Bacterial Communities in the Rhizosphere of Common Bean Plants (Phaseolus vulgaris L.) Grown in an Arable Soil Amended with TiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Common Bean, Soil, and Nanoparticles
2.2. Experimental Design and Greenhouse Experiment
2.3. Soil and Nanoparticle Characterization
2.4. DNA Extraction and PCR Amplification
2.5. rDNA Sequence Analysis
2.6. Data Accessibility
2.7. Phylogenetic and Statistical Analyses
3. Results
3.1. Plant Soil Characteristics
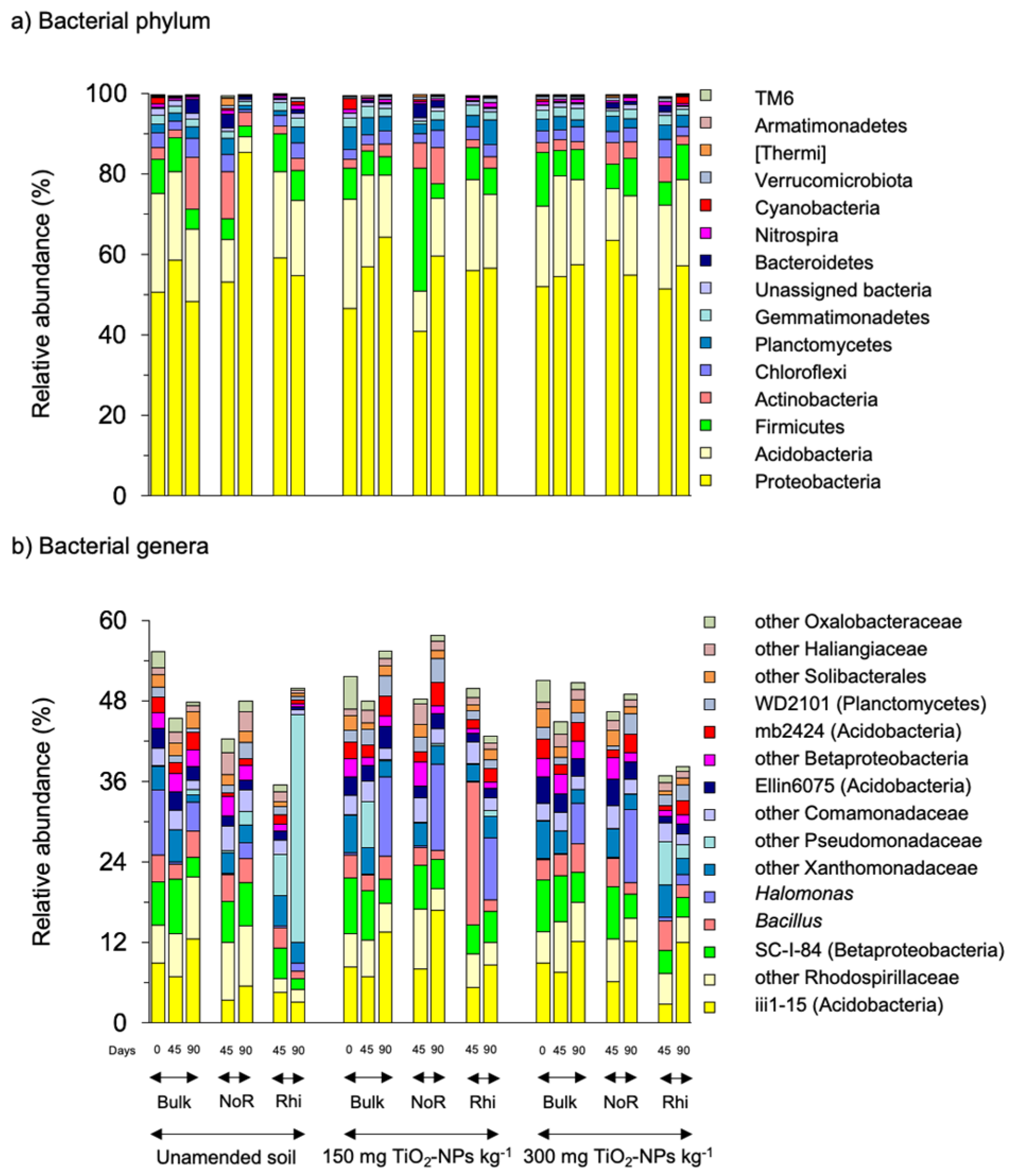
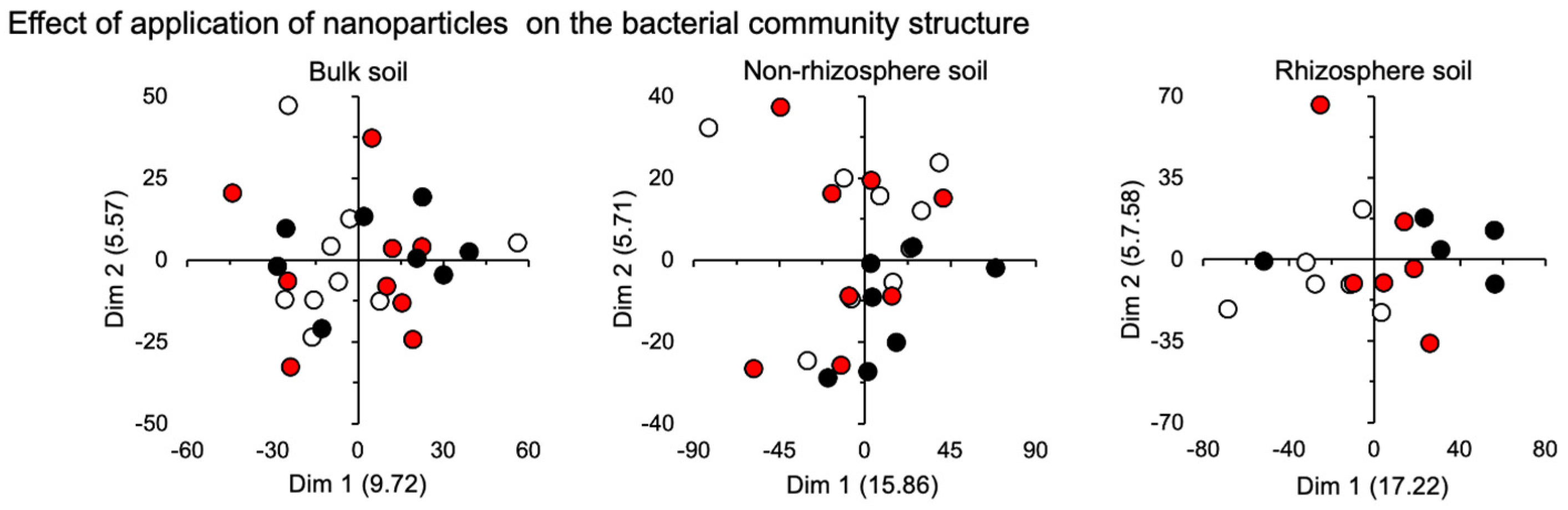
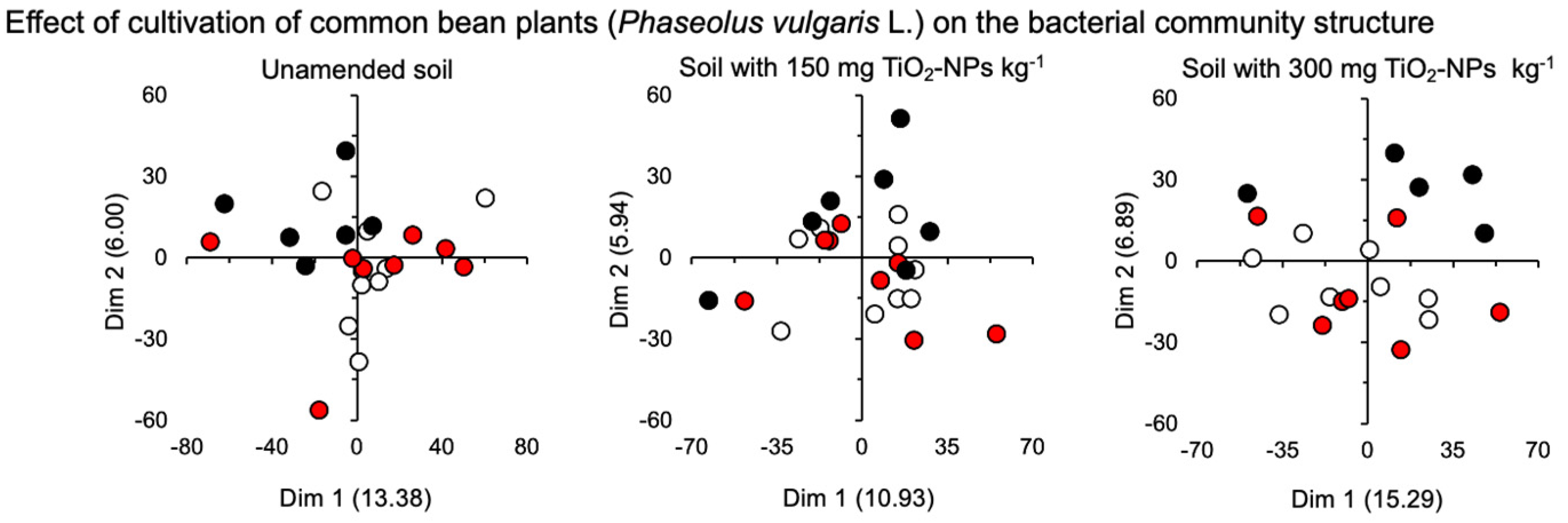
3.2. Bacterial Community
4. Discussion
4.1. Soil and Plant Characteristics
4.2. Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C.; Mirkin, C.A.; Hersam, M.C. Nanotechnology research directions for societal needs in 2020: Summary of international study. J. Nanopart. Res. 2011, 13, 897–919. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1–17. [Google Scholar] [CrossRef]
- Osseweijer, P. Sunscreens with Titanium Dioxide (TiO2) Nano-Particles: A Societal Experiment. NanoEthics 2010, 4, 103–113. [Google Scholar]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment; ACS Publications: Washington, DC, USA, 2009. [Google Scholar]
- Moll, J.; Klingenfuss, F.; Widmer, F.; Gogos, A.; Bucheli, T.D.; Hartmann, M.; van der Heijden, M.G.A. Effects of titanium dioxide nanoparticles on soil microbial communities and wheat biomass. Soil Biol. Biochem. 2017, 111, 85–93. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Feizi, H.; Moghaddam, P.R.; Shahtahmassebi, N.; Fotovat, A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace Elem. Res. 2012, 146, 101–106. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Hrubý, M.; Cígler, P.; Kuzel, S. Contribution to understanding the mechanism of titanium action in plant. J. Plant Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; An, Y.; Yoon, H.; Kweon, H. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. Int. J. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Ding, X.G.; Utami, M.S.; Kawashima, Y.; Miyama, Y.; Hashimoto, K. Detoxification of phytotoxic compounds by TiO2 photocatalysis in a recycling hydroponic cultivation system of asparagus. J. Agric. Food Chem. 2008, 56, 4819–4824. [Google Scholar] [CrossRef] [PubMed]
- Seeger, E.M.; Baun, A.; Kästner, M.; Trapp, S. Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J. Soils Sediments 2009, 9, 46–53. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Speciat. Bioavail. 2018, 30, 123–134. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Changmei, L.; Chaoying, Z.; Junqiang, W.; Guorong, W.; Mingxuan, T. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Bao-Shan, L.; Shao-Qi, D.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, G.; Qian, H.; Liu, P.; Zhao, P.; Hu, Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ. Pollut. 2013, 176, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Qu, C.; Zheng, L.; Yang, F.; Su, M.; Hong, F. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.C.; Sanjay, S.S.; Yadav, R.S. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kappen, P.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kurepa, J.; Smalle, J.A. Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011, 34, 811–820. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 282. [Google Scholar] [CrossRef]
- Larue, C.; Khodja, H.; Herlin-Boime, N.; Brisset, F.; Flank, A.M.; Fayard, B.; Chaillou, S.; Carrière, M. Investigation of titanium dioxide nanoparticles toxicity and uptake by plants. J. Phys Conf. Ser. 2011, 304, 012057. [Google Scholar] [CrossRef]
- Wu, S.G.; Huang, L.; Head, J.; Chen, D.R.; Kong, I.C.; Tang, Y.J. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J. Pet. Environ. Biotechnol. 2012, 3, 126. [Google Scholar]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Ahmad, A.; Alshahrani, T.S. Integrative effects of zinc nanoparticle and PGRs to mitigate salt stress in maize. Agronomy 2023, 13, 1655. [Google Scholar] [CrossRef]
- Chaudhary, I.; Singh, V. Titanium dioxide nanoparticles and its impact on growth, biomass and yield of agricultural crops under environmental stress: A review. Res. J. Nanosci. Nanotechnol. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Silva, S.; Dias, M.C.; Silva, A.M.S. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a)biotic stresses? Toxics 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Huang, Y.C.; Grusak, M.A.; Huang, C.P.; Sherrier, D.J. Effects of nano-TiO2 on the agronomically-relevant Rhizobium–legume symbiosis. Sci. Total Environ. 2014, 466, 503–512. [Google Scholar] [CrossRef] [PubMed]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver nanoparticles (AgNP) in the environment: A review of potential risks on human and environmental health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Fan, R.; Huang, Y.C.; Grusak, M.A.; Huang, C.P.; Sherrier, D.J. Silver nanoparticles, ions, and shape governing soil microbial functional diversity: Nano shapes micro. Front. Microbiol. 2016, 7, 1123. [Google Scholar]
- Samarajeewa, A.; Velicogna, J.; Princz, J.; Subasinghe, R.; Scroggins, R.; Beaudette, L. Effect of silver nano-particles on soil microbial growth, activity and community diversity in a sandy loam soil. Environ. Pollut. 2017, 220, 504–513. [Google Scholar] [CrossRef]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef]
- Heffner, R.A.; Butler, M.J.; Reilly, C.K. Pseudoreplication revisited. Ecology 1996, 77, 2558–2562. [Google Scholar] [CrossRef]
- Medina-Pérez, G. La Nanotecnología Agrícola para la Autosuficiencia Alimentaria, el Cuidado del Ambiente y el Bienestar Social en México: Mitos y Realidades. Ph.D. Thesis, Cinvestav, Mexico City, Mexico, 2019. [Google Scholar]
- Pineda-Tobón, D.M.; Pérez, J.C.; Gaviria-Palacio, D.; Guáqueta-Restrepo, J.J. Fast estimation of chlorophyll content on plant leaves using the light sensor of a smartphone. Dyna 2017, 84, 234–239. [Google Scholar] [CrossRef]
- Lowery, B.; Hickey, W.J.; Arshad, M.A.; Lal, R. Soil water parameters and soil quality. Methods Assess. Soil Qual. 1997, 49, 143–155. [Google Scholar]
- Nadler, A. Methodologies and the practical aspects of the bulk soil EC (σa)—Soil solution EC (σw) relations. Adv. Agron. 2005, 88, 273–312. [Google Scholar]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Huluka, G.; Miller, R. Particle size determination by hydrometer method. South. Coop. Ser. Bull. 2014, 419, 180–184. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1123–1184. [Google Scholar]
- Ceja-Navarro, J.A.; Rivera, F.N.; Patiño-Zúñiga, L.; Govaerts, B.; Marsch, R.; Vila-Sanjurjo, A.; Dendooven, L. Molecular characterization of soil bacterial communities in contrasting zero tillage systems. Plant Soil 2010, 329, 127–137. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformaion of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A laboratory manual. In Cold Spring Harbor Laboratory; Cold Spring Harbor Laboratory Press: Laurel Hollow, NY, USA, 1989. [Google Scholar]
- Valenzuela-Encinas, C.; Neria-González, I.; Alcántara-Hernández, R.J.; Enríquez-Aragón, J.A.; Estrada-Alvarado, I.; Hernández-Rodríguez, C.; Dendooven, L.; Marsch, R. Phylogenetic analysis of the archaeal community in an alkaline-saline soil of the former lake Texcoco (Mexico). Extremophiles 2008, 12, 247–254. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 3 November 2023).
- Chao, A.; Chiu, C.H.; Jost, L. Phylogenetic diversity measures based on Hill numbers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Li, D. Hillr PackAGE: Diversity through Hill Numbers. Version: 0.5.1. 2021. Available online: https://github.com/daijiang/hillR (accessed on 1 March 2021).
- Mair, P.; Wilcox, R.; Schoenbrodt, F. WRS2: A Collection of Robust Statistical Methods. R Package Version 0.9-2.2017. Available online: http://CRAN.R-project.org/package=WRS2 (accessed on 3 November 2023).
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Meth. Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome datasets are compositional: And this is not optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Gloor, G.; Fernandes, A.; Macklain, J.; Albert, A.; Links, M.; Quinn, T.; Wu, J.R.; Wong, R.G.; Lieng, B. ALDEx2 Package: Analysis of Differential Abundance Taking Sample Variation into Account. Version: 1.21.1. 2020. Available online: https://github.com/ggloor/ALDEx_bioc (accessed on 20 April 2020).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR package: Multivariate Exploratory Analysis and Data Mining. Version: 2.3. 2020. Available online: http://factominer.free.fr (accessed on 29 February 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’. Community Ecology Package, Version 2.5-7; 2019, Volume 2. Available online: https://github.com/vegandevs/vegan (accessed on 28 November 2020).
- Kim, H.Y. Statistical notes for clinical researchers: Effect size. Rest. Dent. Endod. 2015, 40, 328–331. [Google Scholar] [CrossRef]
- Burke, D.J.; Zhu, S.; Pablico-Lansigan, M.P.; Hewins, C.R.; Samia, A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils 2014, 50, 1169–1173. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. PPB 2016, 107, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Guyonnet, J.P.; Martins, J.M.; Ginot, M.; Richaume, A. Influence of soil properties on the toxicity of TiO2 nanoparticles on carbon mineralization and bacterial abundance. J. Hazard. Mater. 2015, 283, 529–535. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, L.; Liu, G.; Song, L.; Arenas-Lago, D.; Kong, L.; Peijnenburg, W.; Vijver, M.G. Compositional and functional responses of bacterial community to titanium dioxide nanoparticles varied with soil heterogeneity and exposure duration. Sci. Total Environ. 2021, 773, 144895. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kalia, A.; Sandhu, J.S.; Dheri, G.S.; Kaur, G.; Pathania, S. Interaction of TiO2 nanoparticles with soil: Effect on microbiological and chemical traits. Chemosphere 2022, 301, 134629. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Satoh, M.; Yabe, R.; Takahashi, R.; Tokuyama, T. Identification of genus Nitrosovibrio, ammonia-oxidizing bacteria, by comparison of N-terminal amino acid sequences of phosphoglycerate kinase. J. Biosci. Bioeng. 2004, 98, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.J.; Daebeler, A.; Herbold, C.W.; Kirkegaard, R.H.; Daims, H. Cultivation and genomic characterization of novel and ubiquitous marine nitrite-oxidizing bacteria from the Nitrospirales. ISME J. 2023, 17, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.; Pommier, T. Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef]
- Komendová, R.; Žídek, J.; Berka, M.; Jemelková, M.; Řezáčová, V.; Conte, P.; Kučerík, J. Small-Sized Platinum Nanoparticles in Soil Organic Matter: Influence on Water Holding Capacity, Evaporation and Structural Rigidity. Sci. Total Environ. 2019, 694, 133822. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M.L. Impact on Some Soil Physical and Chemical Properties Caused by Metal and Metallic Oxide Engineered Nanoparticles: A Review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef]
- Ge, Y.; Priester, J.H.; Van De Werfhorst, L.C.; Schimel, J.P.; Holden, P.A. Potential mechanisms and environmental controls of TiO2 nanoparticle effects on soil bacterial communities. Environ. Sci. Technol. 2013, 47, 14411–14417. [Google Scholar] [CrossRef]
- Sharmila Rahale, C.; Lakshmanan ASumithra, M.G.; Ranjith Kumar, E. Humic acid involved chelation of ZnO nanoparticles for enhancing mineral nutrition in plants. Solid State Commun. 2021, 333, 114355. [Google Scholar] [CrossRef]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl. Environ. Microbiol. 2012, 78, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, K.B.; De Los Santos-Ramos, F.J.; Gómez-Acata, E.S.; Luna-Guido, M.; Navarro-Noya, Y.E.; Fernández-Luqueño, F.; Dendooven, L. TiO2 nanoparticles affect the bacterial community structure and Eisenia fetida (Savigny, 1826) in an arable soil. PeerJ 2019, 7, e6939. [Google Scholar] [CrossRef]
- Sun, C.; Hu, K.; Mu, D.; Wang, Z.; Yu, X. The Widespread Use of Nanomaterials: The Effects on the Function and Diversity of Environmental Microbial Communities. Microorganisms 2022, 10, 2080. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of Agricultural Soil with Metal Nanoparticles: Effects on Soil Enzyme Activity and Microbial Community Composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.; Przemieniecki, S.W.; Kurowski, T.; Oćwieja, M. Early plant growth and bacterial community in rhizoplane of wheat and flax exposed to silver and titanium dioxide nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 33820–33826. [Google Scholar] [CrossRef]
- Tapia-García, E.Y.; Hernández-Trejo, V.; Guevara-Luna, J.; Rojas-Rojas, F.U.; Arroyo-Herrera, I.; Meza-Radilla, G.; Vásquez-Murrieta, M.S.; Estrada-de Los Santos, P. Plant growth-promoting bacteria isolated from wild legume nodules and nodules of Phaseolus vulgaris L. trap plants in central and southern Mexico. Microbiol. Res. 2020, 239, 126522. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Identification and characterization of endophytic bacteria isolated from in vitro cultures of peach and pear rootstocks. 3 Biotech 2016, 6, 120. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef]
- Crosbie, D.B.; Mahmoudi, M.; Radl, V.; Brachmann, A.; Schloter, M.; Kemen, E.; Marín, M. Microbiome profiling reveals that Pseudomonas antagonises parasitic nodule colonisation of cheater rhizobia in Lotus. New Phytol. 2022, 234, 242–255. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Banach, A.; Izak, D.; Stępniewska, Z.; Błaszczyk, M. Metagenomic Analysis of Some Potential Nitrogen-Fixing Bacteria in Arable Soils at Different Formation Processes. Microb. Ecol. 2017, 73, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Wegner, C.E.; Luo, Y.; Xiao, K.Q.; Cui, Z.; Zhang, F.; Liesack, W.; Peng, J. Deciphering microbial mechanisms underlying soil organic carbon storage in a wheat-maize rotation system. Sci. Total Environ. 2021, 788, 147798. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L. Oligotrophs versus copiotrophs. Bioessays 2001, 23, 657–661. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Pianka, E.R. On r- and K-Selection. Am. Nat. 1970, 104, 592–597. Available online: http://www.jstor.org/stable/2459020 (accessed on 1 December 2023). [CrossRef]
| TiO2 | Length (cm) | Shoot | Fresh Weight (g) | Dry Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time | -NPs | Nodules | Diameter | ||||||
| (Days) | (mg kg−1) | (Number) | Shoot | Root | (cm) | Shoot | Root | Shoot | Root |
| 45 | 0 | 10 a | 118 c | 60 b | 0.5 a | 41 b | 30 a | 6.4 b | 3.7 a |
| 150 | 15 a | 139 bc | 81 a | 0.4 a | 47 b | 29 a | 6.7 b | 3.6 a | |
| 300 | 20 a | 144 bc | 62 b | 0.5 a | 44 b | 34 a | 6.5 b | 3.7 a | |
| 90 | 0 | 23 a | 221 a | 77 ab | 0.5 a | 78 a | 38 a | 17.1 a | 5.6 a |
| 150 | 35 a | 203 ab | 67 ab | 0.5 a | 77 a | 38 a | 17.0 a | 5.8 a | |
| 300 | 15 a | 255 a | 81 a | 0.5 a | 87 a | 39 a | 19.0 a | 5.9 a | |
| Time | TiO2-NPs | EC | WHC | NH4+ | NO2− | NO3− | ||
|---|---|---|---|---|---|---|---|---|
| (days) | Beans | (mg kg−1) | pH | (dS m−1) | (g kg−1) | (g kg−1) | ||
| 45 | No | 0 | 7.9 ab | 2.23 ab | 625 bcd | 1.3 ab | 0.24 a | 12.5 ab |
| 150 | 7.8 b | 3.09 a | 643 bc | 1.5 ab | 0.18 a | 10.0 ab | ||
| 300 | 7.9 ab | 2.48 ab | 629 bcd | 1.4 ab | 0.19 a | 12.6 a | ||
| Yes | 0 | 7.9 ab | 1.54 b | 659 b | 1.4 ab | 0.25 a | 6.6 ab | |
| 150 | 8.0 ab | 1.62 b | 677 cd | 1.5 ab | 0.17 a | 3.5 ab | ||
| 300 | 7.9 ab | 1.76 b | 840 a | 2.1 a | 0.30 a | 9.5 ab | ||
| 90 | No | 0 | 8.2 ab | ND | 504 e | 1.1 b | 0.18 a | 4.8 ab |
| 150 | 8.1 ab | ND | 616 bcde | 1.3 ab | 0.16 a | 5.9 ab | ||
| 300 | 8.1 ab | ND | 715 b | 1.4 ab | 0.20 a | 10.6 ab | ||
| Yes | 0 | 8.3 a | ND | 598 cde | 1.6 ab | 0.23 a | 4.6 ab | |
| 150 | 8.0 ab | ND | 517 ed | 1.7 ab | 0.26 a | 1.7 b | ||
| 300 | 8.1 ab | ND | 594 cde | 2.1 a | 0.18 a | 1.8 b | ||
| q = 0 | q = 1 | q = 2 | ||||
|---|---|---|---|---|---|---|
| Soil | F Value | p Value | F value | p Value | F Value | p Value |
| Effect of nanoparticles | ||||||
| Bulk soil | 1.26 | 0.332 | 3.85 | 0.057 | 0.00 | 1.000 |
| Non rhizosphere soil | 1.22 | 0.338 | 0.35 | 0.713 | 0.00 | 1.000 |
| Rhizosphere soil | 6.23 | 0.045 * | 7.43 | 0.034 * | 0.00 | 1.000 |
| Effect of cultivation of bean plants | ||||||
| Unamended | 0.50 | 0.634 | 0.65 | 0.561 | 0.00 | 1.000 |
| Amended with 150 mg TiO2-NPs kg−1 | 0.73 | 0.512 | 0.63 | 0.563 | 0.00 | 1.000 |
| Amended with 300 mg nTiO2-NPs kg−1 | 3.53 | 0.107 | 19.67 | 0.003 ** | 0.00 | 1.000 |
| perMANOVA | |||
|---|---|---|---|
| R2 | F Value | p Value | |
| Nanoparticles (0, 150, 300 mg TiO2-NPs kg−1) | 0.022 | 1.53 | 0.005 ** |
| Bean plant (bulk, non-rhizosphere, rhizosphere) | 0.038 | 1.31 | 0.016 * |
| Time (day, 0, 45, 90) | 0.020 | 1.35 | 0.800 |
| Interaction nanoparticles and bean plant | 0.032 | 1.11 | 0.127 |
| Interaction nanoparticles and time | 0.017 | 1.19 | 0.091 |
| Interaction bean plant and time | 0.034 | 1.17 | 0.065 |
| Interaction nanoparticles, bean plant and time | 0.031 | 1.05 | 0.240 |
| Effect of nanoparticles | |||
| Bulk soil | 0.038 | 0.96 | 0.602 |
| Non-rhizosphere soil | 0.042 | 0.97 | 0.391 |
| Rhizosphere soil | 0.105 | 1.75 | 0.005 ** |
| Effect of bean plant | |||
| Unamended soil | 0.102 | 1.13 | 0.124 |
| Soil with 150 mg TiO2 kg−1 | 0.089 | 1.02 | 0.300 |
| Soil with 300 mg TiO2 kg−1 | 0.125 | 1.21 | 0.056 |
| Unamended soil versus soil amended with 150 mg TiO2 kg−1 soil. |
| Bulk soil: other Enterobacteriaceae (−0.8 a, b * c) Non-rhizosphere soil: other Gemmataceae (−1.0*), Afifella (−0.8) Rhizosphere soil: Pseudoxanthomonas (−0.8) |
| Unamended soil versus soil amended with 300 mg TiO2-NPs kg−1 soil. |
| Bulk soil: other Piscirickettsiaceae (−1.2 *), Steroidobacter (−1.1 *), other Solibacterales (−0.8), Thermomonas (−0.8), other Alcaligenaceae (−0.8) Non-rhizosphere soil: other iii1-15 (Acidobacteria-6, −1.2 *), other [Entotheonellaceae] (−1.1 *), mb2424 (Acidobacteria, −1.0 *), PK29 (Acidobacteria, −1.0 *), other Oxalobacteraceae (−0.9 *), WD2101 (Planctomycetes, −0.9 *), S0208 (Chlorofle S0208xi, −0.9 *) Ellin6075 (Acidobacteria, −0.8 *), Bacillus (−0.8), A4b (Chloroflexi, −0.8), other Syntrophobacteraceae (−0.8), other Gemmatimonadetes (−0.8) Rhizosphere soil: other Pseudomonadaceae (−1.0), Pseudoxanthomonas (−0.9), Devosia (−0.9), Pseudomonas (−0.8), other iii1-15 (0.8), mb2424 (0.8), PK29 (0.8), Candidatus Solibacter (0.8), Ellin6067 (0.8), Halomonas (0.9), RB41 (0.9 *), RB25 (0.9), Acidobacteria-5 (1.4 *), Ellin6075 (1.5 *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Pérez, G.; Afanador-Barajas, L.; Pérez-Ríos, S.; Navarro-Noya, Y.E.; Luna-Guido, M.; Fernández-Luqueño, F.; Dendooven, L. Bacterial Communities in the Rhizosphere of Common Bean Plants (Phaseolus vulgaris L.) Grown in an Arable Soil Amended with TiO2 Nanoparticles. Agronomy 2024, 14, 74. https://doi.org/10.3390/agronomy14010074
Medina-Pérez G, Afanador-Barajas L, Pérez-Ríos S, Navarro-Noya YE, Luna-Guido M, Fernández-Luqueño F, Dendooven L. Bacterial Communities in the Rhizosphere of Common Bean Plants (Phaseolus vulgaris L.) Grown in an Arable Soil Amended with TiO2 Nanoparticles. Agronomy. 2024; 14(1):74. https://doi.org/10.3390/agronomy14010074
Chicago/Turabian StyleMedina-Pérez, Gabriela, Laura Afanador-Barajas, Sergio Pérez-Ríos, Yendi E. Navarro-Noya, Marco Luna-Guido, Fabián Fernández-Luqueño, and Luc Dendooven. 2024. "Bacterial Communities in the Rhizosphere of Common Bean Plants (Phaseolus vulgaris L.) Grown in an Arable Soil Amended with TiO2 Nanoparticles" Agronomy 14, no. 1: 74. https://doi.org/10.3390/agronomy14010074






