Lupinus mutabilis Breeding in the Andes of Ecuador, Peru, and Bolivia: A Review
Abstract
:1. Introduction
2. Tarwi Plant Characteristics
3. The Cultivation of Tarwi in Ecuador, Peru, and Bolivia
4. Genetic Diversity of L. mutabilis in the Andean Region
- L. mutabilis, chocho common name (northern Peru and Ecuador), with a high degree of branching, very late maturing, greater hairiness in leaves and stems; some ecotypes behave as biennials, tolerant to anthracnose.
- L. mutabilis, tarwi common name (central and southern Peru), with few branches, medium late maturing, somewhat tolerant to anthracnose.
- L. mutabilis, tauri common name (altiplano of Peru and Bolivia), with few branches, smaller plant (1–1.40 m), with a developed main stem, susceptible to anthracnose.
- Early Titicaca, late Titicaca, Cochabamba.
- Early South (Chuquisaca-Potosí).
- Late South (Potosí).
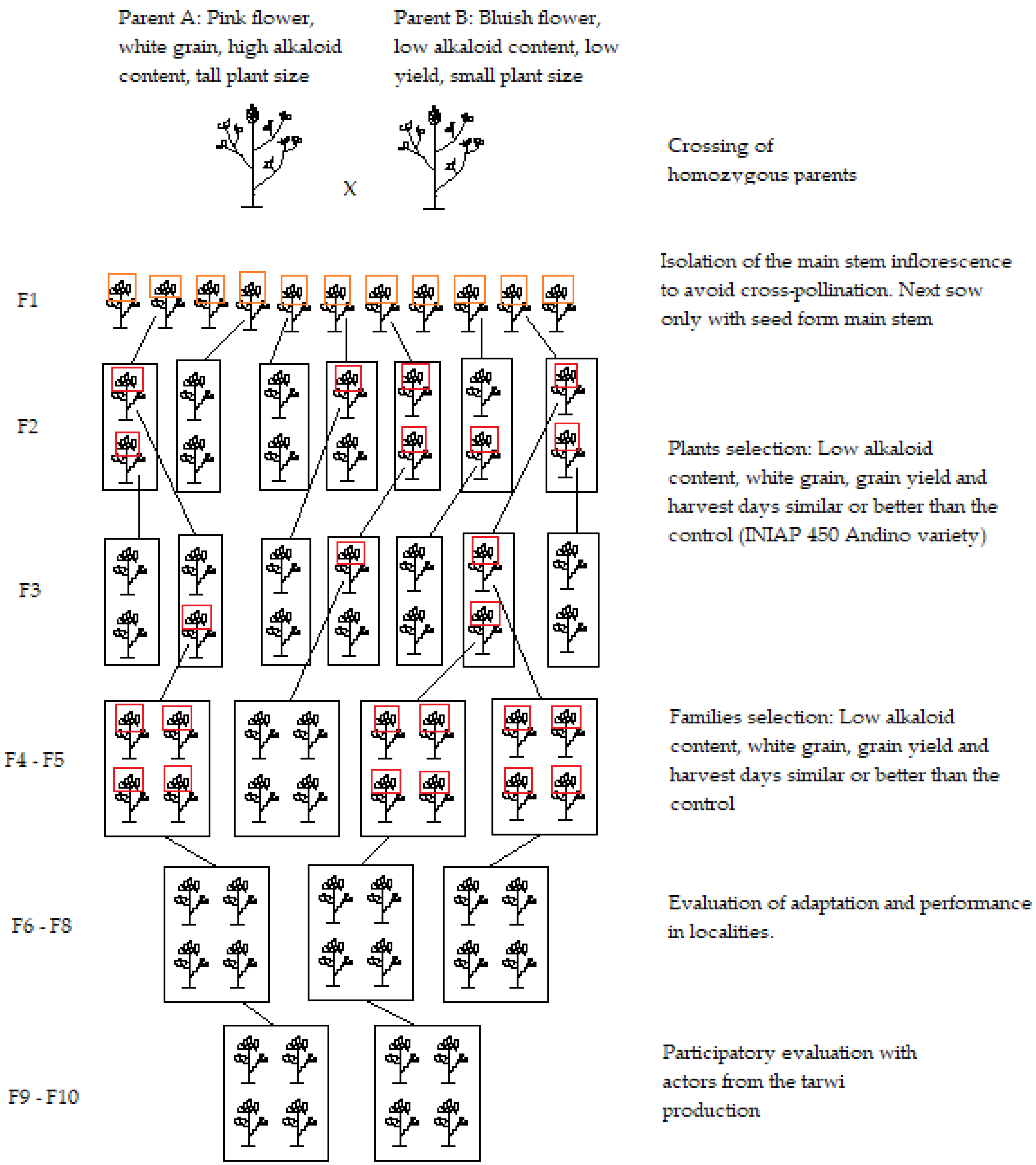
5. Breeding Methods Used in the Region
6. Breeding Targets in the Region
6.1. Breeding for Low Content Alkaloid
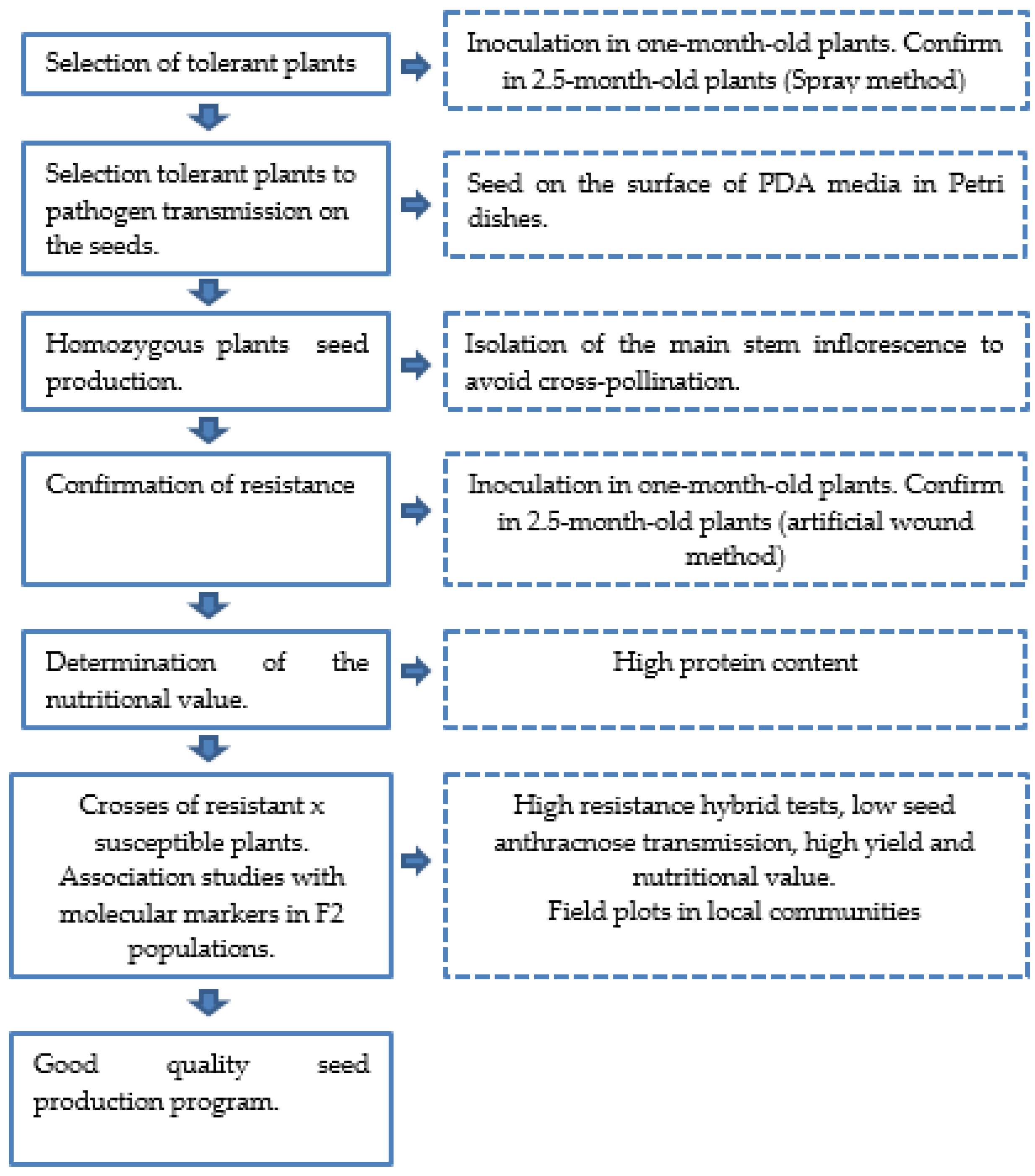
6.2. Breeding for Resistance to Anthracnose
7. Improved Varieties of L. mutabilis Obtained in the Andean Region
7.1. Bolivia
7.2. Peru
7.3. Ecuador
8. Brief Overview of Tarwi Breeding Progress in Europe
9. Breeding Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jacobsen, S.-E.; Mujica, A. El Tarwi (Lupinus mutabilis Sweet.) y sus parientes silvestres. Botánica Económica Los Andes Cent. 2006, 28, 458–482. [Google Scholar]
- Mujica, A. Capítulo 1. La planta de Tarwi. In Lupinus mutabilis (Tarwi), Leguminosa andina Con Gran Potencial Industrial, 1st ed.; Amparo Zavaleta (Compiladora); Fondo Editorial de la Universidad Nacional Mayor de San Marcos: Lima, Peru, 2018; ISBN 978-9972-46-620-5. [Google Scholar]
- Mercado, G. Memoria Foro Virtual: Los Caminos Del Tarwi y La Integración Andina: Bolivia, Peru y Ecuador; IPDRS: La Paz, Bolivia, 2018; 60p. [Google Scholar]
- Blanco, O. Genetic variability of tarwi (Lupinus mutabilis Sweet). In Agricultural and Nutritional Aspects of Lupines; Gross, R., Bunting, E.S., Eds.; GTZ: Eschborn, Germany, 1982; pp. 33–49. [Google Scholar]
- Camarena, F. El tarwi o chocho. In Paradigmas: Desafíos y Oportunidades De Los Cultivos Andinos Frente a Los Tratados De Libre Comercio; Concytec: Lima, Peru, 2011; pp. 32–36. [Google Scholar]
- Jacobsen, S.-E.; Mujica, A. Geographical distribution of the Andean lupin (Lupinus mutabilis Sweet). Plant Genet. Res. 2008, 155, 1–8. [Google Scholar]
- Canahua-Murillo, A.; Roman-Canahua, P. Tarwi. Leguminosa andina de gran potencial. Rev. Agroecol. 2016, 32, 20–21. [Google Scholar]
- Lucas, M.M.; Stoddard, F.; Annicchiarico, P.; Frias, J.; Martinez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Frias, J.; et al. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Peralta, E.; Mazón, N.; Murillo, A.; Rodríguez, D. Manual Agrícola de Granos Andinos: Chocho, Quinua, Amaranto y Ataco. Cultivos, Variedades y Costos de Producción, 4th ed.; Publicación Miscelánea No. 69. INIAP. Programa Nacional de Leguminosas y granos Andinos; Estación experimental Santa Catalina: Quito, Ecuador, 2014; p. 72. [Google Scholar]
- Sinche-Serra, M.; Velazquez, J.; Aguilar-Aguilar, J.; Ramirez, T. Promising Lines of Lupinus mutabilis Sweet Derived from Mutation Induction with Ionizing Radiations. In Proceedings of the Abstracts of International Symposium on Plant Mutation Breeding and Biotechnology, Viena, Austria, 27–31 August 2018. IAEA-CN-263-115. [Google Scholar]
- Molina-Poveda, C.; Lucas, M.; Jover, M. Evaluation of the potential of Andean lupin meal (Lupinus mutabilis Sweet) as an alternative to fish meal in juvenile Litopenaeus vannamei diets. Aquaculture 2013, 410–411, 148–156. [Google Scholar] [CrossRef]
- Gross, R.; Von Baer, E.; Koch, R.; Marquard, L.; Trugo, L.; Wink, M. Chemical composition of a new variety of the Andean lupin (Lupinus mutabilis cv. Inti) with low alkaloid content. J. Food Compos. Anal. 1988, 1, 353–361. [Google Scholar] [CrossRef]
- Gabriel, J.; Vallejos, J.; Mamani, P.; Angulo, A. Mejora genética del tarwi (Lupinus mutabilis Sweet) en Bolivia. Rev. Agric. 2018, 57, 1–9. [Google Scholar]
- Baldeón, M.; Fornasini, M.; Muñoz, E.; Villacrés, E. Medical advances in the consumption of Lupinus mutabilis sweet, chocho/tarwi, in Ecuador. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 38. [Google Scholar]
- Vegas, N.; Vegas, C.; Peña, P. Capítulo 3. Potencial tecnológico de las semillas de tarwi. In Lupinus mutabilis (Tarwi), Leguminosa andina Con Gran Potencial Industrial, 1st ed.; Amparo Zavaleta (Compiladora); Fondo Editorial de la Universidad Nacional Mayor de San Marcos: Lima, Peru, 2018; ISBN 978-9972-46-620-5. [Google Scholar]
- Zamora, H.; Zamora-Burbano, A.; Ribeiro, L.; Lopes, T.; Coral, J.; Pasquini, D. Potencial do tremoço andino (Lupinus mutabilis) para produção de biodiesel via rota metílica: Uma revisão. Rev. Virtual Quim. 2020, 12, 852–866. [Google Scholar]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Gross, R. El cultivo y la utilización del Tarwi: Lupinus mutabilis Sweet. In Producción y Protección Vegetal, N° 36; Estudio FAO: Roma, Italy, 1982. [Google Scholar]
- Rojas, J. El Cultivo de Tarwi (Lupinus mutabilis Sweet) En El Estado Plurinacional de Bolivia. Info INIAF [Online]. 2016, Vol. 1, N. 7, pp. 88–100. Available online: http://www.revistasbolivianas.org.bo/pdf/rciii/v1n7/v1n7_a14.pdf (accessed on 9 May 2021).
- Tapia, M.; Fries, A. Guía de Campo de Los Cultivos Andinos; FAO: Lima, Peru, 2007. [Google Scholar]
- Tapia, M.E. El Tarwi, Lupino Andino. Tarwi, Tauri o Chocho. 2015. Available online: http://fadvamerica.org/wp-content/uploads/2017/04/TARWI-espanol.pdf (accessed on 14 May 2021).
- Peralta, E. El Chocho En Ecuador “Estado Del Arte”; INIAP, PRONALAEG: Quito, Ecuador, 2016. [Google Scholar]
- Chipana, G.; Trigo, R.; Bosque, H.; Jacobsen, S.E.; Mercado, G.; Rodríguez, J.; Callisaya, I.; Contreras, E.; Condori, J. EL tarwi (Lupinus mutabilis) y su importancia social y económica en las familias del altiplano norte de Bolivia. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 54. [Google Scholar]
- Gandarillas, A.; Vallejos, J.; Mamani, O. El tarwi: Un cultivo con nuevas oportunidades en Bolivia. Rev. Agric. 2018, 57, 31–39. [Google Scholar]
- INEC (Instituto Nacional de Estadísticas y Censos). III Censo Nacional Agropecuario. Resultados Nacionales; INEC: Quito, Ecuador, 2000; p. 56. [Google Scholar]
- Atchison, G.W.; Nevado, B.; Eastwood, R.J.; Contreras-Ortiz, N.; Reynel, C.; Madriñán, S.; Filatov, D.A.; Hughes, C.E. Lost crops of the Incas: Origins of domestication of the Andean pulse crop tarwi, Lupinus mutabilis. Am. J. Bot. 2016, 103, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Huaringa, A. Caracterización fenotípica preliminar de 14 ecotipos de tarwi provenientes de Puno, Cusco y La Libertad y Evaluación preliminar de la capacidad simbiótica de cepas nativas de los ecotipos de tarwi. In Proceedings of the Simposio Regional del Chocho o Tarwi, Quito, Ecuador, 29 November–1 December 2016. [Google Scholar]
- Rivera, M.; Pinzón, J.; Caicedo, C.; Murillo, A.; Mazón, N.; Peralta, E. Catálogo del banco de germoplasma de chocho (Lupinus mutabilis Sweet) y otras especies de lupinos. In Programa Nacional de Leguminosas; INIAP, Estación Experimental Santa Catalina: Quito, Ecuador, 1998. [Google Scholar]
- Grin-Global. Banco Nacional de Germoplasma de Bolivia. 2018. Available online: http://germoplasma.iniaf.gob.bo/gringlobal/search.aspx (accessed on 14 May 2021).
- Knudsen, H. Directorio de Colecciones de Germoplasma en América Latina y el Caribe. Primera edición. International Plant Genetic Resources Institute (IPGRI), Roma, Italia. 2000. Available online: https://cgspace.cgiar.org/handle/10568/104098?show=full (accessed on 20 August 2023).
- Neves-Martins, J.; Silva, P.; Sousa, R. Evaluation of Lupinus mutabilis accessions for protein and oil in Portugal. In Agrimed Research Programme—Lupinus mutabilis: Its Adaptation and Production under European Pedoclimatic Conditions; Commission of the European Communities: Luxembourg, 1992; pp. 1–10. [Google Scholar]
- Chirinos-Arias, M.; Jiménez, E.J.; Vilca-Machaca, S.L. Analysis of Genetic Variability among thirty accessions of Andean Lupin (Lupinus mutabilis Sweet) using ISSR molecular markers. Sci. Agropecu. 2015, 6, 17–30. [Google Scholar] [CrossRef]
- Guilengue, N.; Alves, S.; Talhinhas, P.; Neves-Martins, J. Genetic and Genomic Diversity in a Tarwi (Lupinus mutabilis Sweet) Germplasm Collection and Adaptability to Mediterranean Climate Conditions. Agronomy 2020, 10, 21. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Rodriguez, D.; Murillo, A.; van Dinter, B.J.; Torres, A.F.; Gordillo-Romero, M.; Torres, M.L.; Neves-Martins, J.; Paulo, M.J.; et al. Diversity and Agronomic Performance of Lupinus mutabilis Germplasm in European and Andean Environments. Front. Plant Sci. 2022, 13, 903661. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, A.; Lippolis, A.; van Loo, E.N.; Paulo, M.-J.; Trindade, L.M. A genome wide association study to dissect the genetic architecture of agronomic traits in Andean lupin (Lupinus mutabilis). Front. Plant Sci. 2023, 13, 1099293. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M. Cultivos Andinos Sub Explotados y Su Aporte a La Alimentación, 2nd ed.; FAO: Santiago, Chile, 2000. [Google Scholar]
- Cowling, W.; Buirchell, B.; Tapia, M. Lupin. Lupinus L. Promoting the Conservation and USE of Underutilized and Neglected Crops 23; IPK, Gatersleben/IPGRI: Rome, Italy, 1998; 100p. [Google Scholar]
- Vallejos, J.; Gabriel, J.; Angulo, A.; Cadima, X.; Mamani, P. New tarwi (Lupinus mutabilis Sweet) crop obtained by stratified masal selection. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 114. [Google Scholar]
- Caicedo, C.; Murillo, A.; Peralta, E.; Pinzón, J.; Rivera, M. INIAP-450 Andino, Variedad de Chocho Para La Zona Centro/Norte de La Sierra Ecuatoriana; Revista informativa del Instituto Nacional Autónomo de Investigaciones Agropecuarias: Quito, Ecuador, 2000; pp. 28–29. [Google Scholar]
- Lalama, M.; Nieto, C. Avance de la investigación en cultivos andinos en INIAP. In Proceedings of the III Congreso Internacional de Cultivos Andinos. IBTA–CIID, La Paz, Bolivia, 8–12 February 1982; pp. 425–432. [Google Scholar]
- Peralta, E.; Murillo, A.; Mazón, N. Linea Del Tiempo. In Mejoramiento Genético de Los Granos Andinos en Ecuador: Quinua, Chocho, Amaranto y Ataco. Programa de Leguminosas y Granos Andinos; Publicación miscelánea No. 420; INIAP, Estación Experimental Santa Catalina: Quito, Ecuador, 2015. [Google Scholar]
- Poehlman, J.M.; Sleeper, D.A. Breeding Field Crops, 4th ed.; Iowa State University Press: Ames, IA, USA, 1995. [Google Scholar]
- Eastwood, R.J.; Hughes, C.E. Origins of domestication of Lupinus mutabilis in the Andes. In Proceedings of the 12th International Lupin conference, Freemantle, Australia, 14–18 September 2008; pp. 373–379. [Google Scholar]
- Mujica, A.; Chura, E.; Apaza, J.; Chuquimia, D.; Moscoso, G.; Calandri, E.; Montoya, P.; Grasso, F.; Guzmán, C. Selección de cultivares nacionales de tarwi (Lupinus mutabilis sweet) por rendimiento, precocidad, contenido de aceite y proteína en Puno, Peru. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 166. [Google Scholar]
- Mujica, A.; Moscoso, G.; Chuquimia, D.; Romero, T.; Astete, A.; Calandri, E.; Montoya, P. Capítulo 1. Selección de cultivares de tarwi (Lupinus mutabilis Sweet.) por rendimiento, precocidad, contenido de aceite y proteína en Puno Peru. In Agrárias: Pesquisa e Inovaco Nas Que Alimentam o Mundo; Editora Artemis: Curitiba, Brasil, 2021; pp. 1–13. [Google Scholar]
- Murillo, A.; Rivera, M.; Peralta, E.; Mazon, N.; Vargas, F. Avances preliminares en el mejoramiento genético de chocho (Lupinus mutabilis Sweet) para resistencia a antracnosis. In Proceedings of the XII Congreso Internacional de Cultivos Andinos. INIAP, PUCE, Quito, Ecuador, 23–27 July 2006. [Google Scholar]
- Carvajal-Larenas, F.; Linnemann, A.; Nout, M.; Koziol, M.; van Boekel, A. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Rychel, S.; Książkiewicz, M. Development of gene-based molecular markers tagging low alkaloid pauper locus in white lupin (Lupinus albus L.). J. Appl. Genet. 2019, 60, 269–281. [Google Scholar] [CrossRef]
- Frick, K.; Kamphuis, L.G.; Siddique, K.H.; Singh, K.B.; Foley, R.C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef]
- Falconí, C. Lupinus mutabilis in Ecuador with Special Emphasis on Anthracnose Resistance. Ph.D. Thesis, Wageningen University, Wageningen, NL, USA, 2012. [Google Scholar]
- Guilengue, N.; Neves-Martins, J.; Talhinhas, P. Response to Anthracnose in a Tarwi (Lupinus mutabilis) Collection Is Influenced by Anthocyanin Pigmentation. Plants 2020, 9, 583. [Google Scholar] [CrossRef]
- Brown, A.; Sreenivasaprasad, S.; Timmer, L. Molecular characterization of slow-growing orange and key lime anthracnose strains of Colletotrichum from citrus as C. acutatum. Phytopathology 1996, 86, 523–527. [Google Scholar] [CrossRef]
- Gondran, J.; Bateman, G.L.; Milford, G.F.J.; Bayer, J.; Beerepoot, L.; Boller, B.; Caligari, P.D.S.; Carrasco-López, J.M.; Crowley, J.G.; Rocha, J.J.P.; et al. Anthracnose of white lupin: European prospects for a future sustainable crop. In Proceedings of the 8th International Lupin Conference, Pacific Grove, CA, USA, 11–16 May 1996. [Google Scholar]
- Plata, G.; Gandarillas, A. Enfermedades que afectan al cultivo del tarwi (Lupinu mutabilis) en Bolivia. Rev. Agric. 2018, 57, 62–72. [Google Scholar]
- Rodríguez, D.; Vega, L.; Murillo, A.; Peralta, E. Identificación de fuentes de resistencia a la antracnosis (Colletotrihum acutatum) del chocho Lupinus mutabilis Sweet) en el banco de germoplasma del INIAP. In Proceedings of the Memorias IV Congreso Mundial de La Quinua y I Simposio de Granos Andinos, Ibarra, Ecuador, 8–12 July 2013. [Google Scholar]
- Guaytarilla, P.; Falconí, C. Selección por arquitectura de la planta y resistencia a la Antracnosis de 7 Genotipos de Chocho (Lupinus mutabilis). In Proceedings of the Memorias IX Congreso de Ciencia y Tecnología ESPE, Sangolqui, Ecuador, 28–30 May 2014; pp. 63–70. [Google Scholar]
- PADER–COSUDE. Cadena de Valor de Tarwi. Taller “Agenda de Responsabilidad Compartida de la Cadena de Valor de Tarwi”. Mancomunidad del Altiplano Andino de Norte Potosí. Bolivia. 58p. 2006. Available online: https://fdocuments.ec/document/cadena-de-valor-de-tarwi-tarwipdf-2006-3-15-como-tarwi-seco-mote-harina.html?page=5 (accessed on 30 July 2023).
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; et al. State and Progress of Andean Lupin Cultivation in Europe: A Review. Agronomy 2020, 10, 1038. [Google Scholar] [CrossRef]
- Peralta, E.; Rivera, M.; Murillo, A.; Mazón, N.; Monar, C. INIAP 451 Guaranguito, Nueva variedad de chocho para la provincia de Bolívar. In Boletín Divulgativo No. 382; INIAP, Estación Experimental Santa Catalina: Quito, Ecuador, 2010. [Google Scholar]
- Huaringa, J.; Ubillus, M.; Rojas, V.; Sotelo, M. Rendimiento en grano seco, desamargado y proteína de cinco ecotipos promisorios de tarwi, Lupinus mutabilis Swwet cultivados en Vicos Marcará, Ancash, Peru. In Proceedings of the Libro de Resúmenes del VII Congreso Mundial de La Quinua y Otros Granos Andinos, Iquique, Chile, 25–28 March 2019. [Google Scholar]
- Mujica, A.; Jacobsen, S.; Izquierdo, J. Andean lupin (Lupinus mutabilis Sweet) forty years of research in Peru. In Proceedings of the X International Lupin Conference. Wild and Cultivated Lupins from the Tropics to the Poles, Laugarvatn, Iceland, 19–24 June 2002; p. 106. [Google Scholar]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Intern. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Alcon, M. Obtención de fenotipos similares al tarwi domesticado a partir de sus parientes silvestres. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 107. [Google Scholar]
- Tapia, M. Biodiversidad del Género Lupinus en los Andes. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 138. [Google Scholar]
- Morales, T.; Quintana, F. Lupinus silvestres del Ecuador. In Proceedings of the XV International Lupin Conference: Developing Lupin Crop into a Modern and Sustainable Food and Feed Source, Cochabamba, Bolivia, 18–21 March 2019; p. 131. [Google Scholar]
- Bonifacio, A.; Aroni, G.; Villca, M. Adaptación y perspectivas de aprovechamiento del lupino silvestre en sistemas de producción del altiplano. Rev. Agric. 2018, 57, 10–18. [Google Scholar]
- Pakendorf, K.W.; van Rensburg, P.J. Selection for low alkaloid mutants in Lupinus mutabilis. In Proceedings of the Abstracts of International Symposium on Induced Mutation as a Tool for Crop Plant Improvement, Viena, Austria, 9–13 March 1981. STI/PUB/591. [Google Scholar]
- Stawwinski, S.; Rybinski, W.; Starzycki, M. Mutants of Andean lupin (Lupinus mutabilis Sweet) with improved architecture habit. In Proceedings of the XVIth EUCARPIA Genetic Resources Section Workshop, Poznan, Poland, 16–20 May 2001; pp. 93–95. [Google Scholar]
- Galek, R.; Sawicka-Sienkiewicz, E.; Zalewski, D.; Stawiński, S.; Spychała, K. Searching for low alkaloid forms in the Andean lupin (Lupinus mutabilis) collection. Czech J. Genet. Plant Breed. 2017, 53, 55–62. [Google Scholar] [CrossRef]




| Germplasm Bank | Country | Number of Accessions Conserved | Reference |
|---|---|---|---|
| Experimental Station La Molina, INIA, Lima | Peru | 50 | [30] |
| Experimental Station Andenes, INIA, Cusco | Peru | 20 | [30] |
| Experimental Station Baños del Inca, INIA, Cajamarca | Peru | 347 | [30] |
| Experimental Station Canaan-Huamanga, INIA, Cajamarca | Peru | 11 | [30] |
| Universidad Nacional del Altiplano (UNAP) | Peru | 319 | [30] |
| Centro de Investigación de Cultivos Andinos (CICA), Cusco | Peru | 1800 | [6] |
| Centro de Investigación y Producción, Camacani-UNA, Puno | Peru | 260 | [6] |
| Experimental Station Santa Ana, INIA, Huancayo | Peru | 1725 | [30] |
| Instituto Nacional de Investigaciones Agropecuarias, Mejía | Ecuador | 381 | [28] |
| Universidad Tecnica de Ambato (UTA), Ambato | Ecuador | 50 | [30] |
| Fundación para la Promoción e Investigación de Productos Andinos, La Paz | Bolivia | 20 | [6] |
| Centro de Investigaciones Fitoecogenéticas (CIFP), Pairumani, Cochabamba | Bolivia | 114 | [6,30] |
| Universidad Mayor de San Andrés (UMSA), La Paz | Bolivia | 340 | [6] |
| Instituto Nacional de Innovación Agropecuaria y Forestal (INIAF) | Bolivia | 123 | [29,30] |
| Universidad Nacional de Colombia (UNC), Bogotá. | Colombia | 20 | [6] |
| Instituto Colombiano Agropecuario (ICA), Bogotá | Colombia | 40 | [6] |
| Cenrtro Regional de Investigacion Carillanca, INIA, Temuco | Chile | 1 | [6,30] |
| Universidad Astral de Chile, Valdivia | Chile | 3 | [6,30] |
| Universidad de Temuco | Chile | 180 | [6] |
| Instituto Nacional de Tecnología Agropecuaria (INTA), Buenos Aires | Argentina | 180 | [6] |
| Total | 5984 |
| Primer | Sequence | Reference |
|---|---|---|
| (AG)8YT | AGAGAGAGAGAGAGAGYT | [32,33] |
| (AG)8YC | AGAGAGAGAGAGAGAGYC | [32,33] |
| (AG)8 | AGAGAGAGAGAGAGAG | [32] |
| (AG)8YG | AGAGAGAGAGAGAGAGYG | [33] |
| (GA)8YC | GAGAGAGAGAGAGAGAYC | [32] |
| (GA)8YG | GAGAGAGAGAGAGAGAYG | [32] |
| (GA)8YT | GAGAGAGAGAGAGAGAYT | [33] |
| (GA)8C | GAGAGAGAGAGAGAGAC | [32] |
| (GT)8YC | GTGTGTGTGTGTGTGTYC | [33] |
| DBD(AC)7 | DBDACACACACACACAC | [32] |
| HVH(TG)9 | HVHTGTGTGTGTGTGTGTGTG | [32] |
| HVH(TG)7 | HVHTGTGTGTGTGTGTG | [33] |
| Markers | Traits | Chr | Position | Environment |
|---|---|---|---|---|
| M11034 | Flowering time (days), Pods on the main stem (number) | LG13 | 5,615,305 | All Env, PT, NL-Sc |
| M7399 | Flowering time (days) | LG08 | 7,668,768 | All Env, NL-Wi |
| M7412 | Flowering time (days) | LG08 | 7,670,152 | All Env |
| M7413 | Flowering time (days) | LG08 | 7,680,541 | All Env |
| M7670 | Flowering time (days) | LG08 | 16,132,804 | All Env |
| M396 | Plant height (cm) | LG01 | 15,690,000 | EC |
| M10925 | Vegetative yield (g) | LG12 | 18,150,269 | NL-Sc |
| M14832 | Pods on the main stem (number) | LG18 | 3,860,231 | EC |
| M14675 | Pods on the main stem (number) | LG17 | 20,841,361 | NL-Wi |
| M7272 | Pods on the main stem (number) | LG08 | 4,062,540 | All Env |
| Year | No. of Accessions Evaluated | Study Conditions | Inoculation Method | Tolerant Genotypes | Reference |
|---|---|---|---|---|---|
| 2007 | 126 | Greenhouse | Spraying with an inoculum concentration 106 UFC/mL | Neither | [41] |
| 2013 | 70 | Greenhouse | Spraying 3 months after sowing with an inoculum concentration of 105 UFC/mL | ECU-674, ECU-701, ECU-702, ECU-2332 ECU-713 | [56] |
| 2014 | 7 | Field | Spraying at 2 and 3 months after sowing with an inoculum concentration of 105 UFC/mL | ECU-2658, ECU-2700 | [57] |
| Country of Origin | Name of the Variety | Locality of Selection | Institutions | Characteristics | Reference |
|---|---|---|---|---|---|
| Peru | “Alta gracia” | E. E. Santa Ana | INIA-Huancayo | High yield | [2,21] |
| Peru | “Andenes-80” | E. E. Andenes-Cusco | INIA-CUSCO | High yield | [2,21] |
| Peru | “Carlos Ochoa” | Kayra-Cusco | CICA-UNSAAC | High yield | [2] |
| Peru | “Cajamarca” | Cajamarca | Universidad Nacional de Cajamarca | --- | [21] |
| Peru | “Cholo fuerte” | Áncash | CEDEP | Grain white | [21,27] |
| Peru | “Cusco” | Kayra-Cusco | CICA-Universidad Nacional San Antonio Abad del Cusco (UNSAAC) | White flower | [2] |
| Peru | “Cusco 1” | Cusco | UNSAAC | Medium grain and white | [21,27] |
| Peru | “Cusco 2” | Cusco | UNSAAC | Low content of alkaloids | [21] |
| Peru | “Fortunato” “Herrera” | Kayra-Cusco | CICA-UNSAAC | High yield | [2] |
| Peru | “H6” | E. E. Huancayo | Universidad Nacional | High yield | [2,21] |
| Peru | “Huamachuco” | E. E. Baños del Inca | INIA-CAJAMARCA | Anthracnose tolerant, late | [2] |
| Peru | “Kayra” | E. E. Andenes | INIA-CUSCO | High yield | [2] |
| Peru | “Puno” | E. E. Ilpa | INIA-PUNO | Early, small plant | [2,36] |
| Peru | “Sacacatani | E. E. Camacani | UNA-Puno | High yield, late | [2] |
| Peru | “SCG-1 al SCG-4” | E. E. Camacani | UNA-Puno | Early | [2] |
| Peru | “SCG-25” | Kayra-Cusco | CICA-UNSAAC | High yield, White and black seed | [2,36] |
| Peru | “SCG-9” | Kayra-Cusco | CICA-UNSAAC | High yield, white seed | [2,36] |
| Peru | “Yunguyo” | E. E. Ilpa | INIA-PUNO | High yield | [2,21] |
| Bolivia | “Chumpi tarwi” | Potosí | --- | Local dark brown grain ecotypes | [19] |
| Bolivia | “Tarwi Ñawi” | Potosí | --- | Local grain ecotypes of white color and dark color in the part of the embryo | [19] |
| Bolivia | “Toralapa” | E. E. Payrumani | INIAP-Cochabamba | High yield, late | [2,59] |
| Bolivia | “Carabuco” | E. E. Payrumani | INIAP-Cochabamba | High yield, late | [2,19] |
| Bolivia | “Jayata” | Cochabamba | PROINPA-Cochabamba | Uniform maturation, white seed | [13,38] |
| Bolivia | “Candela” | Cochabamba | PROINPA-Cochabamba | Low content of alkaloids | [13] |
| Ecuador | “INIAP-450 Andino” | E. E. Santa Catalina | INIAP-Ecuador | High yield | [39] |
| Ecuador | “INIAP-451 Guaranguito” | E. E. Santa Catalina | INIAP-Ecuador | Foliar disease tolerant | [60] |
| Chile | “Inti” | E. E. Gorbea | Semillas Baer | Free of alkaloids | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ortega, D.; Zambrano, J.L.; Pereira-Lorenzo, S.; Torres, A.; Murillo, Á. Lupinus mutabilis Breeding in the Andes of Ecuador, Peru, and Bolivia: A Review. Agronomy 2024, 14, 94. https://doi.org/10.3390/agronomy14010094
Rodríguez-Ortega D, Zambrano JL, Pereira-Lorenzo S, Torres A, Murillo Á. Lupinus mutabilis Breeding in the Andes of Ecuador, Peru, and Bolivia: A Review. Agronomy. 2024; 14(1):94. https://doi.org/10.3390/agronomy14010094
Chicago/Turabian StyleRodríguez-Ortega, Diego, José Luis Zambrano, Santiago Pereira-Lorenzo, Andrés Torres, and Ángel Murillo. 2024. "Lupinus mutabilis Breeding in the Andes of Ecuador, Peru, and Bolivia: A Review" Agronomy 14, no. 1: 94. https://doi.org/10.3390/agronomy14010094
APA StyleRodríguez-Ortega, D., Zambrano, J. L., Pereira-Lorenzo, S., Torres, A., & Murillo, Á. (2024). Lupinus mutabilis Breeding in the Andes of Ecuador, Peru, and Bolivia: A Review. Agronomy, 14(1), 94. https://doi.org/10.3390/agronomy14010094









