How Tillage System Affects the Soil Carbon Dioxide Emission and Wheat Plants Physiological State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Soil Conditions
2.2.1. Soil Properties
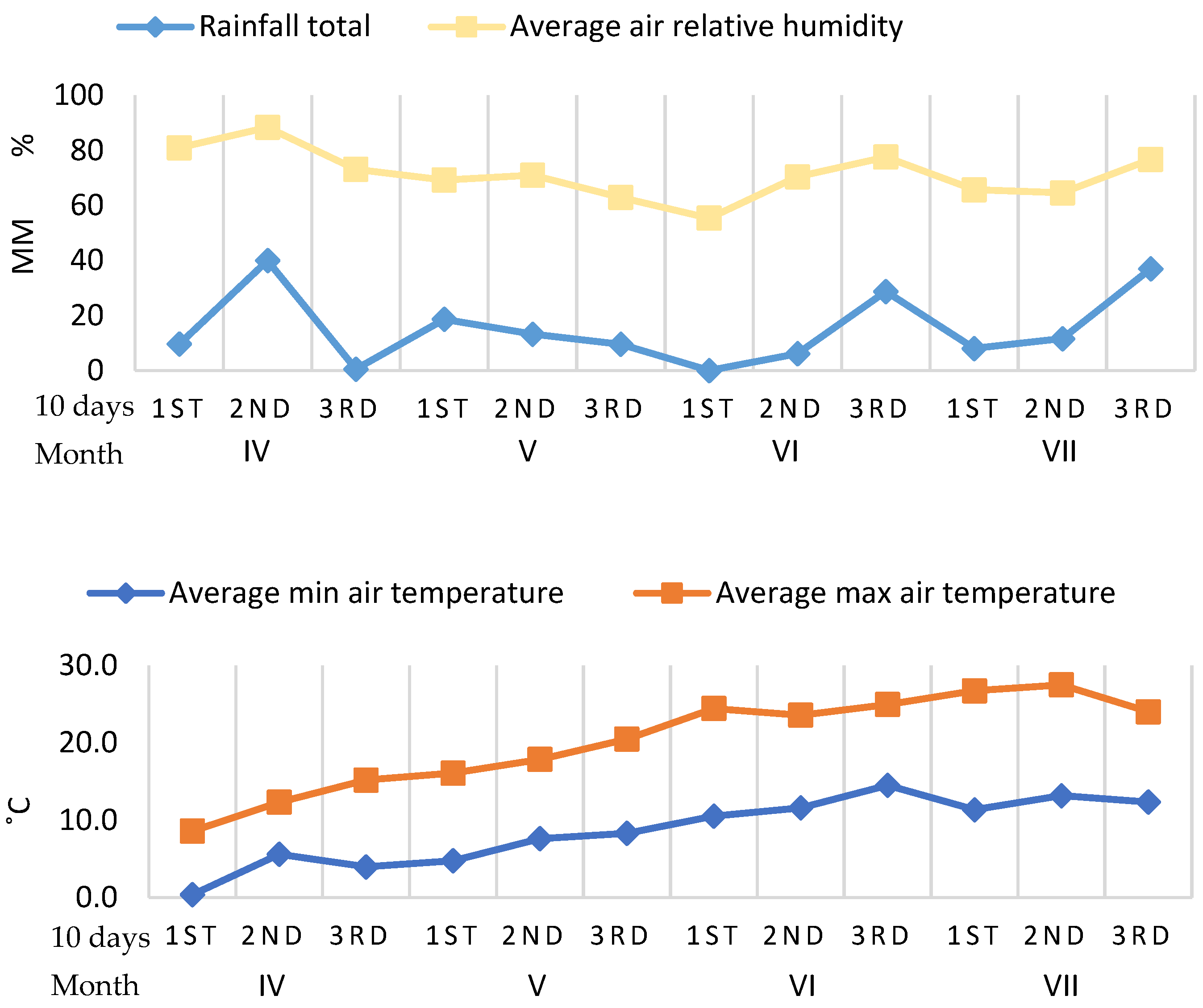
2.2.2. Soil Moisture and Temperature
2.2.3. Soil Respiration
- u = molar air flow in mol s−1
- ∆C—difference in CO2 concentration through soil pot, dilution corrected, µmol mol−1.
- us = molar flow of air per square meter of soil, mol m−2 s−1.
- ∆C—difference in CO2 concentration through soil pot, dilution corrected, µmol mol−1.
- us—molar flow of air per square meter of soil, mol m−2 s−1
- ∆e—differential water vapor concentration, mbar, dilution corrected
- p—atmospheric pressure, mBar
2.3. Plant Conditions
2.3.1. Chlorophyll Fluorescence
2.3.2. Normalized Difference Vegetation Index-NDVI
2.4. Statistical Analysis
3. Results
3.1. Soil
3.1.1. Chemical Properties of the Soil
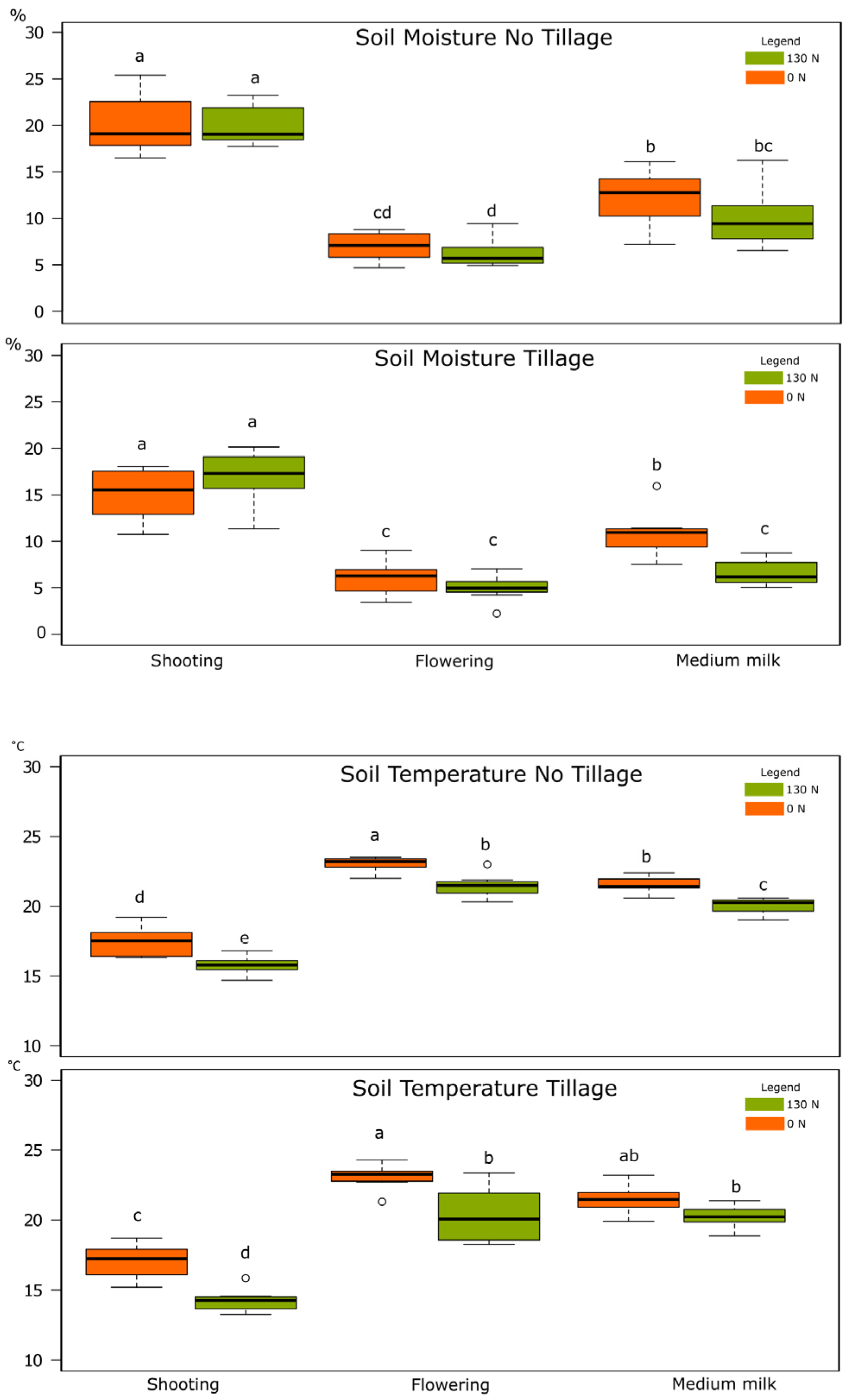
3.1.2. Soil Moisture and Temperature
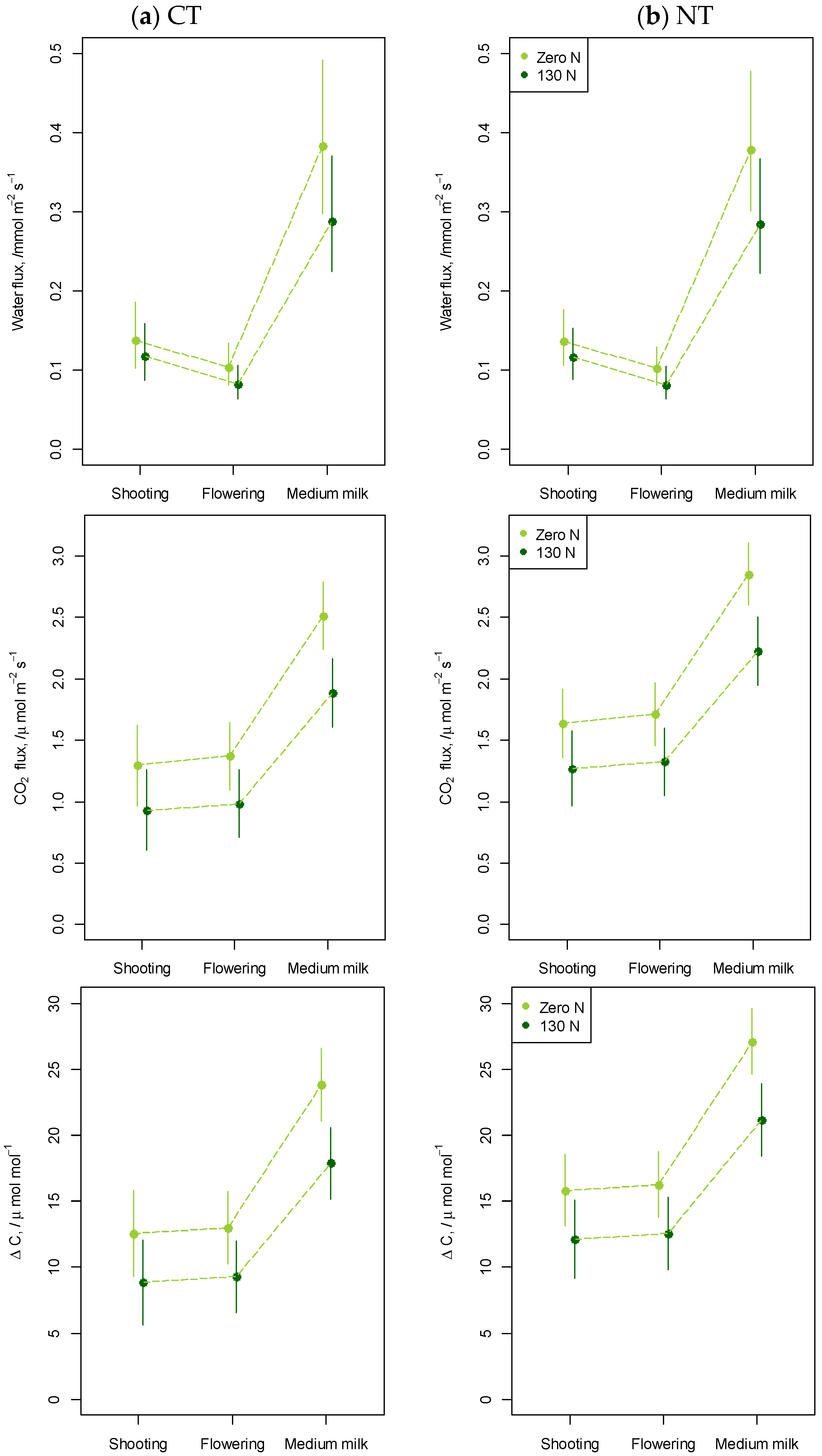
3.1.3. Soil Respiration
3.2. Plants
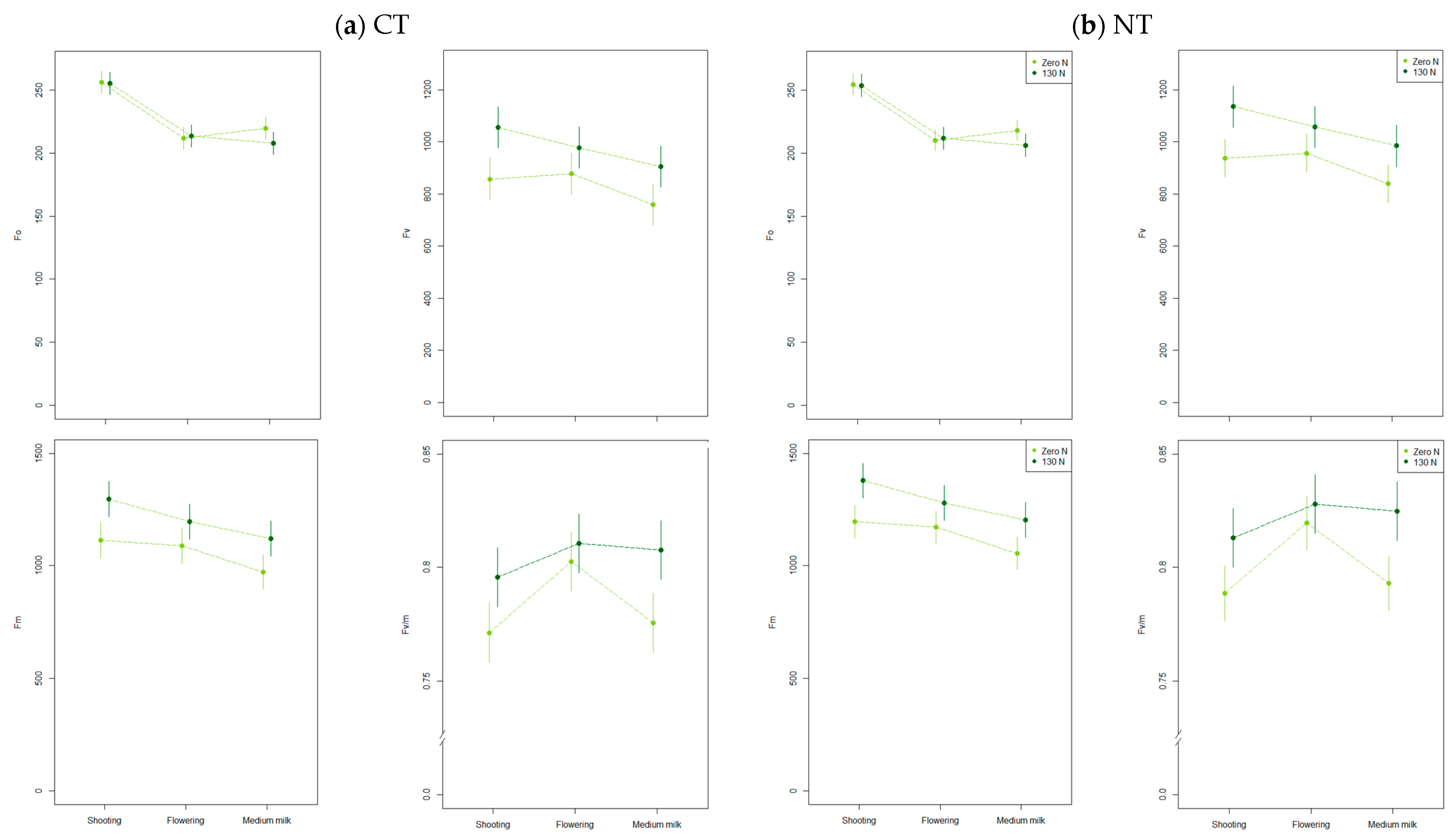
3.2.1. Chlorophyll Fluorescence
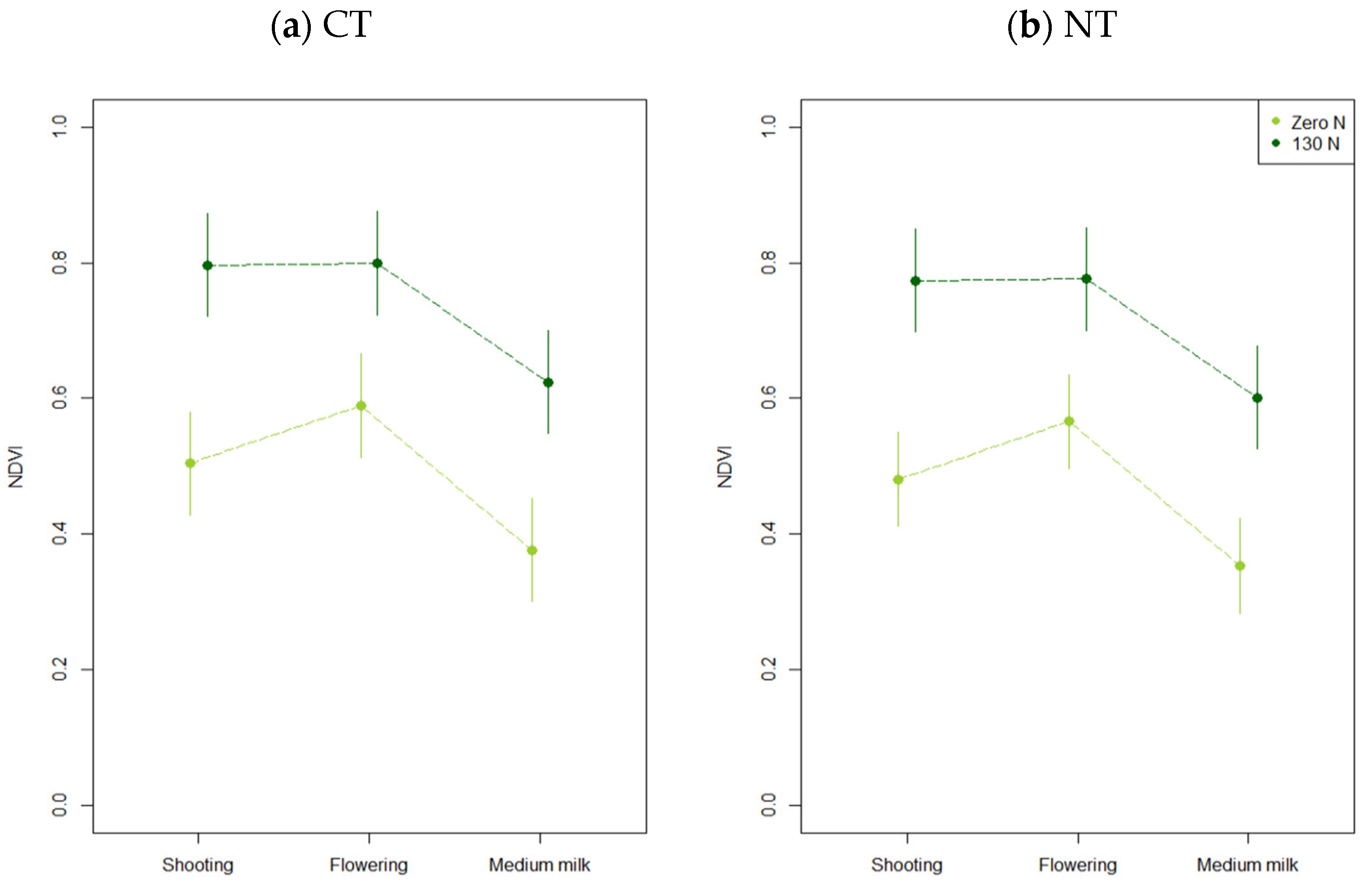
3.2.2. Normalized Difference Vegetation Index-NDVI
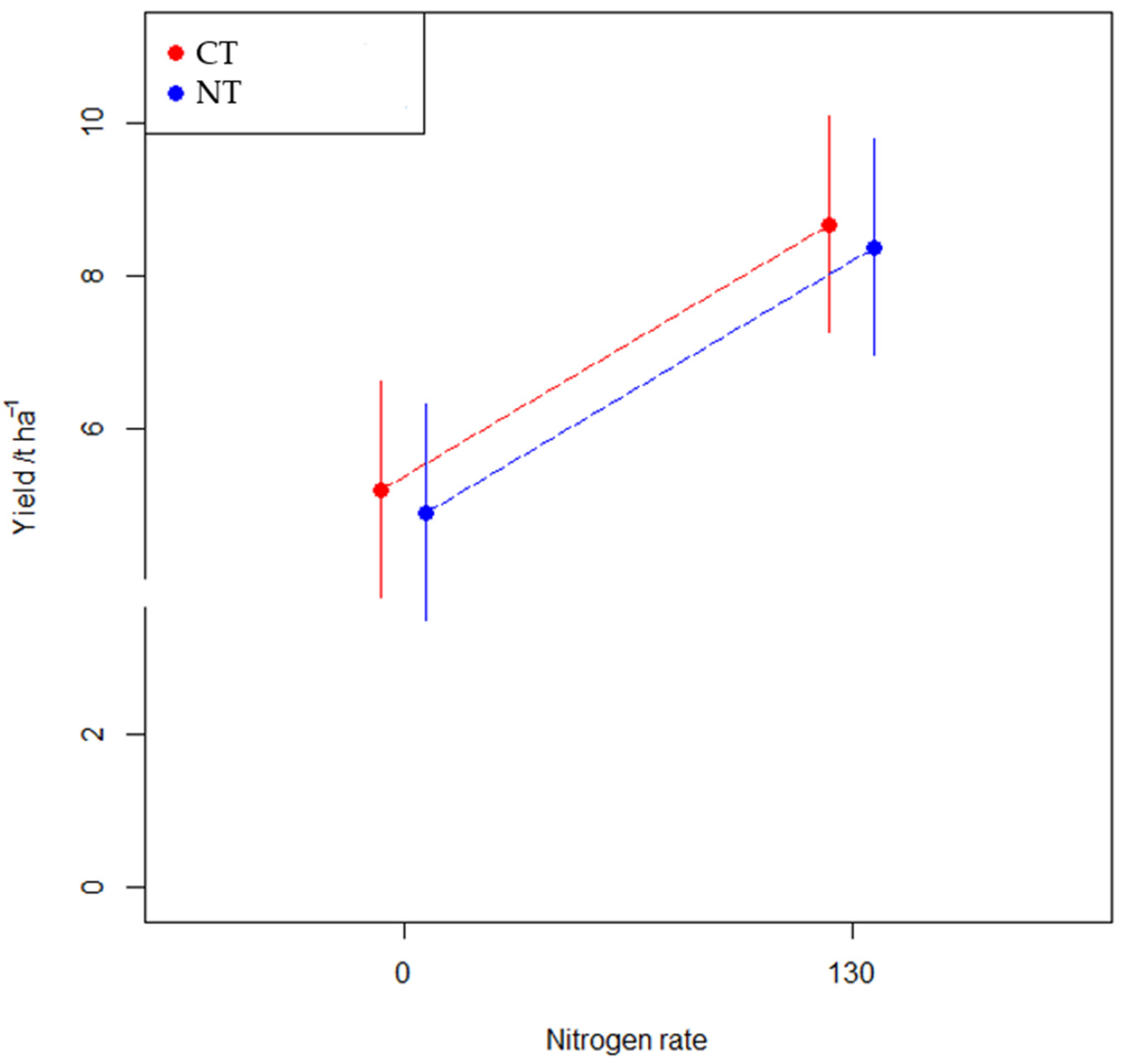
3.2.3. Grain Yield
4. Discussion
4.1. Growth Conditions
4.2. Plants Chlorophyll Fluorescence
4.3. Plants Grain Yield
4.4. Soil Respiration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buczek, J.; Migut, D.; Jańczak-Pieniążek, M. Effect of soil tillage practice on photosynthesis, grain yield and quality of hybrid winter wheat. Agriculture 2021, 11, 479. [Google Scholar] [CrossRef]
- Morris, N.L.; Miller, P.C.H.; Orson, J.H.; Froud-Williams, R.J. The adoption of non-inversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—A review. Soil Tillage Res. 2010, 108, 1–15. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Dotterweich, M. The history of human-induced soil erosion: Geomorphic legacies, early descriptions and research, and the development of soil conservation—A global synopsis. Geomorphology 2013, 201, 1–34. [Google Scholar] [CrossRef]
- WMO State of the Global Climate 2023, 1st ed.; World Meteorological Organization: Geneva, Switzerland, 2024; ISBN 9789263113474.
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Tomczyk, A.M.; Bednorz, E. The extreme year—Analysis of thermal conditions in Poland in 2018. Theor. Appl. Clim. 2020, 139, 251–260. [Google Scholar] [CrossRef]
- Ustrnul, Z.; Marosz, M.; Biernacik, D.; Walus, K.; Chilińska, A.; Wasielewska, K.; Kusek, K. Climate Monitoring Bulletin of Poland Year 2023, 1st ed.; Institute of Meteorology and Water Management—National Research Institute: Warsaw, Poland, 2024. (In Polish) [Google Scholar]
- Okoniewska, M.; Szuminska, D. Changes in potential evaporation in the years 1952–2018 in North-Western Poland in terms of the impact of climatic changes on hydrological and hydrochemical conditions. Water 2020, 12, 877. [Google Scholar] [CrossRef]
- Pinskwar, I.; Chorynski, A.; Kundzewicz, Z.W. Severe drought in the spring of 2020 in Poland—More of the same? Agronomy 2020, 10, 1646. [Google Scholar] [CrossRef]
- Rodrigues, C.I.D.; Brito, L.M.; Nunes, L.J.R. Soil carbon sequestration in the context of climate change mitigation: A Review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon management and climate change. Carbon Manag. 2013, 4, 439–462. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A Review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Zhang, K.; Maltais-Landry, G.; Liao, H.L. How Soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 2021, 156, 108219. [Google Scholar] [CrossRef]
- Johnston, C.A.; Groffman, P.; Breshears, D.D.; Cardon, Z.G.; Currie, W.; Emanuel, W.; Gaudinski, J.; Jackson, R.B.; Lajtha, K.; Nadelhoffer, K.; et al. Carbon cycling in soil. Front. Ecol. Environ. 2004, 2, 522–528. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Agren, G.I.; Sebastià, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef]
- Moraru, P.I.; Teodor, R. Effect of tillage systems on soil moisture, soil temperature, soil respiration and production of wheat, maize and soybean crops. J. Food Agric. Environ. 2012, 10, 445–448. [Google Scholar]
- Liang, G.; Houssou, A.A.; Wu, H.; Cai, D.; Wu, X.; Gao, L.; Li, J.; Wang, B.; Li, S. Seasonal patterns of soil respiration and related soil biochemical properties under nitrogen addition in winter wheat field. PLoS ONE 2015, 10, e0144115. [Google Scholar] [CrossRef]
- Derrouch, D.; Chauvel, B.; Felten, E.; Dessaint, F. Weed management in the transition to conservation agriculture: Farmers’ response. Agronomy 2020, 10, 843. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Molnar, J.J.; Fazio, R.A.; Sydnor, E.; Lowe, M.J. Barriers to adoption of sustainable agriculture practices: Change agent perspectives. Renew. Agric. Food Syst. 2009, 24, 60–71. [Google Scholar] [CrossRef]
- Derpsch, R.; Franzluebbers, A.J.; Duiker, S.W.; Reicosky, D.C.; Koeller, K.; Friedrich, T.; Sturny, W.G.; Sá, J.C.M.; Weiss, K. Why do we need to standardize no-tillage research? Soil Tillage Res. 2014, 137, 16–22. [Google Scholar] [CrossRef]
- Murphy, B.W. Soil Organic Matter and Soil Function—Review of the Literature and Underlying Data; Department of the Environment: Canberra, Australia, 2014; p. 155. [Google Scholar]
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; van Groenigen, K.J.; Lee, J.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. When does no-till yield more? A global Meta-Analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef]
- Małecka, I.; Blecharczyk, A.; Sawinska, Z.; Swędrzyńska, D.; Piechota, T. Winter wheat yield and soil properties response to long-term non-inversion tillage. J. Agric. Sci. Technol. 2015, 17, 1571–1584. [Google Scholar]
- Malecka, I.; Blecharczyk, A.; Sawinska, Z.; Dobrzeniecki, T. The effect of various long-term tillage systems on soil properties and spring barley yield. Turk. J. Agric. For. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Swędrzyńska, D.; Małecka, I.; Blecharczyk, A.; Swędrzyński, A.; Starzyk, J. Effects of various long-term tillage systems on some chemical and biological properties of soil. Pol. J. Environ. Stud. 2013, 22, 1835–1844. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Korbas, M.; Mrówczyński, M. (Eds.) Metodyka Integrowanej Produkcji Pszenicy Ozimej i Jarej; Plant Health and Inspection Service: Warszawa, Poland, 2014; p. 90. Available online: http://www.piorin.gov.pl (accessed on 6 July 2024).
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Schachtschabel, P. Methode Schachtschabel. In Methodenbuch, Band I. Die Untersuchung von Böden, 3. Auflage; Thun, R., Herrmann, R., Knickmann, E., Eds.; Neumann Verlag: Berlin, Germany, 1955. [Google Scholar]
- Tiurin, L.V. Studies in the Genesis and Geography of Soils; Dokuchaiev Soil Institute: Moscow, Russia, 1935; p. 139. [Google Scholar]
- LCpro-SD Portable. Photosynthesis System. Instruction Manual; Software Issue 1.01 Onwards; Issue 4; Copyright ADC BioScientific Ltd.: Broxbourne, UK, 2012. [Google Scholar]
- Chapter 2.·The OS5p+ Hardware. In Preliminary 2021 Edition OS5p+ Operators Guide; Optisciences Inc.: Hudson, NH, USA, 2021.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 6 July 2024).
- Pinheiro, J.; Bates, D.R.; Core Team. Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-163. 2024. Available online: https://CRAN.R-project.org/package=nlme (accessed on 6 July 2024).
- Lenth, R.V. Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.8.5. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 6 July 2024).
- Janusauskaite, D.; Kadziene, G. Influence of different intensities of tillage on physiological characteristics and productivity of crop-rotation plants. Plants 2022, 11, 3107. [Google Scholar] [CrossRef]
- Li, Y.; Hou, R.; Tao, F. Wheat morphophysiological traits and radiation use efficiency under interactive effects of warming and tillage management. Plant Cell Environ. 2021, 44, 2386–2401. [Google Scholar] [CrossRef]
- Hou, X.; Li, R.; Jia, Z.; Han, Q. Rotational tillage improves photosynthesis of winter wheat during reproductive growth stages in a semiarid region. Agron. J. 2013, 105, 215–221. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Tillage and crop effects on seasonal dynamics of soil CO2 evolution, water content, temperature, and bulk density. Appl. Soil Ecol. 1995, 2, 95–109. [Google Scholar] [CrossRef]
- Alvarez, R. A review of nitrogen fertilizer and conservation tillage effects on soil organic carbon storage. Soil Use Manag. 2005, 21, 38–52. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Mineral surfaces and soil organic matter. Eur. J. Soil Sci. 2003, 54, 219–236. [Google Scholar] [CrossRef]
- Brennan, J.; Hackett, R.; McCabe, T.; Grant, J.; Fortune, R.A.; Forristal, P.D. The effect of tillage system and residue management on grain yield and nitrogen use efficiency in winter wheat in a cool Atlantic climate. Eur. J. Agron. 2014, 54, 61–69. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Grzesiak, S.; Grzesiak, M.T.; Hura, T.; Marcińska, I.; Rzepka, A. Changes in root system structure, leaf water potential and gas exchange of maize and triticale seedlings affected by soil compaction. Environ. Exp. Bot. 2013, 88, 2–10. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Janowiak, F.; Szczyrek, P.; Kaczanowska, K.; Ostrowska, A.; Rut, G.; Hura, T.; Rzepka, A.; Grzesiak, S. Impact of soil compaction stress combined with drought or waterlogging on physiological and biochemical markers in two maize hybrids. Acta Physiol. Plant. 2016, 38, 109. [Google Scholar] [CrossRef]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identifcation of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Feng, W.; He, L.; Zhang, H.; Guo, B.; Zhu, Y.; Wang, C.; Guo, T. Assessment of plant nitrogen status using chlorophyll fluorescence parameters of the upper leaves in winter wheat. Eur. J. Agron. 2015, 64, 78–87. [Google Scholar] [CrossRef]
- Baker, N.R. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possi-bilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Li, Y.; Hou, R.; Tao, F. Interactive effects of different warming levels and tillage managements on winter wheat growth, physiological processes, grain yield and quality in the North China Plain. Agric. Ecosyst. Environ. 2020, 295, 106923. [Google Scholar] [CrossRef]
- Stępień-Warda, A. Effect of soil cultivation system on the efficiency of the photosynthetic apparatus in maize leaves (Zea mays L.). Pol. J. Agron. 2020, 43, 57–62. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Ma, Y.; Li, Q. Response of photosynthetic active radiation interception, dry matter accumulation, and grain yield to tillage in two winter wheat genotypes. Arch. Agron. Soil Sci. 2019, 66, 1103–1114. [Google Scholar] [CrossRef]
- Janusauskaite, D. Leaf senescence of winter wheat and spring wheat as influenced by tillage and fertilization management. Acta Physiol. Plant. 2022, 44, 74. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Alsajri, F.A.; Irby, T.; Krutz, J.; Golden, B. Quantifying soil moisture deficit effects on soybean yield and yield component distribution patterns. Irrig. Sci. 2018, 36, 241–255. [Google Scholar] [CrossRef]
- Nehe, A.S.; Misra, S.; Murchie, E.H.; Chinnathambi, K.; Sing Tyagi, B.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and protein concentration in Indian wheat cultivars. Field Crop. Res. 2020, 251, 107778. [Google Scholar] [CrossRef]
- Noulas, C.; Herrera, J.M.; Tziouvalekas, M.; Qin, R. Agronomic assessment of nitrogen use efficiency in spring wheat and interrelations with leaf greenness under field conditions. Comm. Soil Sci. Plant Anal. 2018, 49, 763–781. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Early anthesis and delayed but fast leaf senescence contribute to individual grain dry matter and water accumulation in wheat. Field Crop. Res. 2016, 187, 24–34. [Google Scholar] [CrossRef]
- Lin, Y.C.; Hu, Y.G.; Ren, C.Z.; Guo, L.C.; Wang, C.L.; Jiang, Y.; Wang, X.J.; Phendukani, H.; Zeng, Z.H. Effects of nitrogen application on chlorophyll fluorescence parameters and leaf gas exchange in naked oat. J. Integr. Agric. 2013, 12, 2164–2171. [Google Scholar] [CrossRef]
- Živčak, M.; Olšovska, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestič, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef]
- Seddaiu, G.; Iocola, I.; Farina, R.; Orsini, R.; Iezzi, G.; Roggero, P.P. Long term effects of tillage practices and N fertilization in rainfed Mediterranean cropping systems: Durum wheat, sunflower and maize grain yield. Eur. J. Agron. 2016, 77, 166–178. [Google Scholar] [CrossRef]
- Kan, Z.R.; Qi, J.Y.; Liu, Q.Y.; He, C.; Virk, A.L.; Lal, R.; Zhang, H.L. Effects of conservation tillage on wheat growth duration and grain yield in the North China Plain. Arch. Agron. Soil Sci. 2020, 68, 1019–1033. [Google Scholar] [CrossRef]
- Viljevac Vuletic, M.; Marcek, T.; Španic, V. Photosynthetic and antioxidative strategies of flag leaf maturation and its impact to grain yield of two field-grown wheat varieties. Theor. Exp. Plant Physiol. 2019, 31, 387–399. [Google Scholar] [CrossRef]
- Macák, M.; Candráková, E.; Ðalovi’c, I.; Prasad, P.V.V.; Farooq, M.; Korczyk-Szabó, J.; Kováčik, P.; Šimanský, V. The influence of different fertilization strategies on the grain yield of field peas (Pisum sativum L.) under conventional and conservation tillage. Agronomy 2020, 10, 1728. [Google Scholar] [CrossRef]
- Woźniak, A.; Gos, M. Yield and chemical quality of spring wheat and soil properties as affected by tillage system. Plant Soil Environ. 2014, 60, 141–145. [Google Scholar] [CrossRef]
- Hofmeijer, M.A.J.; Krauss, M.; Berner, A.; Peigne, J.; Mäder, P.; Armengot, L. Effects of reduced tillage on weed pressure, nitrogen availability and winter wheat yields under organic management. Agronomy 2019, 9, 180. [Google Scholar] [CrossRef]
- Cociu, A.I.; Alionte, E. Effect of different tillage systems on grain yield and its quality of winter wheat, maize and soybean under different weather conditions. Rom. Agric. Res. 2017, 34, 59–67. [Google Scholar]
- Woźniak, A.; Rachoń, L. Effect of tillage systems on the yield and quality of winter wheat grain and soil properties. Agriculture 2020, 10, 405. [Google Scholar] [CrossRef]
- Fernández, F.G.; Sorensenb, B.A.; Villamil, M.B. A comparison of soil properties after five years of no-till and strip-till. Agron. J. 2015, 107, 1339–1346. [Google Scholar] [CrossRef]
- Malhi, S.S.; Grant, C.A.; Johnston, A.M.; Gill, K.S. Nitrogen fertilization management for no-till cereal production in Canadian Great Plains: A review. Soil Tillage Res. 2001, 60, 101–122. [Google Scholar] [CrossRef]
- Oorts, K.; Merckx, R.; Gréhan, E.; Labreuche, J.; Nicolardot, B. Determinants of annual fluxes of CO2 and N2O in long-term no-tillage and conventional tillage systems in northern France. Soil Tillage Res. 2007, 95, 133–148. [Google Scholar] [CrossRef]
- Cooper, H.V.; Sjögersten, S.; Lark, R.M.; Girkin, N.T.; Vane, C.H.; Calonego, J.C.; Rosolem, C.; Mooney, S.J. Long-term zero-tillage enhances the protection of soil carbon in tropical agriculture. Eur. J. Soil Sci. 2021, 72, 2477–2492. [Google Scholar] [CrossRef]
- Zhang, X.; Gregory, A.S.; Whalley, W.R.; Coleman, K.; Neal, A.L.; Bacq-Labreuil, A.; Mooney, S.J.; Crawford, J.W.; Soga, K.; Illangasekare, T.H. Relationship between soil carbon sequestration and the ability of soil aggregates to transport dissolved oxygen. Geoderma 2021, 403, 115370. [Google Scholar] [CrossRef]
- Pringle, M.J.; Lark, R.M. Spatial analysis of model error, illustrated by soil carbon dioxide emissions. Vadose Zone J. 2006, 5, 168–183. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Johnson, B.G. Interacting effects of temperature, soil moisture and plant biomass production on ecosystem respiration in a northern temperate grassland. Agric. For. Meteorol. 2005, 130, 237–253. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Yin, X. Tillage and crop residue effects on soil carbon and carbon dioxide emission in corn–soybean rotations. J. Environ. Qual. 2005, 34, 437–445. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Janssens, I.A.; Carrara, A.; Meiresonne, L.; Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol. 2003, 23, 1263–1270. [Google Scholar] [CrossRef]
- Lee, M.; Nakane, K.; Nakatsubo, T.; Mo, W.; Koizumi, H. Effects of rainfall events on soil CO2 flux in a cool temperate deciduous broad-leaved forest. Ecol. Res. 2002, 17, 401–409. [Google Scholar] [CrossRef]
- Anokye, J.; Abunyewa, A.A.; Jørgensen, U.; Kaba, J.S.; Twum-Ampofo, K.; Dawoe, E.; Barnes, V.R.; Plauborg, F.; Pedersen, S.M.; Berg, T.R.; et al. Mitigation of greenhouse gas emissions through shade systems and climate-smart soil fertility interventions in cocoa landscapes in the semi-deciduous ecological zone of Ghana. Soil Adv. 2024, 1, 100001. [Google Scholar] [CrossRef]
- Sainju, U.M.; Jabro, J.D.; Stekens, W.B. Soil carbon dioxide emission and carbon content as affected by irrigation, tillage, cropping system, and nitrogen fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Feiziene, D.; Feiza, V.; Kadziene, G.; Vaideliene, A.; Povilaitis, V.; Deveikyte, I. CO2 fluxes and drivers as affected by soil type, tillage and fertilization. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 311–328. [Google Scholar] [CrossRef]
- Wilson, H.M.; Al-Kaisi, M.M. Crop rotation and nitrogen fertilization effect on soil CO2 emissions in central Iowa. Appl Soil Ecol. 2008, 39, 264–270. [Google Scholar] [CrossRef]
- Curtin, D.; Wang, H.; Selles, F.; McConkey, B.G.; Campbell, C.A. Tillage effects on carbon fluxes in continuous wheat and fallow–wheat rotations. Soil Sci. Soc. Am. J. 2000, 64, 2080–2086. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, X.; Chen, X.; McLaughlin, N.B.; Zhang, S.; Wei, S.; Sun, B.; Liang, A. Long-term conservation tillage influences the soil microbial community and its contribution to soil CO2 emissions in a Mollisol in Northeast China. J. Soils Sediments 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Sosulski, T.; Skowrońska, M.; Szczepaniak, J. Impact of reduced tillage on CO2 emission from soil under maize cultivation. Soil Tillage Res. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Omonode, R.A.; Vyn, T.J.; Smith, D.R.; Hegymegi, P.; Gál, A. Soil carbon dioxide and methane fluxes from long-term tillage systems in continuous corn and corn–soybean rotations. Soil Tillage Res. 2007, 95, 182–195. [Google Scholar] [CrossRef]
- Fortin, M.C.; Rochette, P.; Pattey, E. Soil carbon dioxide fluxes from conventional and no-tillage small-grain cropping systems. Soil Sci. Soc. Am. J. 1996, 60, 1541–1547. [Google Scholar] [CrossRef]
- Elder, J.W.; Lal, R. Tillage effects on gaseous emissions from an intensively farmed organic soil in North Central Ohio. Soil Tillage Res. 2008, 98, 45–55. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Farooq, T.H.; Sahar, N.E.; Shahzad, S.M.; Altaf, M.M.; Ashraf, M. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total Environ. 2021, 750, 142299. [Google Scholar] [CrossRef] [PubMed]
- Gelybó, G.; Barcza, Z.; Dencső, M.; Potyó, I.; Kása, I.; Horel, Á.; Pokovai, K.; Birkás, M.; Kern, A.; Hollós, R.; et al. Effect of tillage and crop type on soil respiration in a long-term field experiment on chernozem soil under temperate climate. Soil Tillage Res. 2022, 216, 105239. [Google Scholar] [CrossRef]
- Dong, W.; Liu, E.; Wang, J.; Yan, C.; Li, J.; Zhang, Y. Impact of tillage management on the short- and long-term soil carbon dioxide emissions in the dryland of Loess Plateau in China. Geoderma 2017, 307, 38–45. [Google Scholar] [CrossRef]
- Regina, K.; Alakukku, L. Greenhouse gas fluxes in varying soils types under conventional and no-tillage practices. Soil Tillage Res. 2010, 109, 144–152. [Google Scholar] [CrossRef]
| Plots | Factor I | Factor II | ||
|---|---|---|---|---|
| Tillage System | Fertilization (kg·ha−1) | Time of Measurements | ||
| BBCH | Growth Phase | Date | ||
| CT—Conventional Tillage | 130 N | 32 | shooting | 10.05.2023 |
| CT—Conventional Tillage | 0 N | 65 | flowering | 01.06.2023 |
| NT—No Tillage | 0 N | 75 | medium milk | 06.07.2023 |
| NT—No Tillage | 130 N | |||
| Tillage System | Fertilization | P2O5 (mg/100 g) | K2O (mg/100 g) | Mg (mg/100 g) | pH in 1M KCl | SOC% |
|---|---|---|---|---|---|---|
| CT | 0 N | >35 | 10.7 | 6.0 | 6.6 | 0.90 |
| CT | 130 N | >35 | 11.2 | 5.6 | 6.6 | 0.86 |
| NT | 0 N | >35 | 15.1 | 8.3 | 6.8 | 0.99 |
| NT | 130 N | >35 | 15.9 | 7.8 | 6.6 | 1.03 |
| Parameter | p Values | |
|---|---|---|
| Average Soil Moisture (%) | Soil Temperature (°C) | |
| W flux (mmol m−2 s−1) | 0.7700 | 0.3170 |
| CO2 flux (µmol m−2 s−1) | <0.0001 | 0.3560 |
| ∆C (µmol mol−1) | <0.0001 | 0.4520 |
| F0 | 0.0047 | 0.2788 |
| Fm | 0.9177 | 0.0231 |
| Fv | 0.6599 | 0.0320 |
| Fv/m | 0.0679 | 0.0595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawinska, Z.; Radzikowska-Kujawska, D.; Blecharczyk, A.; Świtek, S.; Piechota, T.; Cieślak, A.; Cardenas, L.M.; Louro-Lopez, A.; Gregory, A.S.; Coleman, K.; et al. How Tillage System Affects the Soil Carbon Dioxide Emission and Wheat Plants Physiological State. Agronomy 2024, 14, 2220. https://doi.org/10.3390/agronomy14102220
Sawinska Z, Radzikowska-Kujawska D, Blecharczyk A, Świtek S, Piechota T, Cieślak A, Cardenas LM, Louro-Lopez A, Gregory AS, Coleman K, et al. How Tillage System Affects the Soil Carbon Dioxide Emission and Wheat Plants Physiological State. Agronomy. 2024; 14(10):2220. https://doi.org/10.3390/agronomy14102220
Chicago/Turabian StyleSawinska, Zuzanna, Dominika Radzikowska-Kujawska, Andrzej Blecharczyk, Stanisław Świtek, Tomasz Piechota, Adam Cieślak, Laura M. Cardenas, Aranzazu Louro-Lopez, Andrew S. Gregory, Kevin Coleman, and et al. 2024. "How Tillage System Affects the Soil Carbon Dioxide Emission and Wheat Plants Physiological State" Agronomy 14, no. 10: 2220. https://doi.org/10.3390/agronomy14102220







