Agronomic and Physiological Performance of the Indica Rice Varieties Differing in Tolerance to Low Phosphorus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Experimental Design
2.3. Sampling and Measurement
2.3.1. Tiller Number, Leaf Area, and Shoot Biomass
2.3.2. Phosphorus Content and Non-Structural Carbohydrate Remobilization
2.3.3. Leaf Photosynthetic Rate
2.3.4. Root Traits and Phytohormones
2.3.5. Final Harvest
2.4. Statistical Analysis
3. Results
3.1. Grain Yield and PUE
3.2. Non-Structural Carbohydrates (NSC) Remobilization
3.3. Tiller Number, Leaf Area Index, and Leaf Photosynthetic Rate
3.4. Shoot and Root Dry Weight
3.5. Root Oxidative Activity and Root Acid Phosphatase Activity
3.6. Indole-3-Acetic Acid and Zeatin + Zeatin Riboside Contents in Roots and Leaves
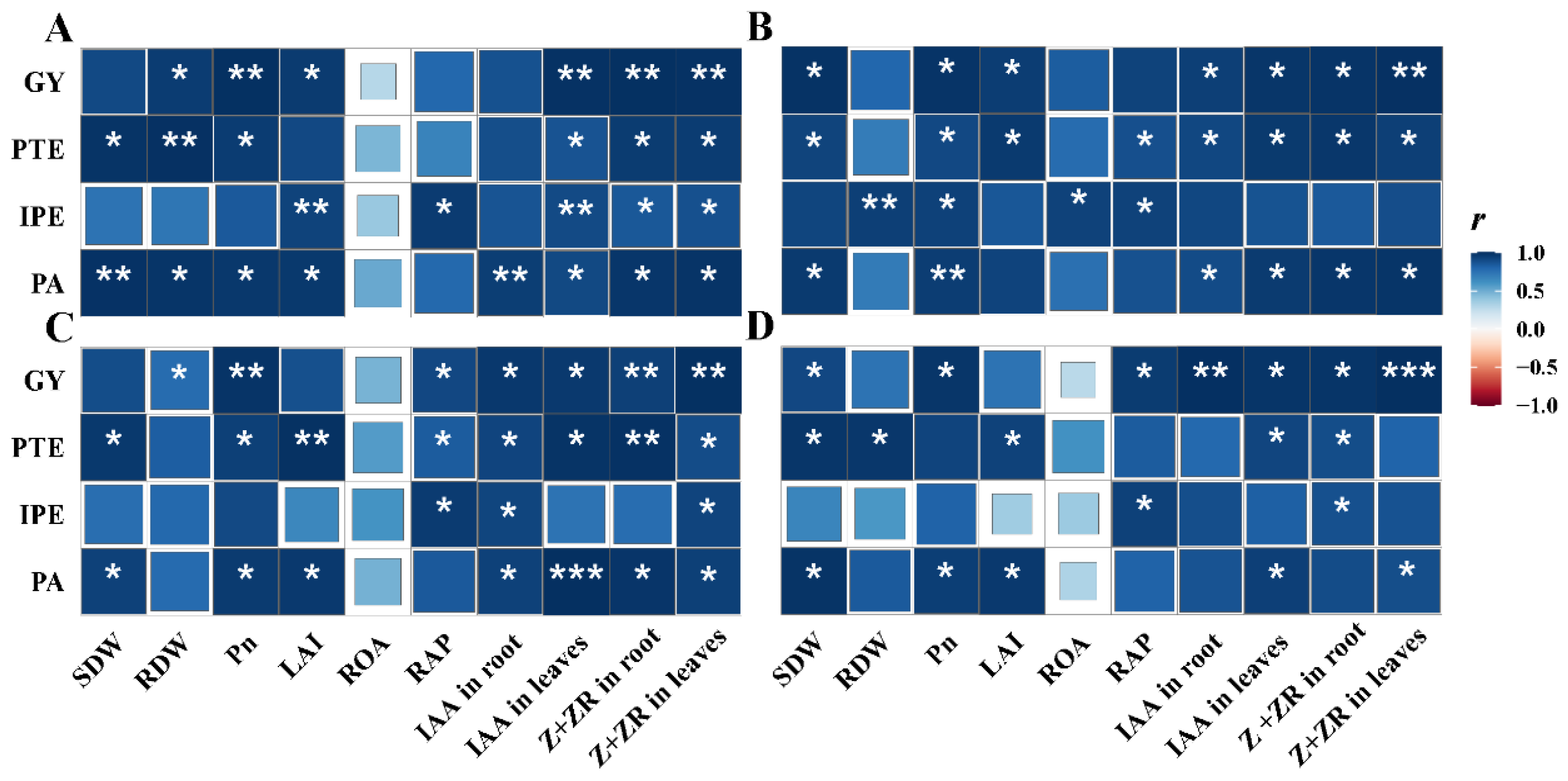
3.7. Correlations of Rice Morphological and Physiological Traits with PUE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Qaswar, M.; Chai, R.; Ahmed, W.; Jing, H.; Han, T.; Liu, K.; Ye, X.; Xu, Y.; Anthonio, C.K.; Zhang, H. Partial substitution of chemical fertilizers with organic amendments increased rice yield by changing phosphorus fractions and improving phosphatase activities in fluvo-aquic soil. J. Soils Sediment. 2020, 20, 1285–1296. [Google Scholar] [CrossRef]
- Prathap, V.; Suresh, K.; Tyagi, A. Comparative proteome analysis of phosphorus-responsive genotypes reveals the proteins differentially expressed under phosphorous starvation stress in rice. Int. J. Biol. Macromol. 2023, 234, 123760. [Google Scholar] [CrossRef] [PubMed]
- Rinasoa, S.; Rakotoson, T.; Rabeharisoa, L.; Tsujimoto, Y.; Nishigaki, T. Farmyard manure application increases lowland rice yield in phosphorus-deficient soils, but not in soils with high pH and phosphorus-fixing capacity. Field Crop. Res. 2023, 296, 108906. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners: The roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef]
- Kumar, S.; Pallavi; Chugh, C.; Seem, K.; Kumar, S.; Vinod, K.K.; Mohapatra, T. Characterization of contrasting rice (Oryza sativa L.) genotypes reveals the Pi-efficient schema for phosphate starvation tolerance. BMC Plant Biol. 2021, 21, 282. [Google Scholar] [CrossRef]
- Song, T.; Das, D.; Hu, Q.; Yang, F.; Zhang, J. Alternate wetting and drying irrigation and phosphorus rates affect grain yield and quality and heavy metal accumulation in rice. Sci. Total Environ. 2021, 752, 141–862. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, H.; Yang, X.; Yuan, P.; Wang, C.; Shi, L. Optimal phosphorus management strategies to enhance crop productivity and soil phosphorus fertility in rapeseed–rice rotation. Chemosphere 2023, 337, 139392. [Google Scholar] [CrossRef]
- Prathap, V.; Kumar, S.; Meena, N.L.; Maheshwari, C.; Dalal, M.; Tyagi, A. Phosphorus Starvation Tolerance in Rice Through Combined Physiological, Biochemical, and Proteome Analyses. Rice Sci. 2023, 30, 613–631. [Google Scholar] [CrossRef]
- Song, T.; Xu, F.; Yuan, W.; Chen, M.; Hu, Q.; Tian, Y.; Xu, W. Combining alternate wetting and drying irrigation with reduced phosphorus fertilizer application reduces water use and promotes phosphorus use efficiency without yield loss in rice plants. Agr. Water Manag. 2019, 223, 105–686. [Google Scholar] [CrossRef]
- Wu, P.; Shou, H.; Xu, G.; Lian, X. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr. Opin. Plant Biol. 2013, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.L.; Jia, P.; Feng, S.W.; Wang, Y.T.; Zheng, J.; Ou, S.N.; Li, J.T. Remarkable effects of microbial factors on soil phosphorus bioavailability: A country-scale study. Global Chang. Biol. 2022, 28, 4459–4471. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.A.; Wang, G. Metagenomic strategies uncover the soil bioavailable phosphorus improved by organic fertilization in Mollisols. Agr. Ecosyst. Environ. 2023, 349, 108462. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.A.; Liu, L.; Wang, D.; Wang, E. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.; Yun, W.; Xia, Y.; You, Z.; Rillig, M.C. Arbuscular mycorrhiza contributes to the control of phosphorus loss in paddy fields. Plant Soil 2020, 447, 623–636. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Hou, S.; Thiergart, T.; Vannier, N.; Mesny, F.; Ziegler, J.; Pickel, B.; Hacquard, S. A microbiota–root–shoot circuit favours Arabidopsis growth over defence under suboptimal light. Nat. Plants 2021, 7, 1078–1092. [Google Scholar] [CrossRef]
- Feng, F.; Li, Y.; Qin, X.; Liao, Y.; Siddique, K.H. Changes in rice grain quality of indica and japonica type varieties released in China from 2000 to 2014. Front. Plant Sci. 2017, 8, 1863. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, S.; Wang, W.; Zhang, W.; Gu, J.; Liu, L.; Zhang, H.; Wang, Z.; Yang, J. Tolerance to low phosphorus was enhanced by an alternate wetting and drying regime in rice. Food Energy Secur. 2021, 10, e294. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wu, M.; Liu, G.; Zhang, Z.; Yang, J. Effects of irrigation schedules and phosphorus fertilizer rates on grain yield and quality of upland rice and paddy rice. Environ. Exp. Bot. 2021, 186, 104465. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Plaxton, W.C.; Lambers, H.; Siebers, M.; Marambe, B.; Wasaki, J. Molecular mechanisms underpinning phosphorus-use efficiency in rice. Plant Cell Environ. 2018, 41, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Vellaikumar, S.; Malarvizhi, P. Phosphorus use efficiency of selected rice varieties. Trends Biochem. Sci. 2017, 10, 6662–6677. [Google Scholar]
- Rose, T.J.; Mori, A.; Julia, C.C.; Wissuwa, M. Screening for internal phosphorus utilization efficiency: Comparison of genotypes at equal shoot P content is critical. Plant Soil 2016, 401, 79–91. [Google Scholar] [CrossRef]
- Kumar, S.; Seem, K.; Kumar, S.; Vinod, K.K.; Chinnusamy, V.; Mohapatra, T. Pup1 QTL regulates gene expression through epigenetic modification of DNA under phosphate starvation stress in rice. Front. Plant Sci. 2022, 13, 871890. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ruan, W.; Li, C.; Huang, F.; Zeng, M.; Liu, Y.; Yu, Y.; Ding, X.; Wu, Y.; Mo, X. Integrative comparison of the role of the PHOSPHATE RESPONSE1 subfamily in phosphate signaling and homeostasis in rice. Plant Physiol. 2015, 168, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Men, C.; Qiao, S.; Wang, W.; Gu, J.; Liu, L.; Zhang, Z.; Zhang, H.; Wang, Z.; Yang, J. Tolerance to low phosphorus in rice varieties is conferred by regulation of root growth. Crop J. 2022, 8, 534–547. [Google Scholar] [CrossRef]
- Lv, B.; Wei, K.; Hu, K.; Tian, T.; Zhang, F.; Yu, Z.; Ding, Z. MPK14-mediated auxin signaling controls lateral root development via ERF13-regulated very-long-chain fatty acid biosynthesis. Mol. Plant. 2021, 14, 285–297. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell Mol. Biol. Lett. 2016, 21, 1–19. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Azhar, B.; Aroosa, Z.; Zeenat, A.; Aman, S. Screening of rice varieties based on remodeling of root architecture linked to enhanced phosphorus transporters and ethylene signaling for better phosphorous acquisition under limiting conditions. Sains Malays. 2021, 50, 1621–1638. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Chen, B.; Wang, W.; Sun, H.; Sun, H.; Zhao, Q. OsCKX2 regulates phosphate deficiency tolerance by modulating cytokinin in rice. Plant Sci. 2022, 319, 111257. [Google Scholar] [CrossRef] [PubMed]
- Mae, T.; Ohira, K. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol. 1981, 22, 1067–1074. [Google Scholar]
- Zhang, W.; Yu, J.; Xu, Y.; Wang, Z.; Liu, L.; Zhang, H.; Gu, J.; Zhang, J.; Yang, J. Alternate wetting and drying irrigation combined with the proportion of polymer-coated urea and conventional urea rates increases grain yield, water and nitrogen use efficiencies in rice. Field Crop. Res. 2021, 268, 108165. [Google Scholar] [CrossRef]
- Yoshida, S.; Forna, D.; Cock, J.; Gomez, K. Laboratory manual for physiological studies of rice. Int. Rice Res. Ins. 1976, 23, 61–66. [Google Scholar]
- Ramasamy, S.; Ten Berge, H.F.M.; Purushothaman, S. Yield formation in rice in response to drainage and nitrogen application. Field Crop. Res. 1997, 51, 65–82. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Elke, V.; Terry, R.; Kazuki, S.; Kwanho, J.; Matthias, W. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutr. Cycl. Agroecosys. 2016, 104, 413–427. [Google Scholar]
- Wang, K.; Cui, K.; Liu, G.; Luo, X.N.; Huang, J. Low straw phosphorus concentration is beneficial for high phosphorus use efficiency for grain production in rice recombinant inbred lines. Field Crop. Res. 2017, 203, 65–73. [Google Scholar] [CrossRef]
- Irfan, M.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Siddique, K.H.; Xu, M. Phosphorus (P) use efficiency in rice is linked to tissue-specific biomass and P allocation patterns. Sci. Rep. 2020, 10, 4278. [Google Scholar] [CrossRef]
- Vejchasarn, P.; Lynch, J.P.; Brown, K.M. Genetic variability in phosphorus responses of rice root phenotypes. Rice 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source-sink relations. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar] [CrossRef]
- Verbeeck, M.; Houben, E.; De Bauw, P.; Rakotoson, T.; Merckx, R.; Smolders, E. Root biomass explains genotypic differences in phosphorus uptake of rainfed rice subjected to water and phosphorus stresses. Plant Soil 2023, 486, 253–271. [Google Scholar] [CrossRef]
- Huang, G.; Hu, H.; van de Meene, A.; Zhang, J.; Dong, L.; Zheng, S.; Zhang, D. AUXIN RESPONSE FACTORS 6 and 17 control the flag leaf angle in rice by regulating secondary cell wall biosynthesis of lamina joints. Plant Cell 2021, 33, 3120–3133. [Google Scholar] [CrossRef]
- Shah, S.; Cai, L.; Li, X.; Fahad, S.; Wang, D. Influence of cultivation practices on the metabolism of cytokinin and its correlation in rice production. Food Energy Secur. 2023, 12, e488. [Google Scholar] [CrossRef]
- Pan, X.; Liu, S.; Li, F.; Li, M. Effect of low phosphorus stress on leaf photosynthesis in the seedlings of different rice cultivars. Act. Agro Sin. 2003, 29, 770–774. (In Chinese) [Google Scholar]
- Ruan, W.; Guo, M.; Xu, L.; Wang, X.; Zhao, H.; Wang, J.; Yi, K. An SPX-RLI1 module regulates leaf inclination in response to phosphate availability in rice. Plant Cell 2018, 30, 853–870. [Google Scholar] [CrossRef]
- Samejima, H.; Kondo, M.; Ito, O.; Nozoe, T.; Shinano, T.; Osaki, M. Root-shoot interaction as a limiting factor of biomass productivity in new tropical rice lines. Soil. Sci. Plant Nutr. 2004, 50, 545–554. [Google Scholar] [CrossRef]
- Huang, R.; Sun, H.; Liu, S.; Song, W.; Liu, Y.; Yu, C.; Mao, Y.; Zhang, Y.; Xu, G. Rice Root Growth and Auxin Concentration in Response to Phosphate Deficiency. Chin. J. Rice Sci. 2012, 26, 563–568. (In Chinese) [Google Scholar]
- Ding, Y.; Wang, Z.; Mo, S.; Liu, J.; Xing, Y.; Wang, Y.; Ge, C.; Wang, Y. Mechanism of low phosphorus inducing the main root lengthening of rice. J. Plant Growth Regul. 2021, 40, 1032–1043. [Google Scholar] [CrossRef]
- Goncalves, B.X.; Lima-Melo, Y.; dos Santos Maraschin, F.; Margis-Pinheiro, M. Phosphate starvation responses in crop roots: From well-known players to novel candidates. Environ. Exp. Bot. 2020, 178, 104162. [Google Scholar] [CrossRef]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, J.R.; Yang, B.; Vandamme, N.; Verstaen, K.; Smet, W.; Van de Velde, C.; De Rybel, B. Vascular transcription factors guide plant epidermal responses to limiting phosphate conditions. Science 2020, 370, eaay4970. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Yao, H.Y.; Jia, L.H.; Tan, J.F.; Xu, Z.H.; Zheng, W.M.; Xue, H.W. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. New Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, Z.; Wang, Z.; Yang, J.; Zhang, J. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crop. Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Katsura, K.; Maeda, S.; Horie, T.; Shiraiwa, T. Analysis of yield attributes and crop physiological traits of Liangyoupeijiu, a hybrid rice recently bred in China. Field Crop. Res. 2007, 103, 170–177. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; George, T.S.; Feng, G. A core microbiome in the hyphosphere of arbuscular mycorrhizal fungi has functional significance in organic phosphorus mineralization. New Phytol. 2023, 238, 859–873. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef]
- Bhatta, B.B.; Panda, R.K.; Anandan, A.; Pradhan, N.S.N.; Mahender, A.; Rout, K.K.; Ali, J. Improvement of phosphorus use efficiency in rice by adopting image-based phenotyping and tolerant indices. Front. Plant Sci. 2021, 12, 717107. [Google Scholar] [CrossRef]
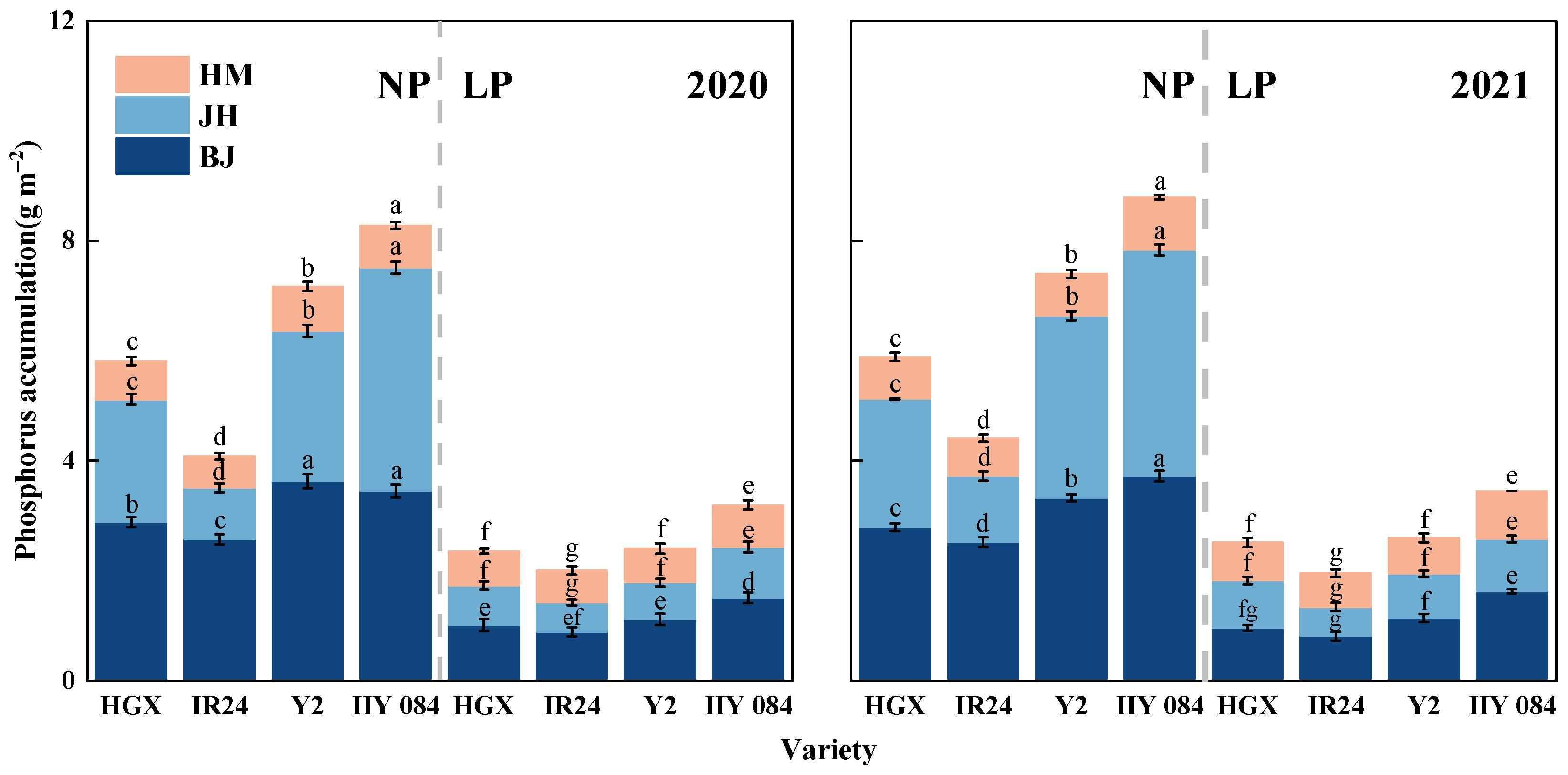




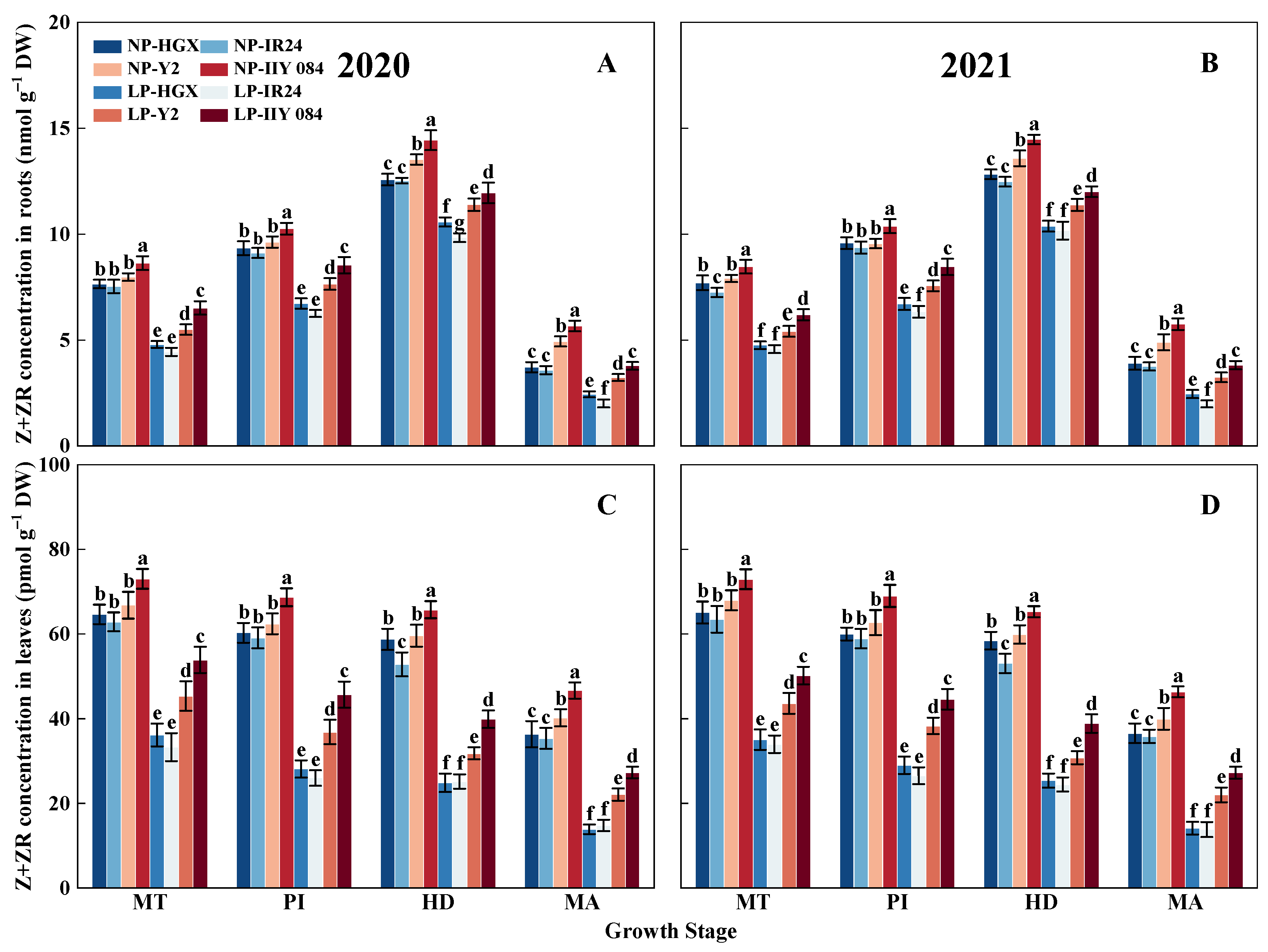
| Year/Treatment | Variety | Panicles per (m2) | Spikelet per Panicle | Total Spikelets (×103 m2) | 1000-Grain Weight (g) | Filled Grains (%) | Grain Yield (g m−2) |
|---|---|---|---|---|---|---|---|
| 2020 | |||||||
| NP | HGX | 310.17 c | 108.87 d | 33.77 d | 24.48 e | 67.82 d | 561.69 e |
| IR24 | 294.98 d | 113.17 d | 33.39 d | 24.08 f | 59.18 e | 476.48 f | |
| Y2 | 291.98 d | 161.57 a | 47.18 c | 25.43 c | 76.75 c | 921.09 c | |
| IIY 084 | 392.24 a | 156.86 b | 61.53 a | 24.99 d | 83.13 ab | 1278.45 a | |
| LP | HGX | 252.89 f | 95.68 e | 24.2 e | 24.96 d | 70.23 d | 424.44 fg |
| IR24 | 254.77 f | 86.91 f | 22.15 f | 24.84 d | 66.14 d | 364.05 g | |
| Y2 | 282.78 e | 121.59 c | 34.38 d | 26.46 b | 79.89 bc | 726.60 d | |
| IIY 084 | 324.48 b | 152.37 b | 49.44 b | 26.85 a | 86.68 a | 1150.66 b | |
| 2021 | |||||||
| NP | HGX | 313.90 c | 116.33 d | 36.5 cd | 24.95 e | 70.40 d | 640.78 e |
| IR24 | 308.80 c | 115.03 d | 35.52 d | 24.38 g | 61.50 e | 524.39 f | |
| Y2 | 303.61 c | 166.32 a | 50.51 b | 25.21 c | 79.22 c | 999.41 c | |
| IIY 084 | 393.35 a | 165.35 a | 65.03 a | 25.06 d | 82.56 b | 1319.31 a | |
| LP | HGX | 255.46 e | 99.35 e | 25.71 e | 24.65 f | 72.93 d | 451.62 g |
| IR24 | 259.30 e | 91.02 f | 23.57 f | 25.07 d | 71.40 d | 410.91 g | |
| Y2 | 288.90 d | 127.00 c | 37.87 c | 26.96 a | 83.68 b | 775.02 d | |
| IIY 084 | 329.40 b | 158.41 b | 52.16 b | 26.68 b | 86.89 a | 1196.22 b | |
| Analysis of variance | |||||||
| Year (Y) | ** | ** | ** | * | * | ** | |
| Treatment (T) | ** | ** | ** | ** | ** | ** | |
| Variety (V) | ** | ** | ** | ** | ** | ** | |
| Y × T | NS | NS | NS | NS | NS | NS | |
| Y × V | NS | NS | NS | NS | NS | NS | |
| T × V | ** | ** | * | ** | * | NS | |
| Y × T × V | NS | NS | NS | ** | NS | NS | |
| Year/Treatment | Variety | PTE (%) | IPE (kg kg−1) | PHI (%) |
|---|---|---|---|---|
| 2020 | ||||
| NP | HGX | 34.54 d | 99.40 h | 39.97 h |
| IR24 | 28.74 e | 127.09 g | 41.02 g | |
| Y2 | 41.12 c | 139.19 f | 42.16 f | |
| IIY 084 | 40.93 c | 164.77 e | 50.54 e | |
| LP | HGX | 45.50 b | 246.90 d | 53.47 d |
| IR24 | 41.27 c | 306.18 c | 56.86 c | |
| Y2 | 48.00 b | 360.33 b | 58.19 b | |
| IIY 084 | 51.12 a | 403.91 a | 63.83 a | |
| 2021 | ||||
| NP | HGX | 38.21 d | 110.27 h | 40.95 f |
| IR24 | 37.32 d | 128.52 g | 40.09 f | |
| Y2 | 42.06 c | 152.18 f | 43.11 e | |
| IIY 084 | 41.51 c | 171.90 e | 46.21 d | |
| LP | HGX | 51.12 b | 274.38 d | 55.53 c |
| IR24 | 42.18 c | 316.41 c | 59.00 b | |
| Y2 | 52.71 ab | 411.35 b | 61.05 a | |
| IIY 084 | 57.08 a | 445.47 a | 61.60 a | |
| Analysis of variance | ||||
| Year (Y) | ** | NS | ** | |
| Treatment (T) | ** | ** | ** | |
| Variety (V) | ** | ** | ** | |
| Y × T | ** | * | NS | |
| Y × V | NS | ** | NS | |
| T × V | ** | ** | NS | |
| Y × T × V | NS | NS | * | |
| Year/Treatment | Variety | NSC at HD (kg·m−2) | NSC at MA (kg·m−2) | NSC Remobilization (%) | NSC Contribution to the Grain (%) | NSC per Spikelet (mg Spikelet−1) |
|---|---|---|---|---|---|---|
| 2020 | ||||||
| NP | HGX | 0.20 e | 0.17 e | 18.84 e | 5.88 e | 5.76 e |
| IR24 | 0.13 g | 0.11 g | 16.16 f | 4.04 f | 3.84 g | |
| Y2 | 0.39 b | 0.28 b | 28.99 c | 15.03 b | 8.09 b | |
| IIY 084 | 0.45 a | 0.29 a | 34.23 b | 10.61 c | 6.94 c | |
| LP | HGX | 0.16 f | 0.13 f | 21.85 d | 8.49 d | 6.40 d |
| IR24 | 0.10 h | 0.09 h | 17.80 e | 6.26 e | 4.48 f | |
| Y2 | 0.37 d | 0.24 d | 34.98 b | 21.87 a | 9.27 a | |
| IIY 084 | 0.43 c | 0.26 c | 40.16 a | 15.46 b | 8.34 b | |
| 2021 | ||||||
| NP | HGX | 0.19 d | 0.17 d | 13.19 f | 4.59 e | 5.30 d |
| IR24 | 0.14 f | 0.12 e | 13.70 f | 3.99 e | 4.00 e | |
| Y2 | 0.41 b | 0.27 a | 34.10 c | 16.38 b | 8.22 b | |
| IIY 084 | 0.42 a | 0.25 b | 40.02 ab | 12.69 c | 6.65 c | |
| LP | HGX | 0.17 e | 0.13 e | 25.16 d | 9.84 d | 6.71 c |
| IR24 | 0.13 g | 0.10 f | 20.27 e | 8.40 d | 5.45 d | |
| Y2 | 0.38 c | 0.24 c | 37.77 bc | 23.68 a | 9.17 a | |
| IIY 084 | 0.41 ab | 0.23 c | 44.05 a | 16.45 b | 8.03 b | |
| Analysis of variance | ||||||
| Year (Y) | NS | ** | * | ** | NS | |
| Treatment (T) | ** | ** | ** | ** | ** | |
| Variety (V) | ** | ** | ** | ** | ** | |
| Y × T | * | * | * | * | NS | |
| Y × V | ** | ** | * | NS | * | |
| T × V | * | ** | NS | ** | NS | |
| Y × T × V | NS | * | * | NS | NS | |
| Year/Treatment | Variety | Number of Tillers and Main Stems (m−2) | PPST(%) | |||
|---|---|---|---|---|---|---|
| Mid-Tillering | Panicle Initiation | Heading | Maturity | |||
| 2020 | ||||||
| NP | HGX | 392.13 a | 525.18 a | 508.37 a | 317.05 a | 68.27 d |
| IR24 | 345.78 b | 450.39 b | 417.20 b | 310.55 a | 61.30 e | |
| Y2 | 389.16 a | 535.45 a | 508.37 a | 311.50 a | 55.99 f | |
| IIY 084 | 333.50 b | 418.26 c | 400.16 c | 312.25 a | 71.69 c | |
| LP | HGX | 208.96 d | 386.53 d | 357.12 d | 269.87 c | 83.95 a |
| IR24 | 163.96 e | 317.63 e | 308.81 e | 272.84 c | 68.34 d | |
| Y2 | 257.34 c | 411.21 cd | 401.00 c | 295.87 b | 71.77 c | |
| IIY 084 | 215.49 d | 322.61 e | 317.68 e | 312.12 a | 81.84 b | |
| 2021 | ||||||
| NP | HGX | 398.31 a | 524.74 b | 508.37 a | 317.90 b | 68.50 e |
| IR24 | 350.20 b | 451.29 c | 417.20 b | 305.69 c | 62.02 f | |
| Y2 | 386.43 a | 536.27 a | 508.37 a | 306.50 c | 55.99 g | |
| IIY 084 | 333.81 c | 416.79 d | 398.00 c | 390.00 a | 71.70 c | |
| LP | HGX | 208.02 e | 383.13 e | 357.12 d | 268.63 e | 83.88 a |
| IR24 | 163.45 f | 316.67 f | 308.81 e | 274.45 d | 69.98 d | |
| Y2 | 254.80 d | 411.41 d | 401.00 c | 297.66 c | 70.78 cd | |
| IIY 084 | 217.92 e | 323.29 f | 315.00 e | 309.68 c | 81.25 b | |
| Analysis of variance | ||||||
| Year (Y) | NS | NS | NS | NS | NS | |
| Treatment (T) | ** | ** | ** | ** | ** | |
| Variety (V) | ** | ** | ** | ** | ** | |
| Y × T | NS | NS | NS | NS | NS | |
| Y × V | NS | NS | NS | NS | NS | |
| T × V | ** | ** | ** | ** | ** | |
| Y × T × V | NS | NS | NS | NS | NS | |
| Year/Treatment | Variety | Stem + Leaf Dry Matter at Heading (g/m2) | Stem + Leaf Dry Matter at Maturity (g/m2) | Dry Matter Translocation Amount (g/m2) | Dry Matter Translocation Rate (%) |
|---|---|---|---|---|---|
| 2020 | |||||
| NP | HGX | 894 d | 815 d | 79 e | 8.85 f |
| IR24 | 866 e | 775 e | 91 e | 10.5 e | |
| Y2 | 1283 a | 1152 a | 131 c | 10.22 ef | |
| IIY 084 | 1222 b | 1066 b | 156 b | 12.76 d | |
| LP | HGX | 717 g | 601 g | 116 d | 16.13 b |
| IR24 | 617 h | 528 h | 89 e | 14.41 c | |
| Y2 | 817 f | 654 f | 163 b | 19.92 a | |
| IIY 084 | 1037 c | 839 c | 198 a | 19.08 a | |
| 2021 | |||||
| NP | HGX | 903 d | 811 d | 91 f | 10.09 e |
| IR24 | 875 e | 775 e | 100 ef | 11.4 d | |
| Y2 | 1284 a | 1146 a | 138 d | 10.73 de | |
| IIY 084 | 1217 b | 1048 b | 169 c | 13.89 c | |
| LP | HGX | 705 g | 596 g | 109 e | 15.47 b |
| IR24 | 619 h | 525 h | 94 f | 15.19 b | |
| Y2 | 824 f | 644 f | 180 b | 21.82 a | |
| IIY 084 | 1038 c | 821 c | 217 a | 20.93 a | |
| Analysis of variance | |||||
| Year (Y) | NS | * | * | * | |
| Treatment (T) | ** | ** | ** | ** | |
| Variety (V) | ** | ** | ** | ** | |
| Y × T | NS | NS | NS | NS | |
| Y × V | NS | NS | NS | NS | |
| T × V | ** | ** | * | ** | |
| Y × T × V | NS | NS | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Qiao, S.; Xu, Y.; Ji, D.; Zhang, W.; Gu, J.; Zhu, K.; Wang, Z.; Zhang, J.; Yang, J. Agronomic and Physiological Performance of the Indica Rice Varieties Differing in Tolerance to Low Phosphorus. Agronomy 2024, 14, 41. https://doi.org/10.3390/agronomy14010041
Sun Z, Qiao S, Xu Y, Ji D, Zhang W, Gu J, Zhu K, Wang Z, Zhang J, Yang J. Agronomic and Physiological Performance of the Indica Rice Varieties Differing in Tolerance to Low Phosphorus. Agronomy. 2024; 14(1):41. https://doi.org/10.3390/agronomy14010041
Chicago/Turabian StyleSun, Zhiwei, Shengfeng Qiao, Yuemei Xu, Dongling Ji, Weiyang Zhang, Junfei Gu, Kuanyu Zhu, Zhiqin Wang, Jianhua Zhang, and Jianchang Yang. 2024. "Agronomic and Physiological Performance of the Indica Rice Varieties Differing in Tolerance to Low Phosphorus" Agronomy 14, no. 1: 41. https://doi.org/10.3390/agronomy14010041
APA StyleSun, Z., Qiao, S., Xu, Y., Ji, D., Zhang, W., Gu, J., Zhu, K., Wang, Z., Zhang, J., & Yang, J. (2024). Agronomic and Physiological Performance of the Indica Rice Varieties Differing in Tolerance to Low Phosphorus. Agronomy, 14(1), 41. https://doi.org/10.3390/agronomy14010041







