Exploring Genetic Diversity in Black Gram (Vigna mungo (L.) Hepper) for Pre-Harvest Sprouting Tolerance
Abstract
:1. Introduction
2. Material and Method
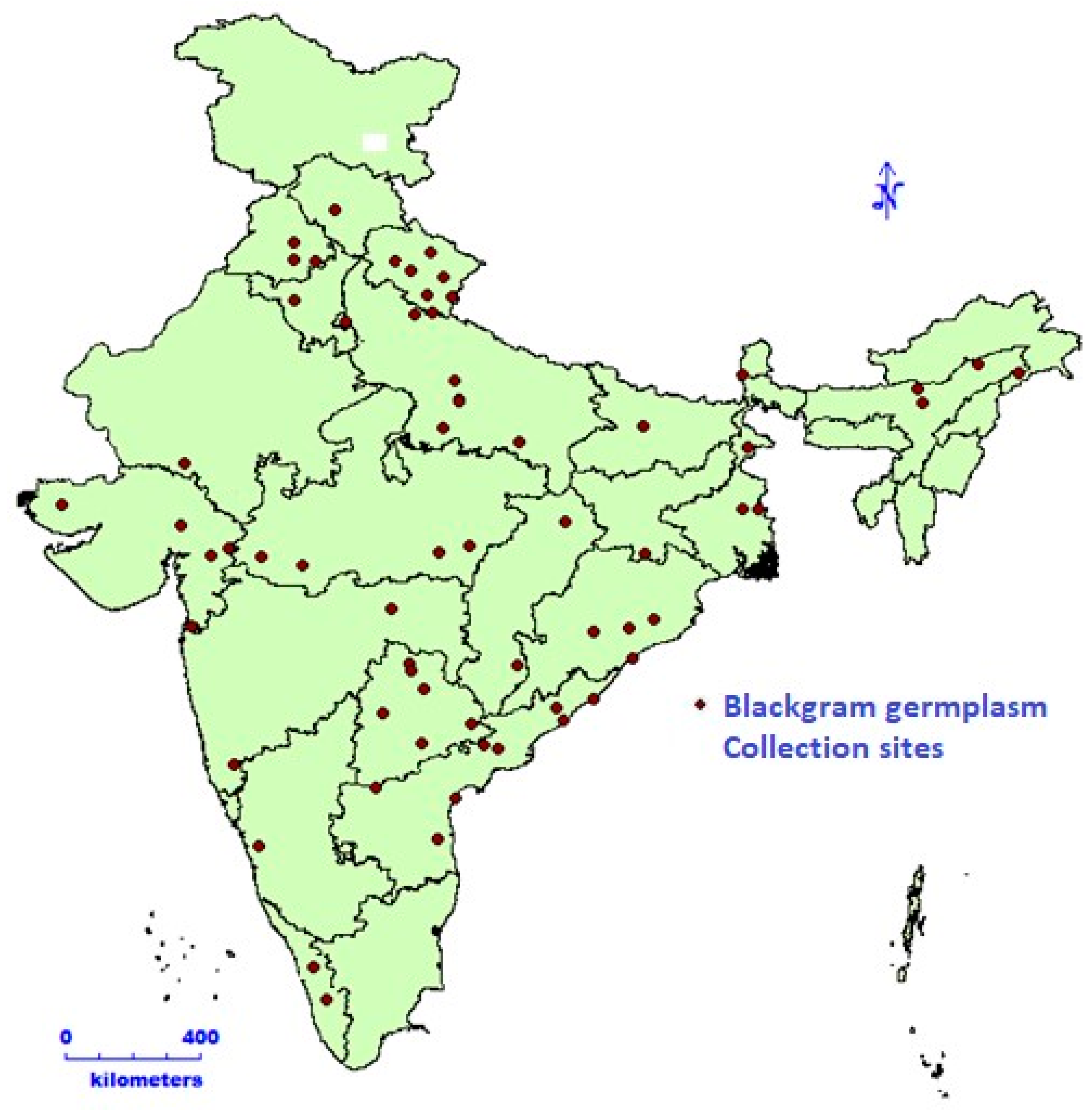
2.1. Field Trial
2.2. Physiological Traits of Pods and Seeds
2.2.1. Water Imbibition in Pod (WIP)
2.2.2. Seed Germination in a Pod (Indicates PHS Value)
2.2.3. Water Imbibition by Seeds (WIS)
2.2.4. Fresh-Seed Germination (FSG)
2.2.5. Physical Traits of Pod and Seed
2.3. Seed Hardness (SH)
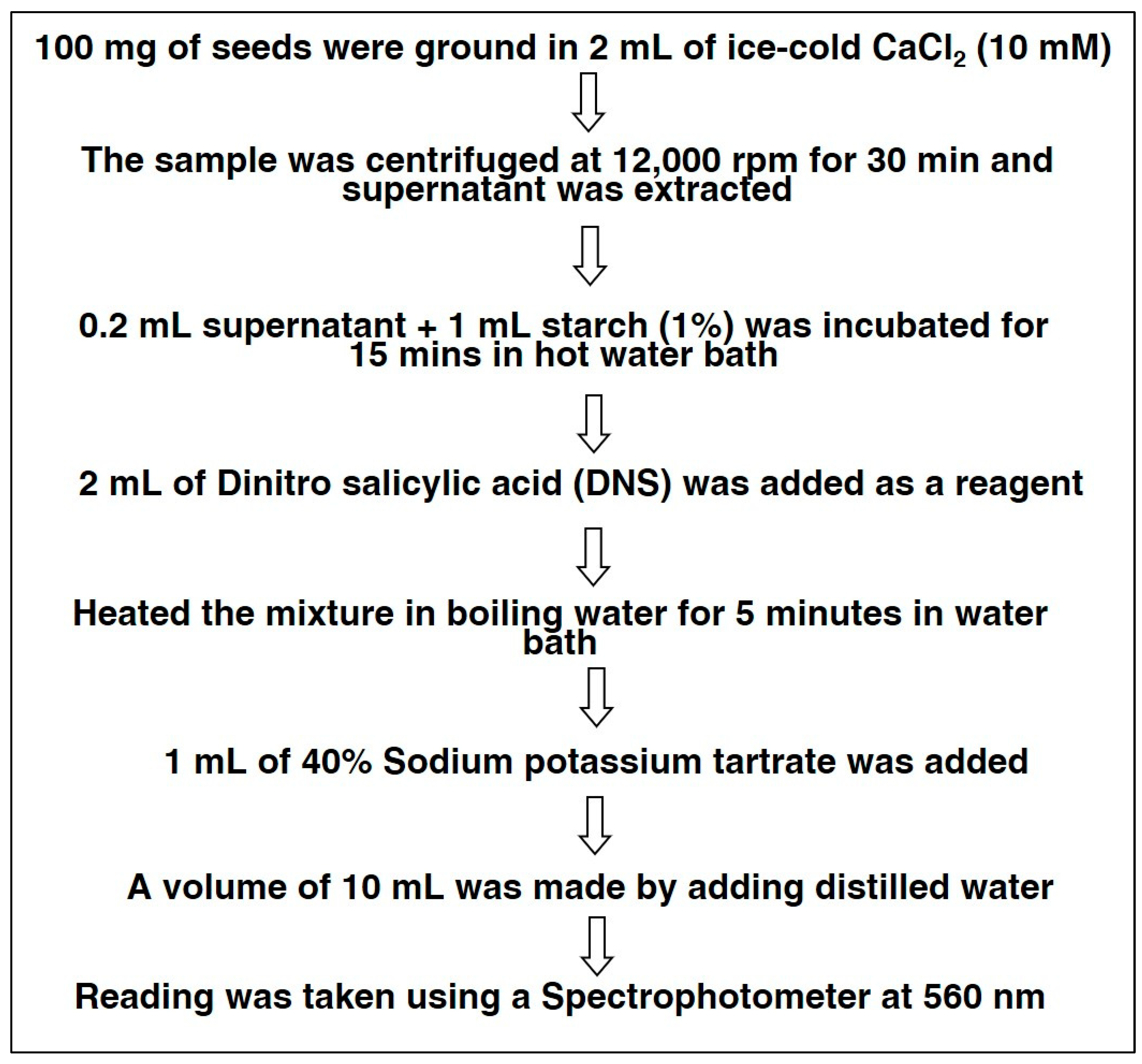
2.3.1. Alpha-Amylase Activity
2.3.2. Statistical Analysis of the Data
3. Results
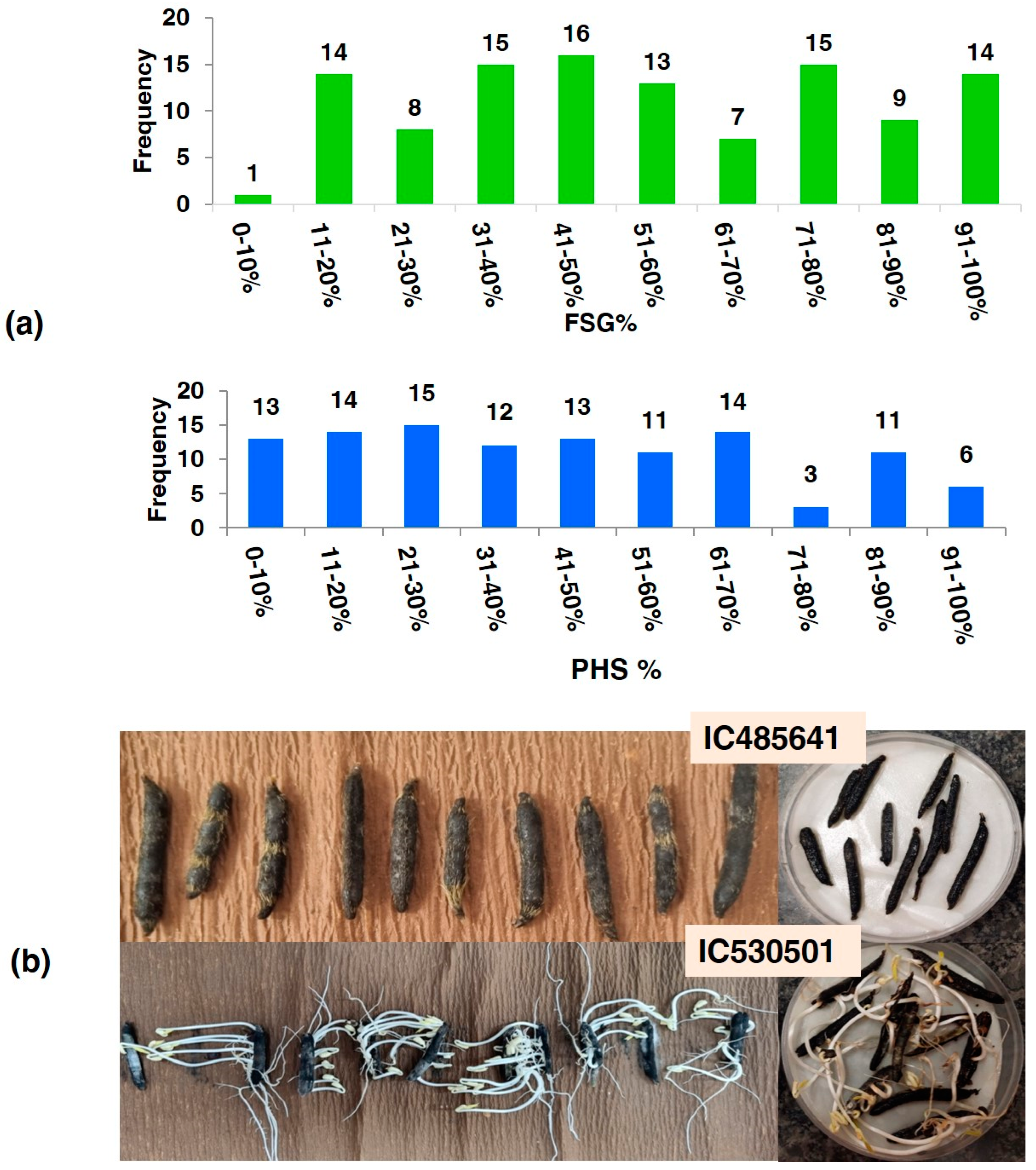
3.1. Genotypic Variation for Morpho–Physiological Traits
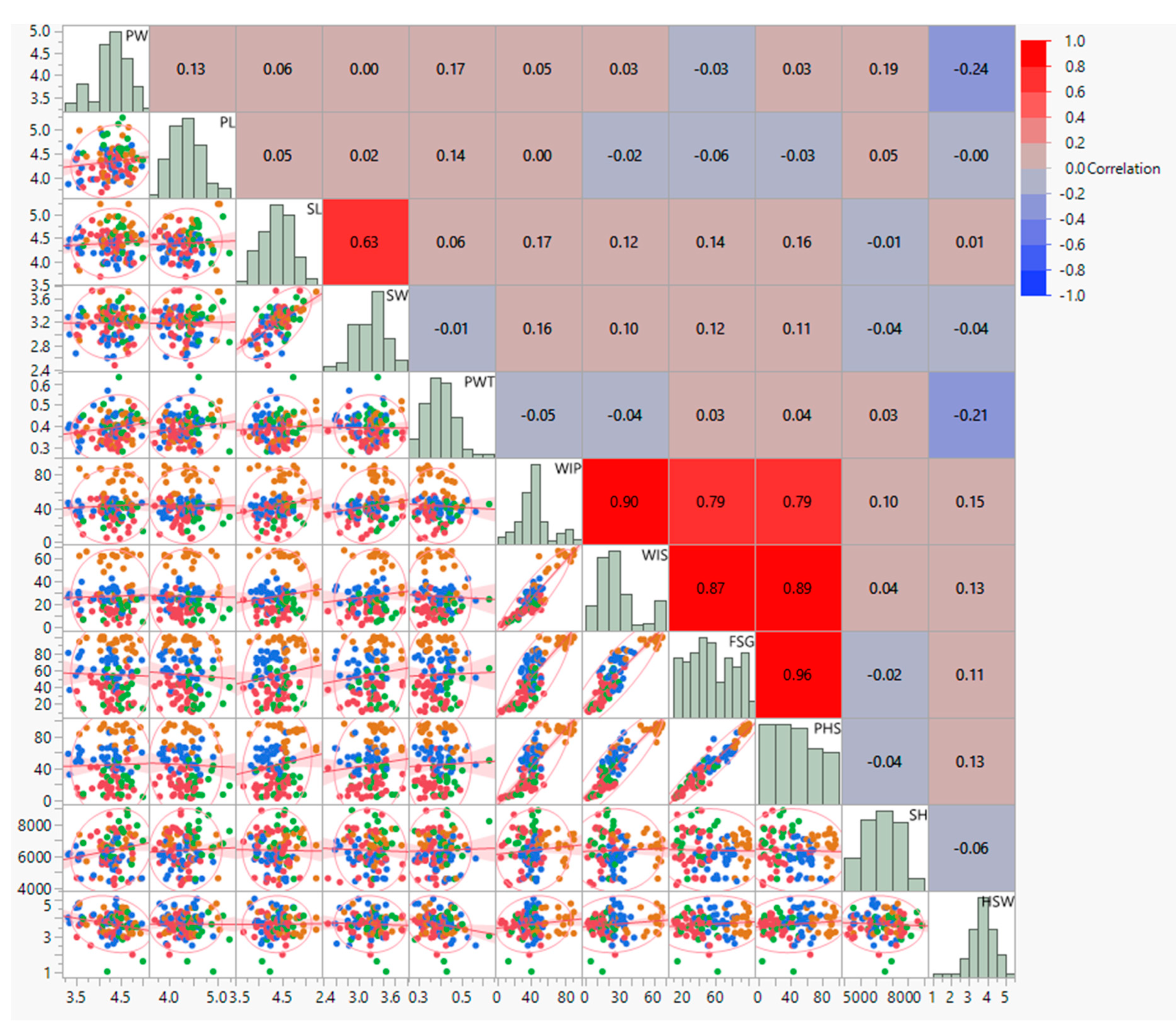
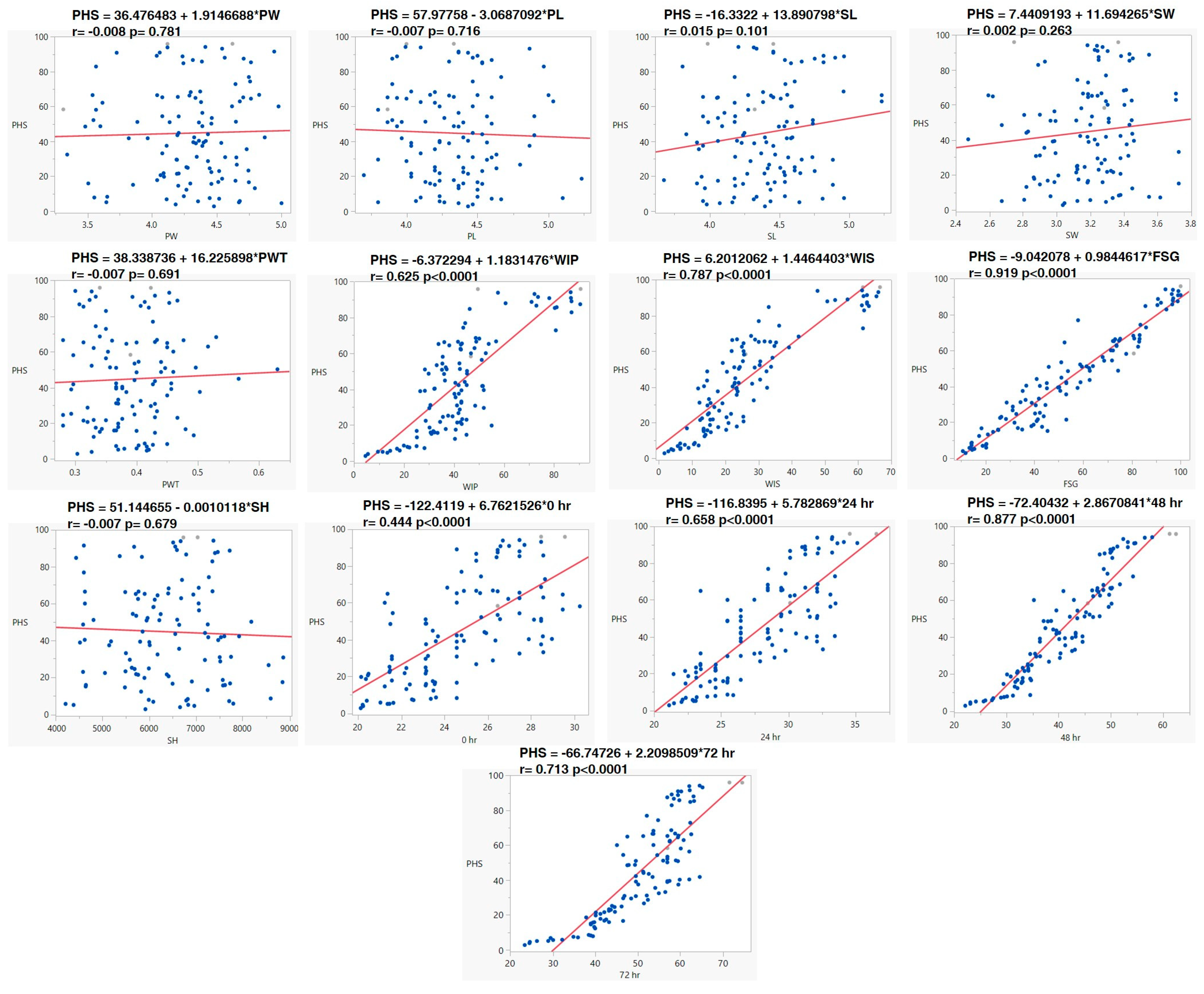
3.2. Correlation and Regression Analysis
3.3. Clustering of Black Gram Accessions
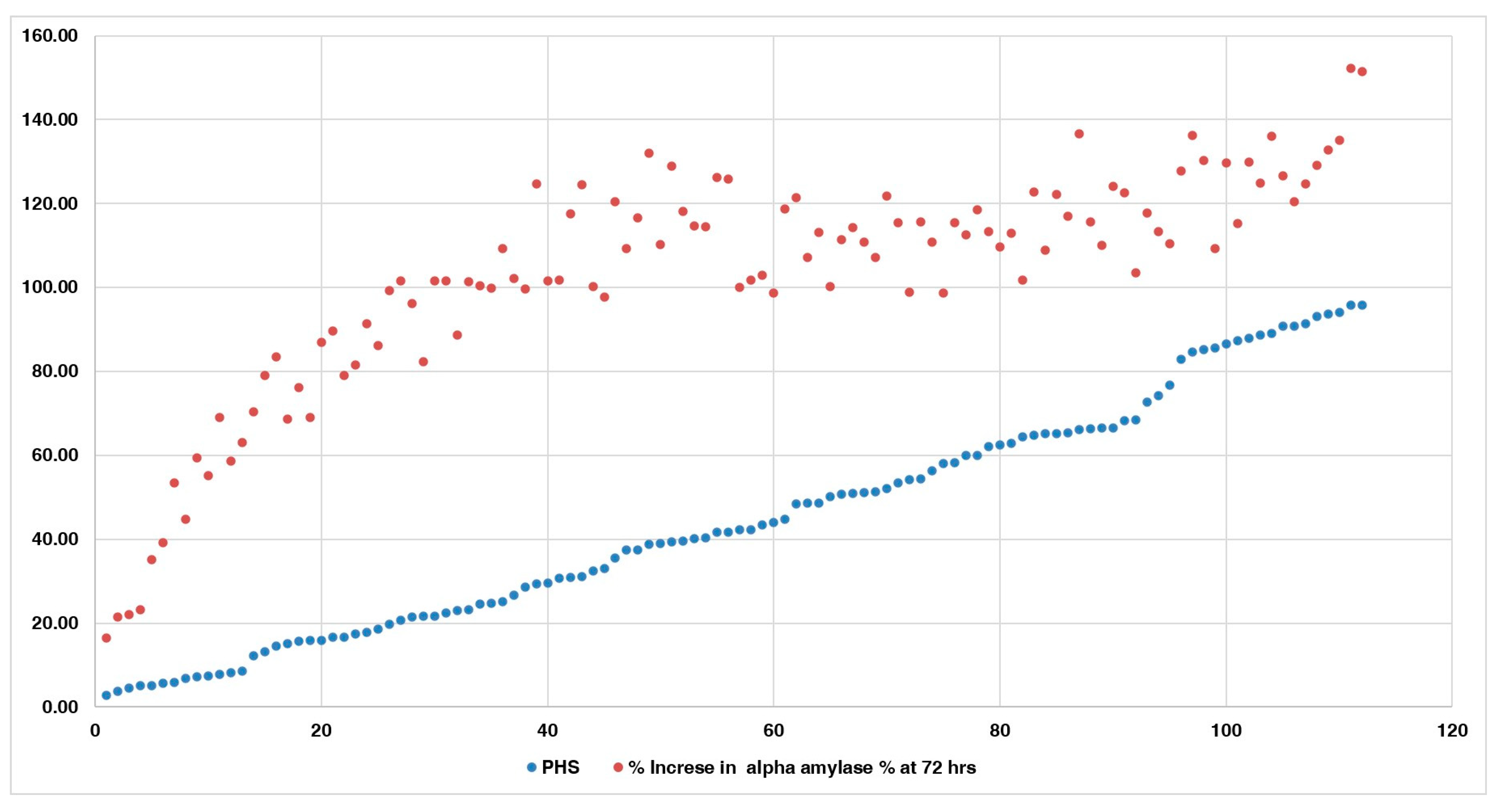
3.4. Changes in Alpha-Amylase Activity during Germination in PHS-Susceptible and -Tolerant Accessions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Das, A.; Pratap, A.; Gupta, D.S. Urdbean. In The Beans and the Peas; Pratap, A., Gupta, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 33–54. [Google Scholar]
- Smýkal, P.; Nelson, M.N.; Berger, J.D.; Von Wettberg, E.J. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef]
- Lamichaney, A.; Katiyar, P.K.; Laxmi, V.; Pratap, A. Variation in pre-harvest sprouting tolerance and fresh seed germination in mungbean (Vigna radiata L.) genotypes. Plant Genet. Resour. Charact. Util. 2018, 16, 437–445. [Google Scholar] [CrossRef]
- Jane, J.; Chen, J. Effects of amylose molecular size and amylopectin branch chain length on paste properties of starch. Cereal. Chem. 1992, 69, 60–65. [Google Scholar]
- Singh, A.; Khulbe, R.K.; Panwar, R.K. Evaluation of black gram (Vigna mungo) germplasm for pre-harvesting sprouting tolerance. J. Food. Legumes 2012, 25, 183–186. [Google Scholar]
- Ahmad, S.; Khulbe, R.; Roy, D. Evaluation of mungbean (Vigna radiata) germplasm for pre-harvest sprouting tolerance. Legum. Res. Int. J. 2014, 37, 259–263. [Google Scholar] [CrossRef]
- Singh, P.; Chourasiya, V.K.; Verma, P. Screening of mungbean (Vigna radiata) germplasm against precocious germination susceptibility. Int. J. Pure. Appl. Biosci. 2017, 5, 1010–1014. [Google Scholar] [CrossRef]
- Dougherty, R.W.; Boerma, H.R. Genotypic variation for resistance to pre-harvest sprouting in soybean. Crop. Sci. 1984, 24, 683–686. [Google Scholar] [CrossRef]
- Adarsh, S.; Jacob, J.; Giffy, T. Role of pulses in cropping systems: A review. Agric. Rev. 2019, 40, 185–191. [Google Scholar]
- Pratap, A.; Sen Gupta, D.; Singh, B.B.; Kumar, S. Development of super early genotypes in green gram [Vigna. radiata. (L.) Wilczek]. Legume. Res. 2013, 36, 105–110. [Google Scholar]
- Gupta, U.S. What’s New About Crop Plants: Novel Discoveries of the 21st Century; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Federer, W.T.; Nair, R.C.; Raghavarao, D. Some Augmented Row-Column Designs. Biometrics 1975, 31, 361. [Google Scholar] [CrossRef]
- Somyong, S.; Ishikawa, G.; Munkvold, J.D.; Tanaka, J.; Benscher, D.; Cho, Y.-G.; Sorrells, M.E. Fine mapping of a preharvest sprouting QTL interval on chromosome 2B in white wheat. Theor. Appl. Genet. 2014, 127, 1843–1855. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, X.; Xia, X.; He, Z. Cloning of seed dormancy genes (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor. Appl. Genet. 2014, 127, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, H.; Cheng, M.P.; Dankwa, K.O. Genome-wide association study for pre-harvest sprouting re-sistance in a large germplasm collection of Chinese wheat landraces. Front. Plant. Sci. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Lamichaney, A.; Pratap, A.; Katiyar, P.K.; Singh, N.P. Genotypic variability studies and identification of pre-harvest sprouting tolerant wild Vigna. Indian J. Agric. Sci. 2021, 91, 335–339. [Google Scholar] [CrossRef]
- Mares, D. Pre-harvest sprouting in wheat. I. Influence of cultivar, rainfall and temperature during grain ripening. Aust. J. Agric. Res. 1993, 44, 1259–1272. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2019; p. 276. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J Food Sci Technol. 2015, 52, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Sunayana, R.; Ramendra, N.S.; Raj, N.S.D. Variation in seed dormancy and α-amylase activity in indian rice (Oryza. sativa) accessions. Indian. J. Agric. Sci. 2013, 83, 56–62. [Google Scholar]
- Gemechu, K.; Endashaw, B.; Muhammad, I.; Emana, G.; Kifle, D.; Fassil, A. Breeding chickpea (Cicer. arietinum. [Fabaceae]) for better seed quality inadvertently increased susceptibility to the adzuki bean beetle (Callosobruchus. chinensis [Coleoptera.: Bruchidae]). Int. J. Trop. Insect. Sci. 2011, 31, 249–261. [Google Scholar]
- Rodríguez, M.V.; Arata, G.J.; Díaz, S.M.; Rentería, S.; Benech-Arnold, R.L. Phenotyping for resistance to pre-harvest sprouting in grain sorghum. Seed Sci. Res. 2021, 31, 178–187. [Google Scholar] [CrossRef]
- Zhang, H.F.; Liu, R.Z.C. Studies on pre-harvest sprouting resistance in winter wheat and its determination. Acta. Agron. Sin. 1989, 15, 116–122. [Google Scholar] [CrossRef]
- Kulwal, P.; Ishikawa, G.; Benscher, D.; Feng, Z.; Yu, L.-X.; Jadhav, A.; Mehetre, S.; Sorrells, M.E. Association mapping for pre-harvest sprouting resistance in white winter wheat. Theor. Appl. Genet. 2012, 125, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Mrva, K. Mapping quantitative trait loci associated with variation in grain dormancy in Australian wheat. Aust. J. Agric. Res. 2001, 52, 1257–1265. [Google Scholar] [CrossRef]
- Argel, P.J.; Paton, C.J. Overcoming legume hard seededness. In Forage Seed Production, Tropical and Subtropical Species; Loch, D.S., Ferguson, J.E., Eds.; CAB International Publishing: Wallingford, UK, 1999; pp. 247–259. [Google Scholar]
- Gore, P.G.; Tripathi, K.; Pratap, A.; Bhat, K.V.; Umdale, S.D.; Gupta, V.; Pandey, A. Delineating taxonomic identity of two closely related Vigna species of section Aconitifoliae: V. trilobata (L.) Verdc. and V. stipulacea (Lam.) Kuntz in India. Genet. Resour. Crop. Evol. 2019, 66, 1155–1165. [Google Scholar] [CrossRef]
- Dorian, Q.F.; Robin, A. Seed dispersal and crop domestication: Shattering, germination and seasonality in evolution under cultivation. Annu. Plant. Rev. 2009, 38, 238–295. [Google Scholar]
- Tripathi, K.; Gore, P.G.; Pandey, A.; Bhardwaj, R.; Singh, N.; Chawla, G.; Kumar, A. Seed morphology, quality traits and imbibition behaviour study of a typical lentil (Lens. culinaris Medik.) from Rajasthan, India. Genet. Resour. Crop. Evol. 2019, 66, 697–706. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Bansal, R.; Gayacharan, C.; Shubha, K.; Kumar, V.; Singh, N.; Pandey, C.D.; Sharma, B.B.; Kumar, A. Identification and revealing the potential traits of the unique germplasm with extended funiculus in pea (Pisum sativum L.). Genet. Resour. Crop. Evol. 2021, 68, 3125–3132. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Bandyopadhyay, A.; Zala, P.V. In situ sprouting and regulation of fresh seed dormancy in spanish type groundnut (Arachis. hypogaea L.). Field. Crops. Res. 2001, 70, 233–241. [Google Scholar] [CrossRef]
- Lamichaney, A.; Hazra, K.K.; Katiyar, P.K.; Parihar, A.K.; Gupta, D.S.; Kumar, A.; Singh, F. Influence of seed and pod biophysical characters on pre-harvest sprouting tolerance in urdbean (Vigna mungo L.). Acta Physiol. Plant. 2023, 45, 48. [Google Scholar] [CrossRef]
- Mori, M.; Uchino, N.; Chono, M.; Kato, K.; Miura, H. Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 2005, 110, 1315–1323. [Google Scholar] [CrossRef]
- Torada, A.; Ikeguchi, S.; Koike, M. Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 2005, 143, 251–255. [Google Scholar] [CrossRef]
- Vijay, L.; Gupta, S. Pre-harvest sprouting tolerance in mungbean. In Pulses News Letter 19; Indian Institute of Pulses Research: Kanpur, India, 2008; pp. 4–5. [Google Scholar]
- Cheralu, C.; Satyanarayana, A.; Kulkarni, N.; Jagdishwar, K.; Reddy, M.S.S. Combining ability analysis for resistance to preharvest sprouting in mungbean (Vigna. radiata L.) WILCZEK). Ind. J. Gen. 1999, 59, 465–472. [Google Scholar]
- Tekrony, D.M.; Egli, D.B.; Phillips, A.D. Effect of field weathering on the viability and vigour of soybean seed. Agronomy 1980, 72, 749–753. [Google Scholar] [CrossRef]
- Rao, K.L.N.; Rao, C.M.; Rao, Y.K. Evaluation of greengram germplasm for tolerance to pre-harvest sprouting. In Environmental Protection; Kumar, A., Nehar, S., Eds.; Daya Publishing House: Delhi, India, 2007; pp. 51–54. [Google Scholar]
- Imrie, B.C.; Williams, R.W.; Lawn, R.J. Breeding for resistance to weather damage in mungbean. In Proceedings of the Second International Symposium on Mungbean; Shanmugasundaram, S., McLean, B.T., Eds.; AVRDC: Taiwan, China, 1988; pp. 130–135. [Google Scholar]
- Reihaneh, A.G.; Mehdi, G.D. Evaluation of Changes in phytase, α-Amylase and protease activities of some legume seeds during germination. In International Conference on Bioscience; Biochemistry and Bioinformatics IPCBEE IACSIT Press: Singapore, 2011; pp. 353–356. [Google Scholar]
- Krishnasamy, V.; Seshu, D.V. Germination after accelerated ageing and associated characters in rice varieties. Seed. Sci. Technol. 1990, 18, 353–359. [Google Scholar]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef]



| Traits | Mean Square (Genotype) | F Value (Genotype) | Significance |
|---|---|---|---|
| PW | 0.423 | 3.221 | 0.00 |
| PL | 0.283 | 2.466 | 0.00 |
| SL | 0.278 | 6.207 | 0.00 |
| SW | 0.184 | 7.003 | 0.00 |
| PWT | 0.012 | 31.651 | 0.00 |
| WIP | 996.24 | 291.805 | 0.00 |
| WIS | 836.005 | 316.248 | 0.00 |
| FSG | 2104.012 | 516.869 | 0.00 |
| PHS | 2216.305 | 864.235 | 0.00 |
| SH | 3.75 × 108 | 748.482 | 0.00 |
| Traits | Minimum | Maximum | Mean | Standard Error | Standard Deviation | Kurtosis | Skewness | CV |
|---|---|---|---|---|---|---|---|---|
| PW | 3.31 | 5 | 4.29 | 0.04 | 0.38 | 0.05 | −0.62 | 8.75 |
| PL | 3.7 | 5.23 | 4.33 | 0.03 | 0.31 | 0.15 | 0.41 | 7.09 |
| SL | 3.67 | 5.23 | 4.39 | 0.03 | 0.3 | −0.01 | 0.18 | 6.93 |
| SW | 2.47 | 3.73 | 3.19 | 0.02 | 0.25 | 0.19 | −0.36 | 7.78 |
| PWT | 0.28 | 0.63 | 0.39 | 0.01 | 0.06 | 0.97 | 0.63 | 16.21 |
| WIP | 4.59 | 90.76 | 43.16 | 1.72 | 18.22 | 0.89 | 0.79 | 42.22 |
| WIS | 1.63 | 66.68 | 26.61 | 1.58 | 16.69 | 0.38 | 1.08 | 62.73 |
| FSG | 10.76 | 100 | 54.58 | 2.5 | 26.48 | −1.12 | 0.15 | 48.52 |
| PHS | 2.75 | 95.85 | 44.69 | 2.57 | 27.18 | −1.01 | 0.27 | 60.82 |
| SH | 4218.71 | 8868.52 | 6378.33 | 100.33 | 1061.81 | −0.46 | 0 | 16.65 |
| 0 h | 20.12 | 30.25 | 24.71 | 0.25 | 2.69 | −1.08 | 0.07 | 10.9 |
| 24 h | 21.12 | 36.54 | 27.93 | 0.36 | 3.82 | −1.12 | 0.06 | 13.69 |
| 48 h | 22.13 | 62.45 | 40.84 | 0.84 | 8.88 | −0.66 | 0.04 | 21.75 |
| 72 h | 23.45 | 74.56 | 50.43 | 0.98 | 10.41 | −0.03 | −0.54 | 20.64 |
| Category | Accessions |
|---|---|
| Tolerant (<10%) | IC485641, IC485425, IC296077, IC250220, IC328775, IC617773, IC253905, IC506657, IC297661, IC616492, IC398973, IC485645, IC530653 |
| Moderately Tolerant (10–30%) | IC279521, IC530450, IC330870, IC393545, IC250187, IC519915, IC010403, IC530446, IC338927, IC519619, IC485407, IC617804, IC485503, IC485417, IC350108, IC001572, IC599542, IC530478, IC039559, IC579620, IC546457, IC047443, IC140814, IC485612, IC485478, IC485495, IC449253 |
| Moderately Susceptible (30–70%) | IC250235, IC519918, IC436717, IC273826, IC449238, IC250190, IC557432, IC617803, IC311876, IC396772, IC021001, IC530613, IC362098, IC248206, IC423034, IC281976, IC321680, IC337021, IC278274, IC436787, IC372188, IC281983, IC073052, IC346276, IC485461, IC608570, IC250262, IC276917, IC343973, IC546467, IC530461, IC280487, IC485580, IC485430, IC332918, IC519933, IC282008, IC396032, IC472035, IC337389, IC530492, IC565297, IC625924, IC330859, IC519705, IC330852, IC297659, IC449264, IC279658, IC570268, IC250225, IC485628 |
| Susceptible (>70%) | IC485487, IC519620, IC393553, IC472004, IC530459, IC398983, IC541882, IC546466, IC519684, IC601237, IC257060, IC320916, IC553493, IC277057, IC530506, IC427990 IC281979, IC398922, IC362582, IC530501 |
| Traits | Group I (PHS < 30%) | Group II (PHS 30–70%) | Group III (PHS >70%) | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R Square | t Stat | p Value | R Square | t Stat | p Value | R Square | t Stat | p Value | R Square | t Stat | p Value | |
| PW | −0.024 | 0.29 | 0.776 | −0.019 | −0.12 | 0.908 | 0.013 | −1.12 | 0.275 | −0.008 | 0.28 | 0.781 |
| PL | −0.018 | −0.54 | 0.592 | −0.007 | −0.80 | 0.426 | −0.030 | −0.66 | 0.514 | −0.007 | −0.36 | 0.716 |
| SL | 0.029 | 1.48 | 0.146 | 0.018 | 1.39 | 0.170 | −0.039 | −0.53 | 0.600 | 0.015 | 1.65 | 0.101 |
| SW | −0.013 | 0.69 | 0.497 | −0.0009 | 0.98 | 0.334 | −0.053 | 0.20 | 0.840 | 0.002 | 1.12 | 0.263 |
| PWT | −0.003 | −0.92 | 0.361 | −0.019 | 0.06 | 0.949 | −0.051 | 0.26 | 0.798 | −0.007 | 0.40 | 0.691 |
| WIP | 0.654 | 8.66 | <0.0001 | 0.053 | 1.96 | 0.055 | 0.028 | 1.25 | 0.227 | 0.625 | 13.66 | <0.0001 |
| WIS | 0.538 | 6.81 | <0.0001 | 0.318 | 4.98 | <0.0001 | 0.211 | 2.47 | 0.023 | 0.787 | 20.29 | <0.0001 |
| FSG | 0.609 | 7.87 | <0.0001 | 0.761 | 12.79 | <0.0001 | 0.474 | 4.26 | 0.0005 | 0.919 | 35.58 | <0.0001 |
| SH | −0.025 | −0.21 | 0.838 | 0.015 | −1.34 | 0.185 | −0.036 | 0.57 | 0.574 | −0.007 | −0.41 | 0.679 |
| 0 h | 0.069 | 1.98 | 0.055 | 0.0007 | 1.02 | 0.313 | 0.086 | 1.67 | 0.112 | 0.444 | 9.48 | <0.0001 |
| 24 h | 0.223 | 3.49 | 0.0012 | 0.015 | 1.35 | 0.183 | 0.336 | 3.26 | 0.004 | 0.658 | 14.68 | <0.0001 |
| 48 h | 0.723 | 10.15 | <0.0001 | 0.453 | 6.58 | <0.0001 | 0.365 | 3.46 | 0.0028 | 0.877 | 28.18 | <0.0001 |
| 72 h | 0.780 | 11.81 | <0.0001 | 0.019 | 1.41 | 0.164 | 0.276 | 3.53 | 0.0024 | 0.713 | 16.66 | <0.0001 |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| PW | 0.004 | 0.198 | 0.690 |
| PL | −0.069 | 0.210 | 0.502 |
| SL | 0.203 | 0.866 | −0.141 |
| SW | 0.183 | 0.849 | −0.247 |
| PWT | 0.034 | 0.159 | 0.524 |
| WIP | 0.835 | 0.013 | 0.068 |
| WIS | 0.895 | −0.064 | 0.047 |
| FSG | 0.914 | −0.054 | −0.012 |
| PHS | 0.951 | −0.053 | 0.022 |
| SH | 0.017 | 0.016 | 0.521 |
| 0 h | 0.825 | −0.017 | 0.001 |
| 24 h | 0.923 | −0.027 | −0.014 |
| 48 h | 0.976 | −0.061 | −0.021 |
| 72 h | 0.933 | −0.085 | 0.002 |
| Var (%) | 47.683 | 11.432 | 9.729 |
| Cum. Percent (%) | 47.683 | 59.116 | 68.845 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, J.; Gore, P.G.; Kumari, J.; Wankhede, D.P.; Jacob, S.R.; Thirumani Venkatesh, A.K.; Nair, R.M.; Tripathi, K. Exploring Genetic Diversity in Black Gram (Vigna mungo (L.) Hepper) for Pre-Harvest Sprouting Tolerance. Agronomy 2024, 14, 197. https://doi.org/10.3390/agronomy14010197
Verma J, Gore PG, Kumari J, Wankhede DP, Jacob SR, Thirumani Venkatesh AK, Nair RM, Tripathi K. Exploring Genetic Diversity in Black Gram (Vigna mungo (L.) Hepper) for Pre-Harvest Sprouting Tolerance. Agronomy. 2024; 14(1):197. https://doi.org/10.3390/agronomy14010197
Chicago/Turabian StyleVerma, Jyotsna, Padmavati G. Gore, Jyoti Kumari, Dhammaprakash P. Wankhede, Sherry R. Jacob, Arun Kumar Thirumani Venkatesh, Ramakrishnan M. Nair, and Kuldeep Tripathi. 2024. "Exploring Genetic Diversity in Black Gram (Vigna mungo (L.) Hepper) for Pre-Harvest Sprouting Tolerance" Agronomy 14, no. 1: 197. https://doi.org/10.3390/agronomy14010197
APA StyleVerma, J., Gore, P. G., Kumari, J., Wankhede, D. P., Jacob, S. R., Thirumani Venkatesh, A. K., Nair, R. M., & Tripathi, K. (2024). Exploring Genetic Diversity in Black Gram (Vigna mungo (L.) Hepper) for Pre-Harvest Sprouting Tolerance. Agronomy, 14(1), 197. https://doi.org/10.3390/agronomy14010197








