−wall thickness (mm), WIP; water imbibition by pod (%), WIS: water imbibition by seed (%), FSG: fresh−seed germination (%), PHS: pre−harvest sprouting (%), SH: seed hardness (g) and α-amylase activity at 0, 24, 48, and 72 h of germination.
3.3. Clustering of Black Gram Accessions
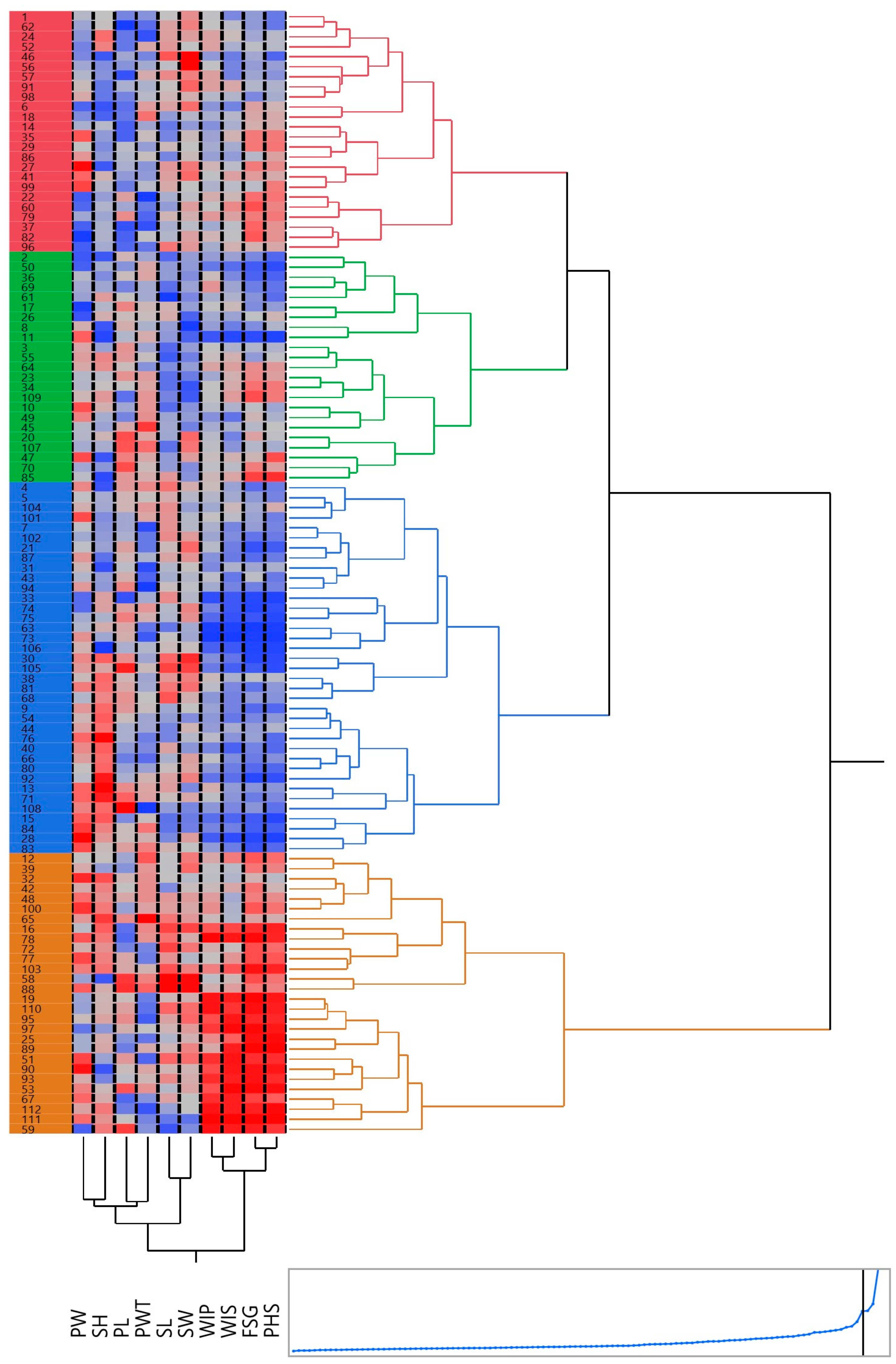
Grouping various accessions into cohesive clusters aids in identifying diverse parents. This approach facilitates the meticulous assessment of all possible pairings among individuals, enabling the amalgamation of distinct gene combinations. Such a method leads to generating the desired progeny through the strategic crossbreeding of diverse parent lines. To evaluate the genetic diversity, a hierarchical cluster analysis was performed, using Ward’s method of minimum variance. The phenotypic relationships between black gram accessions were established using Euclidean distances, based on 14 traits. All the accessions were categorized into four distinct clusters, each cluster containing a varying number of accessions (Figure 6). The clusters were visualized using a heatmap, depicting the trait variations by the different colors in different clusters. The highest number of accessions (43) was recorded in Cluster I, followed by Cluster II with 38 accessions and Cluster IV with 17 accessions, while Cluster III had a minimum, i.e., 14 accessions. PHS-tolerant accessions with less than 10% PHS value were clustered together in Cluster I, while PHS-susceptible accessions were grouped in Cluster IV.
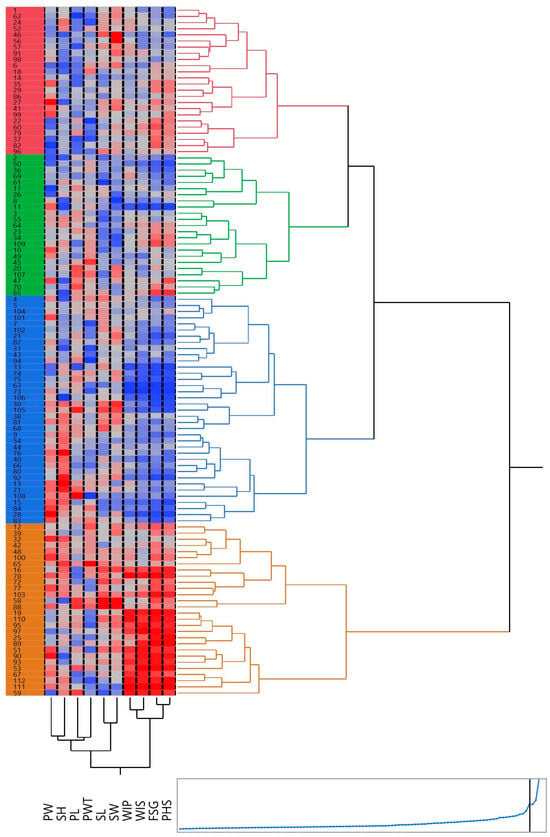
Figure 6.
A dendrogram, paired with a heatmap, produced for 112 blackgram accessions using hierarchical cluster analysis. These accessions are grouped into 4 clusters, each distinguished by a unique color. The two-dimensional heatmap consists of columns and rows: columns represent different traits, while rows signify individual accessions. A brighter red color indicates a higher trait value whereas brighter blue indicates lower trait values.
3.4. Changes in Alpha-Amylase Activity during Germination in PHS-Susceptible and -Tolerant Accessions
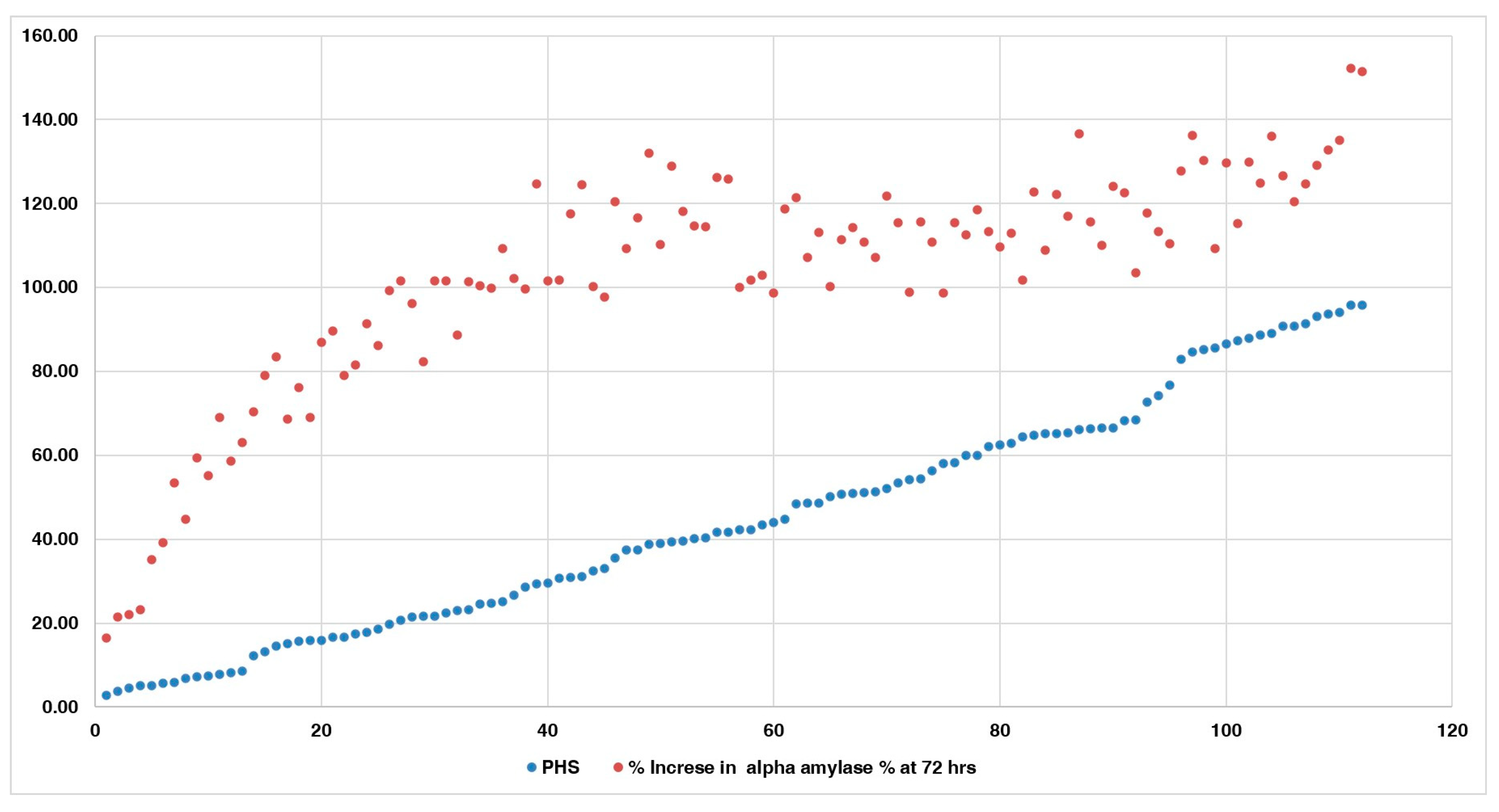
Comparison of alpha-amylase activity with the PHS indicated that accessions with lower PHS and FSG exhibited reduced alpha-amylase activity compared to other accessions with higher PHS and FSG values (Figure 7). Accessions IC485641, IC485425, IC296077, IC250220, IC328775, IC617773, IC253905, IC506657, IC297661, IC616492, IC398973, IC485645, and IC530653 had lower PHS and α-amylase activity than other accessions, especially at 48 and 72 h after germination. These accessions showed an increase of 16.55–63.09% in α-amylase activity at 72 h. However, accessions with high PHS value, ranging from 90.72 to 95.85%, exhibited a greater fold increase in α-amylase activity, of 126.6–151.44% at 72 h, as compared to the initial values. The comparison of the accessions having high and low alpha-amylase activity with PHS is given in Figure 8.
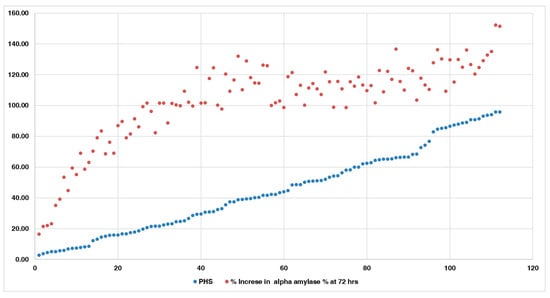
Figure 7.
Increase in alpha−amylase activity of all 112 accessions.
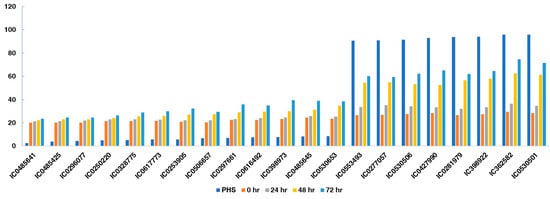
Figure 8.
Correlation of black gram accessions having low- and high-PHS value with alpha-amylase activity, showing relationship of PHS with an increase in alpha-amylase activity at 0, 24, 48, and 72 h of germination of tolerant and susceptible accessions.
4. Discussion
During the process of domestication, both intentional and unintentional selections have greatly impacted seed characteristics. In many instances, such selection resulted in significant improvements in seed traits, including increased seed size, enhanced nutritional quality, and reduced concentration of harmful compounds [2]. These selections often led to significant enhancements in seed attributes, such as increased seed size, improved nutritional content, and reduced levels of harmful substances. Nonetheless, some of these selections inadvertently introduced disadvantages, like increased vulnerability to PHS, various pests, and diseases [22]. PHS is a quantitative trait affected by various factors comprising plant characteristics, seed morphology, physiological aspects, and prevailing climatic conditions [17,23]. One major factor contributing to PHS susceptibility is the absence of seed dormancy, particularly evident under humid and wet conditions [24,25]. Consequently, the presence or absence of seed dormancy has emerged as a pivotal determinant in the resistance to PHS [16,26]. In leguminous plants, the phenomenon of hardseededness or physical seed dormancy can act as a barrier to water absorption, subsequently impeding germination. This trait is generally considered undesirable, because it obstructs the initial stages of seed growth [27,28]. Consequently, breeding efforts have been focused on reducing this characteristic, resulting in seeds that are more susceptible to PHS. In contrast, wild legume species, which have not undergone artificial selection, naturally exhibit stronger dormancy compared to most modern varieties, which tend to lack tolerance to PHS [29,30,31]. (Wild species showing high fresh-seed dormancy had better PHS tolerance [17]). This is corroborated by studies in crops such as black gram [5], green ram [6], rice [21], and groundnut [32]. Extensive research has been conducted to investigate PHS in various crops, but not in pulses, particularly in black gram. Hence, to contribute novel insights into the genetic basis of this significant trait, we assessed 112 black gram accessions. The extensive data collected from this research could serve as a valuable resource to study the seed-dormancy and germination aspects in black gram improvement.
In our research, we conducted fresh-seed germination tests at 25 ± 1 °C for 5 days. Observation of seed germination was recorded daily. The number of seeds that had germinated at the end of the germination test period was used to quantify the germination percentage. Previous investigations on black gram [33] and green gram [3] also used similar controlled conditions for assessing the variation in PHS tolerance. In wheat, fresh-seed germination was assessed at 20 ± 1 °C for a seven-day period. This entailed recording germination rates daily, which were utilized to determine the cumulative germination percentage or germination rate (GR). These metrics were used to determine the level of seed dormancy [16,34,35]. The findings indicated that a GR nearing 100% denoted minimal grain dormancy or higher resistance to PHS, indicating that most grains had germinated. Conversely, a GR close to 0% suggested an elevated amount of seed dormancy or greater PHS tolerance. In our study also, a highly positive association was recorded (r = 0.959 **) between germination rates of seeds within the pod and those removed from the pod, i.e., fresh-seed germination. Nevertheless, we observed that seeds after removal from the pod had a higher germination rate. This observation suggests that the pod wall acts as a barrier, limiting the moisture availability which is vital for germination initiation. The WIP of the pod is a crucial trait, which affects PHS. It determines the moisture levels accessible to the seeds within the pod, thereby influencing their ability to germinate. Correlation analysis indicated a positive relationship between PHS and WIP (r = 0.793 **); similarly, a positive association between WIP with PHS was recorded in green gram accessions [3]. Content of epicuticular wax [36] (and the thickness of the pod wall [6,37,38]) played a role in regulating the water absorption by the pod. This suggests that when screening for genotypes with tolerance to PHS, it may be beneficial to prioritize those with higher pod-wax content and thicker pod walls. Additionally, the multivariate analysis highlighted a direct correlation between PHS and WIP. In studies on green gram [6] and soybean [37] there was a significant negative correlation between pod-wall thickness and its water-imbibing capacity, with respect to PHS. A thicker pod wall is associated with an increased likelihood of seed sprouting within the pod, as the thicker wall has the capacity to absorb and retain a greater amount of moisture, which is favorable for seeds to sprout. A more effective approach to enhance PHS tolerance involves breeding for pods with low-to-medium thickness [39]. However, in the current study, no significant association between pod-wall thickness and PHS was observed. Similarly, a non-significant relation between PHS and pod-wall thickness was reported by [3]. A significant positive correlation among WIP, WIS, and FSG with PHS was recorded. A similar association of WIP, WIS and FSG with PHS has been reported by [3,17], while studying green gram and black gram. Conversely, a non-significant association between seed water imbibition and PHS was reported by [6]. When we categorized black gram germplasm based on PHS values, as PHS < 30% (Group I), 30–70% (Group II) and (PHS > 70% (Group III), it became evident that FSG was the principal factor influencing PHS. This observation aligns with conclusions drawn by [17]. In our study, we observed a positive but non-significant association between seed length, width and PHS. Previous studies reported a significant correlation between PHS and seed length [17,40].
In dried black gram seeds, α-amylase activity is typically low initially, but undergoes rapid activation, and increases during the germination process as it converts starch into sugar. In green gram, a significant rise in α-amylase activity was observed, increasing from 8.1 to 280.2 maltose units per gram of dry matter in the span of 0 to 72 h post germination of the seed. This rise in activity is significant, as α-amylase plays a crucial role in breaking down starch into simpler sugars during germination. Notably, in genotypes susceptible to pre-harvest sprouting (PHS), high α-amylase activity is observed, leading to the hydrolysis of starch, particularly amylopectin, into lower-molecular-weight amylase and amylopectin [41]. In green gram accessions, α-amylase activity at 0 h, 24 h, 48 h and 72 h after germination was measured [3], and the findings suggest that genotypes with lower FSG and PHS values exhibited reduced α-amylase activity, particularly at 48 and 72 h of germination. The PHS-tolerant genotypes at 72 h of germination showed a 15.46–87.49% rise in α-amylase activity, whereas genotypes with higher PHS levels showed a greater fold increase, ranging from 61.35 to 149.45%. In the present investigation, germplasm with high germination rates had the highest alpha-amylase activity, and vice versa. This observation was consistent with prior research findings, indicating that genotypes exhibiting higher seed-germination rates and susceptibility to PHS also tend to have increased levels of α-amylase activity [3,21,42,43]. Notably, a highly positive correlation was observed between α-amylase activity and both FSG and PHS after 24, 48, and 72 h of germination. This underscores the potential of α-amylase activity as a reliable biochemical marker for assessing black gram germplasm for their PHS tolerance. Due to increased weather variability, particularly rainfall during crop maturation, it is anticipated that PHS may emerge as one of the most important constraints in the future. Therefore, finding novel sources of PHS tolerance and introducing them into high-yielding black gram varieties would reduce the loss incurred due to PHS.
5. Conclusions
In the present climate change scenario, where inconsistent and unpredictable rainfall patterns are expected, particularly during crop maturation and harvesting, the potential for increased losses due to PHS becomes a pressing concern. Thus, identified accessions (IC485641, IC485425, IC296077, IC250220, IC328775, IC617773, IC253905, IC506657, IC297661, IC616492, IC398973, IC485645, and IC530653) that exhibit optimal and consistent levels of tolerance to PHS could be harnessed as a promising source of PHS tolerance in black gram. Future research efforts should prioritize the identification of key genes and loci linked to PHS tolerance, the establishment and utilization of PHS-tolerant germplasm resources, and advancements in breeding techniques.
Author Contributions
Conceptualization, J.V., P.G.G. and K.T.; data curation, J.V. and P.G.G.; formal analysis, P.G.G., J.K. and K.T.; funding acquisition, R.M.N.; investigation, J.V.; methodology, J.V.; project administration, K.T.; resources, P.G.G. and K.T.; supervision, K.T.; validation, P.G.G. and K.T.; visualization, K.T.; writing—original draft, J.V., P.G.G. and K.T.; writing—review and editing, J.K., D.P.W., S.R.J., A.K.T.V. and R.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by ICAR-National Bureau of Plant Genetic Resources, New Delhi-110012 and The Graduate School, ICAR-IARI, New Delhi-110012 through the Indian Council of Agricultural Research, India and partly supported by the Department of Biotechnology [grant number: BT/Ag/Network/Pulses-1/2017–18]. Ramakrishnan M. Nair gratefully acknowledges the long-term strategic donors to the World Vegetable Center: Taiwan, United States Agency for International Development (USAID), UK Government’s Foreign, Commonwealth & Development Office (FCDO), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, Japan, and funding from ACIAR Project on International Mungbean Improvement Network (CROP/2019/144).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors acknowledge the Director, ICAR-NBPGR, New Delhi and Dean, ICAR-IARI, New Delhi for their encouragement and support during the study. The First Author also acknowledges the IARI fellowship received during postgraduate study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gupta, S.; Das, A.; Pratap, A.; Gupta, D.S. Urdbean. In The Beans and the Peas; Pratap, A., Gupta, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 33–54. [Google Scholar]
- Smýkal, P.; Nelson, M.N.; Berger, J.D.; Von Wettberg, E.J. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef]
- Lamichaney, A.; Katiyar, P.K.; Laxmi, V.; Pratap, A. Variation in pre-harvest sprouting tolerance and fresh seed germination in mungbean (Vigna radiata L.) genotypes. Plant Genet. Resour. Charact. Util. 2018, 16, 437–445. [Google Scholar] [CrossRef]
- Jane, J.; Chen, J. Effects of amylose molecular size and amylopectin branch chain length on paste properties of starch. Cereal. Chem. 1992, 69, 60–65. [Google Scholar]
- Singh, A.; Khulbe, R.K.; Panwar, R.K. Evaluation of black gram (Vigna mungo) germplasm for pre-harvesting sprouting tolerance. J. Food. Legumes 2012, 25, 183–186. [Google Scholar]
- Ahmad, S.; Khulbe, R.; Roy, D. Evaluation of mungbean (Vigna radiata) germplasm for pre-harvest sprouting tolerance. Legum. Res. Int. J. 2014, 37, 259–263. [Google Scholar] [CrossRef]
- Singh, P.; Chourasiya, V.K.; Verma, P. Screening of mungbean (Vigna radiata) germplasm against precocious germination susceptibility. Int. J. Pure. Appl. Biosci. 2017, 5, 1010–1014. [Google Scholar] [CrossRef]
- Dougherty, R.W.; Boerma, H.R. Genotypic variation for resistance to pre-harvest sprouting in soybean. Crop. Sci. 1984, 24, 683–686. [Google Scholar] [CrossRef]
- Adarsh, S.; Jacob, J.; Giffy, T. Role of pulses in cropping systems: A review. Agric. Rev. 2019, 40, 185–191. [Google Scholar]
- Pratap, A.; Sen Gupta, D.; Singh, B.B.; Kumar, S. Development of super early genotypes in green gram [Vigna. radiata. (L.) Wilczek]. Legume. Res. 2013, 36, 105–110. [Google Scholar]
- Gupta, U.S. What’s New About Crop Plants: Novel Discoveries of the 21st Century; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Federer, W.T.; Nair, R.C.; Raghavarao, D. Some Augmented Row-Column Designs. Biometrics 1975, 31, 361. [Google Scholar] [CrossRef]
- Somyong, S.; Ishikawa, G.; Munkvold, J.D.; Tanaka, J.; Benscher, D.; Cho, Y.-G.; Sorrells, M.E. Fine mapping of a preharvest sprouting QTL interval on chromosome 2B in white wheat. Theor. Appl. Genet. 2014, 127, 1843–1855. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, X.; Xia, X.; He, Z. Cloning of seed dormancy genes (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor. Appl. Genet. 2014, 127, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, H.; Cheng, M.P.; Dankwa, K.O. Genome-wide association study for pre-harvest sprouting re-sistance in a large germplasm collection of Chinese wheat landraces. Front. Plant. Sci. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Lamichaney, A.; Pratap, A.; Katiyar, P.K.; Singh, N.P. Genotypic variability studies and identification of pre-harvest sprouting tolerant wild Vigna. Indian J. Agric. Sci. 2021, 91, 335–339. [Google Scholar] [CrossRef]
- Mares, D. Pre-harvest sprouting in wheat. I. Influence of cultivar, rainfall and temperature during grain ripening. Aust. J. Agric. Res. 1993, 44, 1259–1272. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2019; p. 276. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J Food Sci Technol. 2015, 52, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Sunayana, R.; Ramendra, N.S.; Raj, N.S.D. Variation in seed dormancy and α-amylase activity in indian rice (Oryza. sativa) accessions. Indian. J. Agric. Sci. 2013, 83, 56–62. [Google Scholar]
- Gemechu, K.; Endashaw, B.; Muhammad, I.; Emana, G.; Kifle, D.; Fassil, A. Breeding chickpea (Cicer. arietinum. [Fabaceae]) for better seed quality inadvertently increased susceptibility to the adzuki bean beetle (Callosobruchus. chinensis [Coleoptera.: Bruchidae]). Int. J. Trop. Insect. Sci. 2011, 31, 249–261. [Google Scholar]
- Rodríguez, M.V.; Arata, G.J.; Díaz, S.M.; Rentería, S.; Benech-Arnold, R.L. Phenotyping for resistance to pre-harvest sprouting in grain sorghum. Seed Sci. Res. 2021, 31, 178–187. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, R.Z.C. Studies on pre-harvest sprouting resistance in winter wheat and its determination. Acta. Agron. Sin. 1989, 15, 116–122. [Google Scholar] [CrossRef]
- Kulwal, P.; Ishikawa, G.; Benscher, D.; Feng, Z.; Yu, L.-X.; Jadhav, A.; Mehetre, S.; Sorrells, M.E. Association mapping for pre-harvest sprouting resistance in white winter wheat. Theor. Appl. Genet. 2012, 125, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Mrva, K. Mapping quantitative trait loci associated with variation in grain dormancy in Australian wheat. Aust. J. Agric. Res. 2001, 52, 1257–1265. [Google Scholar] [CrossRef]
- Argel, P.J.; Paton, C.J. Overcoming legume hard seededness. In Forage Seed Production, Tropical and Subtropical Species; Loch, D.S., Ferguson, J.E., Eds.; CAB International Publishing: Wallingford, UK, 1999; pp. 247–259. [Google Scholar]
- Gore, P.G.; Tripathi, K.; Pratap, A.; Bhat, K.V.; Umdale, S.D.; Gupta, V.; Pandey, A. Delineating taxonomic identity of two closely related Vigna species of section Aconitifoliae: V. trilobata (L.) Verdc. and V. stipulacea (Lam.) Kuntz in India. Genet. Resour. Crop. Evol. 2019, 66, 1155–1165. [Google Scholar] [CrossRef]
- Dorian, Q.F.; Robin, A. Seed dispersal and crop domestication: Shattering, germination and seasonality in evolution under cultivation. Annu. Plant. Rev. 2009, 38, 238–295. [Google Scholar]
- Tripathi, K.; Gore, P.G.; Pandey, A.; Bhardwaj, R.; Singh, N.; Chawla, G.; Kumar, A. Seed morphology, quality traits and imbibition behaviour study of a typical lentil (Lens. culinaris Medik.) from Rajasthan, India. Genet. Resour. Crop. Evol. 2019, 66, 697–706. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Bansal, R.; Gayacharan, C.; Shubha, K.; Kumar, V.; Singh, N.; Pandey, C.D.; Sharma, B.B.; Kumar, A. Identification and revealing the potential traits of the unique germplasm with extended funiculus in pea (Pisum sativum L.). Genet. Resour. Crop. Evol. 2021, 68, 3125–3132. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Bandyopadhyay, A.; Zala, P.V. In situ sprouting and regulation of fresh seed dormancy in spanish type groundnut (Arachis. hypogaea L.). Field. Crops. Res. 2001, 70, 233–241. [Google Scholar] [CrossRef]
- Lamichaney, A.; Hazra, K.K.; Katiyar, P.K.; Parihar, A.K.; Gupta, D.S.; Kumar, A.; Singh, F. Influence of seed and pod biophysical characters on pre-harvest sprouting tolerance in urdbean (Vigna mungo L.). Acta Physiol. Plant. 2023, 45, 48. [Google Scholar] [CrossRef]
- Mori, M.; Uchino, N.; Chono, M.; Kato, K.; Miura, H. Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 2005, 110, 1315–1323. [Google Scholar] [CrossRef]
- Torada, A.; Ikeguchi, S.; Koike, M. Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 2005, 143, 251–255. [Google Scholar] [CrossRef]
- Vijay, L.; Gupta, S. Pre-harvest sprouting tolerance in mungbean. In Pulses News Letter 19; Indian Institute of Pulses Research: Kanpur, India, 2008; pp. 4–5. [Google Scholar]
- Cheralu, C.; Satyanarayana, A.; Kulkarni, N.; Jagdishwar, K.; Reddy, M.S.S. Combining ability analysis for resistance to preharvest sprouting in mungbean (Vigna. radiata L.) WILCZEK). Ind. J. Gen. 1999, 59, 465–472. [Google Scholar]
- Tekrony, D.M.; Egli, D.B.; Phillips, A.D. Effect of field weathering on the viability and vigour of soybean seed. Agronomy 1980, 72, 749–753. [Google Scholar] [CrossRef]
- Rao, K.L.N.; Rao, C.M.; Rao, Y.K. Evaluation of greengram germplasm for tolerance to pre-harvest sprouting. In Environmental Protection; Kumar, A., Nehar, S., Eds.; Daya Publishing House: Delhi, India, 2007; pp. 51–54. [Google Scholar]
- Imrie, B.C.; Williams, R.W.; Lawn, R.J. Breeding for resistance to weather damage in mungbean. In Proceedings of the Second International Symposium on Mungbean; Shanmugasundaram, S., McLean, B.T., Eds.; AVRDC: Taiwan, China, 1988; pp. 130–135. [Google Scholar]
- Reihaneh, A.G.; Mehdi, G.D. Evaluation of Changes in phytase, α-Amylase and protease activities of some legume seeds during germination. In International Conference on Bioscience; Biochemistry and Bioinformatics IPCBEE IACSIT Press: Singapore, 2011; pp. 353–356. [Google Scholar]
- Krishnasamy, V.; Seshu, D.V. Germination after accelerated ageing and associated characters in rice varieties. Seed. Sci. Technol. 1990, 18, 353–359. [Google Scholar]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).