1. Introduction
The primary production sector across Europe and beyond faces unprecedented challenges nowadays relating to climatic change, increased consumer demand and the latest energy crisis, on top of already increased production costs [
1,
2,
3]. Additional structural challenges currently faced by the EU fruit and vegetable sector have been proposed to be related to weak organization, a lack of adequate innovation and an unremarkable response to consumer needs [
4]. At the same time, further environmental challenges relating to environmental adaptation of crops to climatic change, biodiversity conservation and food insecurity put additional pressure on the plant-based primary sector [
5,
6]. Options for novel, highly nutritive crops with diverse uses that meet contemporary consumer demands and can deliver high-quality products with minimum environmental impacts and costs are becoming imperative for the sustainability of current and future production systems. Such novel crop alternatives can be either discovered or re-discovered through the utilization and sustainable exploitation of wild-occurring species that are otherwise neglected or underutilized (recently termed neglected and underutilized plant species (NUPs) elsewhere) which have been given considerable scientific recognition in recent years [
7,
8,
9,
10,
11]. Within the category of NUPs fall several regional wild-growing plant species, with profuse advantages relating to environmental resilience, diversified crop quality and high nutraceutical potential [
9,
12].
Such an example of a local NUP germplasm with documented agronomical and nutraceutical potential is Greek
Rosa canina (dogrose) [
13,
14]. Species-wise, dogrose
Rosa canina (Rosaceae) is a small forest tree or shrub that bears hermaphroditic epigynous flowers that produce a type of pseudo-fruits, called rosehips. Across the relatively widespread distribution range of the species in Greece,
R. canina trees flower from late spring to early summer and produce ripe rosehips from late summer to mid-autumn [
15]. Rosehips are widely known and valued for their superior nutraceutical properties as a result of their documented high content of ascorbic acid and antioxidants, among other things [
16]. Indicatively, the antioxidant activity of rosehips has been extensively documented in a diverse array of plant-based nutritional and pharmaceutical studies [
13,
17,
18,
19,
20]. The documented value of rosehips has brought about the significant economic potential of the species both worldwide and regionwide in the Greek horticultural sector, establishing a high exploitation feasibility and an already achieved readiness timescale for commercial agronomic exploitation [
14]. Indicatively, traditional and contemporary uses of rosehips in Greece and elsewhere include culinary and beverage uses [
13,
14]. Additional studies have highlighted the versatility of
R. canina providing data for further use in species enrichment for degraded forest mitigation programs, enhancing biodiversity and soil erosion protection [
21,
22].
R. canina has been shown to enhance the soil’s organic matter, reduce the risk of erosion and enhance soil fertility in forest landscapes [
22]. Furthermore, rosehip residues have been shown to have multilateral potential [
23], including use for biogas production [
24]. As such, it is believed herein that multifaceted uses of rosehips in sectors like landscape management, agro-alimentary and medicinal–cosmetic are warranted.
Based on the above-mentioned versatile potential of rosehips, an integrated domestication framework for the sustainable exploitation of Greek
R. canina has recently been proposed, implementing molecular authentication coupled with propagation and phytochemical evaluation of the fruits of wild-occurring material [
13]. Consequently, the establishment of selected genotypes under experimental cultivation was further evaluated [
14]. The results of the above work refurbished the exploitation feasibility of Greek native
R. canina germplasm, highlighting at the same time the current research gaps and potential limitations.
Considering the domestication steps for Greek
R. canina, as are depicted above, asexual propagation and cultivation establishment have been considered herein the two pivotal steps for further research on enhancing the exploitation framework of this germplasm. In addition, the evaluation of novel environmentally friendly or alternative management methods and inputs for achieving successful propagation and cultivation has also been considered worthy of research, especially when considering the contemporary challenges of environmentally sustainable production systems under a changing climate [
5,
6].
Asexual propagation via cuttings in commercial terms has long been conducted via the application of artificial hormonal substances to enhance rooting, with indole-3-butyric acid (IBA) being the main substance used [
25]. The same holds true for the asexual propagation of native plant material for domestication purposes [
26,
27], including
R. canina [
13,
28]. The use of alternative rooting enhancers to the conventionally used IBA during propagation is a subject that has been given research attention recently [
29,
30], with encouraging evidence for small tree species like
Cornus spp. and the
Rosa sp. [
31,
32]. Indicative examples are coconut water, which includes crucial phytohormones that support the plant’s maintenance and development [
33], along with various rooting gels [
29]. Coconut water is a very versatile substance that, in terms of the current study, contains natural phytohormones of most known groups, including the natural auxin indole-3-acetic acid (IAA) [
34]. A further example of natural plant extracts that contain natural auxin substances is aloe vera gel [
35]. Apart from substances with rooting enhancing properties, the use of arbuscular mycorrhizal fungi (AMF) as alternative rooting enhancers in asexual plant propagation has also been evaluated and discussed in the past [
36], taking advantage of the natural properties of arbuscular mycorrhizal fungi in enhancing plant growth and development or acting as natural bio-fertilizers [
37].
Trial cultivation efforts of small tree or shrub NUP germplasm in Mediterranean environments that also explore the fertilization effects or requirements have thus far shown potential to facilitate integrated sustainable utilization frameworks [
14,
38]. The cultivation of NUPs that are tree species inevitably falls into the principles of orchard management. According to these principles, organic management mainly considers diversification in terms of lower inputs or inputs with a lesser environmental impact for fertilization, crop protection and/or water management, as opposed to conventional management, where traditionally no such limitations apply [
39,
40]. A large body of evidence from the literature supports the environmental sustainability of organic tree crop management compared to conventional systems as part of an integrated or diversified management system [
41,
42,
43,
44].
Prompted by the above-described multifaceted premise and considering the previously proposed exploitation framework for Greek
R. canina [
13,
14], this study firstly investigated whether alternative rooting enhancers in combination with arbuscular mycorrhizal fungi against the conventionally used indole-3-butyric acid (IBA) could be used for asexual propagation with the cuttings of two different
R. canina genotypes; secondly, this study investigated the effects of a diversified organic fertilization regime against conventional fertilization and no fertilization on the fruit yield, fruit size and macro- and micro-nutrient content in the leaves during the trial cultivation of four distinct Greek
R. canina genotypes. The pivotal goal of the study was to enhance the integrated cultivation framework of underutilized Greek
R. canina germplasm as a potential novel crop that can deliver high-quality products with minimum environmental impact.
2. Materials and Methods
2.1. Greek Rosa canina Germplasm
In succession with previous research efforts undertaken at the Institute of Plant Breeding and Genetic Resources (IPBGR), Hellenic Agricultural Organization DIMITRA, Thessaloniki, Greece (ELGO-DIMITRA), concerning the domestication and sustainable utilization of Greek
R. canina germplasm, the current study utilized cultivated
R. canina germplasm that has been previously molecularly authenticated, phytochemically evaluated, asexually propagated, ex situ adapted and established under experimental cultivation as part of a research scheme for evaluating the sustainable exploitation potential of the germplasm [
13,
14]. Four distinct Greek
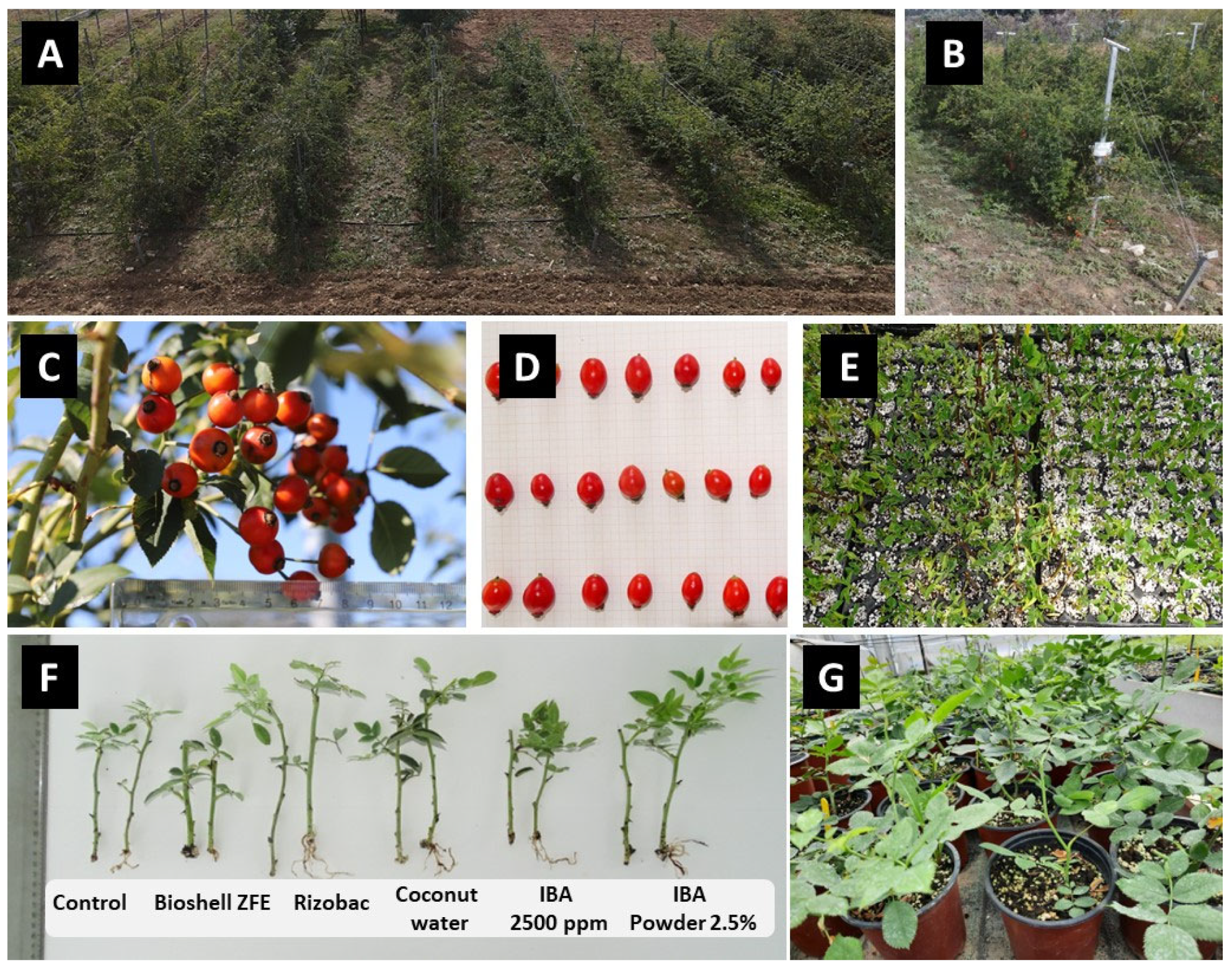
R. canina genotypes have been used herein, consisting of 3-year-old plants that were already established in a pilot orchard-type field cultivation trial (
Figure 1) [
14]. The genotypes used had previously been allocated unique IPEN (International Plant Exchange Network) accession numbers by the IPBGR, ELGO-DIMITRA, as follows: GR-1-BBGK-19,191, GR-1-BBGK-19,193, GR-1-BBGK-19,635 and GR-1-BBGK-19,674 [
13].
2.2. Asexual Propagation
Between the asexual propagation experiments conducted, five alternative rooting enhancers to the conventionally used IBA rooting hormone were tested, which were: (i) Rizobac (a root growth stimulator which contains beneficial soil bacteria in a total population of 1 × 1011 cfu (colony forming units) (Humofert, Athens, Greece)); (ii) Coconut water 75% (containing water, coconut water (5.3%) (coconut cream, water), rice (3.3%), calcium neutral phosphate, stabilizers (guar gum, gellan gum, xanthan gum), sea salt, flavorings, vitamins (B12, D2)); (iii) BIOSHELL ZFE biostimulator (0.2% w/v) (Agroecosystem, Thessaloniki, Greece); (iv) Aloe vera inner leaf natural gel (99.7%) (Forever Living LLC, Athens, Greece) and (v) Vitax organic rooting gel containing natural plant extracts (Vitax, Leicester, UK). The rooting enhancers were applied undiluted based on the manufacturer’s instructions for each product. In addition, the effects of the enrichment of the rooting substrate with externally applied mycorrhizal fungi, consisting of a commercial mix of the Funneliformis mosseae and Rhizoglomus irregulare strains (CLICK NATURE, Italpollina S.p.A., Rivoli, Italy), coupled with the pre-treatment of cuttings with a mycorrhizal inoculator (Glomus spp., Traver, Geenea Med. Ltd., Larnaca, Cyprus) before the application of the rooting enhancer, were also tested in separate experiments.
Three propagation experiments were conducted on the Greek R. canina genotype GR-1-BBGK-19,674 and two propagation experiments on genotype GR-1-BBGK-19,635 under a completely randomized design, which were replicated twice for each experiment during three propagation periods, namely summer 2022, autumn 2022 and summer 2023. In the summer of 2022, the experimentation kicked off with genotype GR-1-BBGK-19,674. Two cutting types were tested, which were 10–12 cm stem sections of primary soft wood from the tip of the stem bearing the stem’s apical meristem (apical cuttings) and internode sections forming the lower parts of the stem (sub-apical cuttings). Each cutting type was treated with six rooting enhancer treatments (which included a control treatment, BIOSHELL ZFE (0.2% w/v), Rizobac, coconut water 75%, 2500 ppm IBA and 0.25% IBA powder), resulting in 12 treatments in total with 30 replicate cuttings each. Consecutively, based on the results of the summer 2022 experiment, in the autumn of 2022, two identical experiments were conducted on the two separate Greek R. canina genotypes mentioned above, where the effects of mycorrhizal enrichment in the substrate were evaluated via the application of two substrate types (peat:perlite (1:3 v/v) and Mycorrhizae-enriched peat:perlite (1:3 v/v), according to the manufacturer’s instructions), each with five rooting enhancer treatments (control, Vitax rooting gel, Rizobac, coconut water 75% and 2500 ppm IBA), resulting in 10 treatments in total and 20 replicate apical cuttings in each treatment for each experiment.
Consecutively, in the summer of 2023, for genotype GR-1-BBGK-19,674, four rooting enhancers were evaluated (control, aloe vera gel (99.7%), Vitax rooting gel and 2500 ppm IBA) with 20 replicate apical cuttings each. At the same time, based on the results of the autumn 2022 experiment for genotype GR-1-BBGK-19,635, in the summer of 2023, the effects of mycorrhizal pre-treatment of the apical cuttings prior to the rooting enhancer treatment were tested via the application of five rooting enhancers (control, coconut water (75%), 2500 ppm IBA, Rizobac and rooting gel) with or without mycorrhizal pre-treatment with ready-made mycorrhizal inoculator solution, resulting in 10 treatments in total of 20 replicate cuttings each.
The “quick-dip method” was used for the application of the rooting enhancer, where the basal tip of each cutting is dipped into the enhancer solution for 5–7 s. The pre-treatment was applied by dipping the basal tip of the cuttings into a 1%
v/
v mycorrhizal inoculator solution prior to the rooting enhancer dip. The parameters measured were the rooting percentage, average root length and number of roots per cutting. The rooting took place for 30 days under intermittent misting with a relative humidity of >85% in a plastic greenhouse with propagation trays filled with peat (Klasmann, KTS 1)–perlite (1:3
v/
v) as the main substrate, unless otherwise stated, across all propagation experiments (
Figure 1).
2.3. Experimental Cultivation of Four Rosa canina Genotypes under Distinct Fertilization Regimes
The trial cultivation setup was established since March 2020 when 8-month-old ex situ adapted plants of the four Greek
R. canina genotypes studied herein were planted under mainstream orchard establishment techniques. These genotypes were previously selected for cultivation based on a multifaceted evaluation [
13,
14]. The site of the cultivation setup has been previously described in [
14,
38]. The cultivation trial consisted of 15 individuals for each genotype planted in rows/plots of 5 plants each, where a separate fertilization regime was applied in each row (5 plants) according to a completely randomized design (
Figure 1 and
Figure S1). The established plants were drip-irrigated on a weekly basis at a rate of 1.92 L/h throughout the 2022 growing season from April to October.
The implemented fertilization regimes were as follows: no fertilization (control) (NF); conventional crop fertilization (CF) and organic crop fertilization (OF). The CF and OF protocols were adjusted based on the soil analysis and were applied throughout the 2022 growing season monthly, similarly to the previous growing seasons of 2020 and 2021 (see
Table S1). The commercial inorganic fertilizers that are typically used in orchard husbandry were employed for the CF regime. The applied fertilization regime consisted of a soil application of 200 g/plant (21% N, 17% P
2O
5, 0.15% Zn and 4% S), a foliar spray application (Mo 1%
w/
v, B 15%
w/
v and Zn 30%
w/
v), a soil application of 200 g/plant (N 13.7%, K
2O 46.3%), a second foliar spray application (2%
w/
w total N, 4%
w/
w P
2O
5, 0.2%
w/
w K
2O, 0.6%
w/
w CaO, 0.09%
w/
w MgO and 0.1%
w/
w S) and a final foliar spray application of 230 g/L CaO.
Similarly, the applied OF regime consisted of a soil application of 50 g/plant organic fertilizer (organic water-soluble N 11%
w/
w, organic C 40%
w/
w, total amino acids 69.2%
w/
w) with additional 4 g/plant organic fertilizer (organic and humic compounds: 68–78%, 5% Ν, 3% Ρ
2O
5, 3–5% CaO, 0.7–1.0% MgO, 1.2 Fe and trace elements), a foliar spray application (Mo 1%
w/
v, B 15%
w/
v, Zn 30%
w/
v), a soil application of 50 g/plant organic fertilizer (organic water-soluble N 11%
w/
w, organic C 40%
w/
w, total amino acids 69.2%
w/
w) with additional 50 g/plant organic fertilizer (30% K
2O, 10% MgO, 42.5% SO
3), a second foliar spray application (2%
w/
w total N, 4%
w/
w P
2O
5, 0.2%
w/
w K
2O, 0.6%
w/
w CaO, 0.09%
w/
w MgO, 0.1%
w/
w S) and a final foliar spray application of 230 g/L CaO (
Table S1).
The growth and productivity of the trees was monitored on a long-term basis throughout the period of the cultivation trial since planting (2020–2022) [
14]. Building upon the previously collected data, in the current study, the rosehip fruit size growth patterns and final yield, coupled with the concentrations of macro- and micro-elements in leaves, were measured across the 2022 growing season, which was the 3rd year since field establishment and the 2nd year since plants had entered the fruit production stage.
2.4. Determination of Rosehip Fruit Size Growth Patterns and Yield of Cultivated Greek Rosa canina Genotypes
Throughout the 2022 growing season, the fruit growth curve was assessed by measuring the fruit length (from the pedicel base to the tip of the fruit), as well as the fruit height and the width in terms of perpendicular and horizontal diameter (mm), respectively, using a digital caliber (
Figure 1). The measurements were conducted at 7 time points following full bloom (days after anthesis—DAA) on a 50-fruit sample, which was averaged per replicate tree on 3 replicate trees for every fertilization regime in every genotype. The fruit size was then determined using the formula:
where
L is the measured length (mm) and
W1 and
W2 are the measured widths in terms of the perpendicular and horizontal diameter, respectively (mm).
The fresh weight of the harvested rosehip fruits was determined in grams per tree (experimental replicate) for all harvested fruits with the use of a digital scale.
2.5. Determination of Macro- and Micro-Elements in the Leaves of Cultivated Greek Rosa canina Genotypes
The concentration of mineral elements (P, K, Ca, Mg, B, Mn, Zn, Fe, Cu) in the
Rosa canina leaves was determined using an inductively coupled plasma optical emission spectrometry (ICP–OES) system (Avio 220 Max, PerkinElmer, Waltham, MA, USA) after each sample’s incineration at 550 °C for 6 h and following ash dissolution in 6 N HCl [
45]. Three biological replicates were conducted for each treatment and the results were expressed in dry weight. The total nitrogen (N) was determined following the Kjeldahl method using a VAPODEST 50 s system (Gerhardt, Königswinter, Germany). The results were expressed in N%.
2.6. Statistical Analysis
In all the propagation experiments, an analysis of variance (GLM MANOVA) was performed according to a completely randomized design to assess the overall treatment effects on the rooting attributes measured (root number, average root length, rooting). Following the results of the GLM MANOVA, the data were split according to the experimental design in each experiment and were analyzed separately to dissect the specific treatment effects for each applied factor, and differences between means were determined via Duncan’s multiple range post hoc test (p < 0.05) in each case as appropriate. Similarly, for the cultivation data of fruit size, an analysis of variance was performed to assess the overall genotype and fertilization treatment effects on fruit size, coupled with discreet analysis for each measurement date to pinpoint any specific treatment effects on fruit growth during the cropping period, and means were compared via Duncan’s multiple range test (p < 0.05).
For the final fruit yield data (fruit weight), an analysis of variance was conducted to determine the genotype and fertilization effects on the fruit weight, and mean comparison was conducted via Duncan’s multiple range test (p < 0.05). Similarly, for the leaf macro- and micro-element content data, an analysis of variance was conducted to determine the genotype and fertilization effects on each measured element, followed by mean comparison via Duncan’s multiple range test (p < 0.05)
The statistical software used for all analyses was IBM-SPSS v.21 and the graphs were formulated using Microsoft Excel 365.