Abstract
Straw returning enhances soil fertility and increases corn yield, but the impact on soil fertility varies with different incorporation methods. To explore the optimal straw-returning method, this study, based on a long-term field experiment, investigated the following different corn-straw-returning methods: deep plowing and straw returning (B), rotary tillage and straw returning (RT), crushing and mixing straw returning (TM), pulverized cover straw returning (C), high-stubble-retention straw returning (LHS), strip cover (S), and flat no-tillage without straw returning (CK). High-throughput sequencing technology was employed to analyze the soil bacterial community composition and structural changes under different straw-returning methods. The study further explored the relationships between the soil bacterial community and nutrient content. The results indicated that different straw-returning methods altered the composition and structure of the soil bacterial community. The TM treatment significantly increased the richness and diversity of the soil bacterial communities. Shredding and covering (C and TM) effectively improved the soil nutrient content and bacterial community structure. In the C treatment, the abundance of Blastococcus, Nocardioides, and Microvirga increased the most, by 241.02%, 77.79%, and 355.08%, respectively, compared with CK. In the TM treatment, Pseudarthrobacter showed the highest abundance, increasing by 343.30%. The genes involved in soil carbon hydrolysis (pulA), nitrification (hao), organic nitrogen degradation and synthesis (gudB), and the nitrogen limitation response (glnR) significantly decreased by 56.21%, 78.75%, 66.46%, and 67.40%, respectively, in the C treatment. The genes involved in soil carbon hydrolysis (IMA), carbon fixation (pccB-A), methane metabolism (moxF), nitrate reduction in soil (nirD), organic nitrogen degradation and synthesis (gdh, ureAB, ureE), and phosphate absorption (glpT) significantly increased by 93.37%, 92.68%, 95.00%, 23.42%, 35.40%, 114.21%, 59.14%, and 75.86%, respectively, in the C treatment. The nitrate reduction gene (nrfA) significantly increased by 80.27% in the TM treatment. Therefore, we concluded that straw primarily stimulates the activity of bacterial communities and regulates the bacterial community by changing the relative abundance of the soil microorganisms and functional genes, thereby improving the soil nutrient content. This study considered pulverized cover straw returning and crushing and mixing straw returning to be the most reasonable methods.
1. Introduction
China ranks first in the world in straw production, and straw contains abundant nutrients, lignin, and cellulose. In addition, straw plays an irreplaceable role in improving soil structures and regulating soil water, fertilizer, air, heat, and microbial activity. Direct straw returning remains one of the main methods of straw returning. The primary direct-incorporation methods include deep tillage, plowing and burying, shredding and mixing, crushing and covering, leaving high stubble, and strip covering [1,2]. Studies have shown that rotary mixing and straw mulching can increase corn yields and improve soil fertility [3,4]. Burying straw is more favorable when increasing organic matter, total nitrogen, and total potassium, while straw mulching is more beneficial for increasing alkaline hydrolysis nitrogen, phosphorus, and potassium [5]. Research also suggests that the uniform mixing of straw can increase soil organic-matter contents more than straw mulching under the same application amount of straw. Combined with autumn plowing and rotary tillage, soil fertility can be enhanced [6]. No-tillage straw mulching is more advantageous than no-tillage and straw shredding under drought conditions at improving wheat yields [7]. Previous study indicates that straw mulching significantly enhances corn’s carbon and nitrogen nutrient sources and improves the physical conditions of soil [8]. However, there is no consensus on the effects of straw mulching and uniform mixing in current research; this requires further investigation. Therefore, this experimental study is essential.
Straw returning enhances soil fertility, impacting soil microbial communities in particular. Research has shown that straw returning increases soil microbial diversity, and microbial activity promotes the decomposition of organic matter and the turnover of soil nutrients [9,10]. Straw mulching can stimulate the ability of soil microbes to degrade organic materials and promote soil nutrient turnover [11]. Studies have also confirmed that straw mulching is more conducive to improving the soil environment and increasing microbial activity [11,12]. However, some research has suggested that short-term straw shredding and mulching only alter the community structure of soil bacteria [13,14]. No-tillage straw returning and plowing can significantly increase soil microbial diversity [15]. Deep-tillage straw returning enhances soil microbial community functional diversity by influencing soil nutrients, optimizing the microbial community structure, promoting organic matter and nutrient cycling, and improving soil fertility [16,17]. Soil microbes are essential for soil ecosystems, and participate in many crucial physiological and biochemical reactions. Bacteria, the most abundant and dominant part of microorganisms, play a vital role in soil ecosystem functions and nutrient cycling [18,19,20,21]. Therefore, exploring the changes in soil microbial community compositions and structures under different straw-returning methods can help with the selection of the best straw-returning method from a microbial perspective.
Although there has been extensive research on the impact of straw returning on soil microbial community structures and soil nutrients, a comprehensive understanding of the effects of different straw-returning methods is lacking. Further investigations are needed to explore the influence of various straw-returning methods on soil microbial diversity and soil nutrients. Considering this, our study established a long-term field experiment with different straw-returning treatments, utilizing Illumina sequencing technology combined with bioinformatics analyses to examine the impact of maize-straw returning on the bacterial diversity and soil chemical properties of black calcareous soil. The objectives of this study were as follows: (1) to elucidate the effects of different maize-straw-returning methods on the chemical properties of chernozems and bacterial community structures; (2) to clarify the relationship between different maize-straw-returning methods, soil chemical properties, and bacterial diversity in chernozems; and (3) to identify the optimal straw-returning method, providing a theoretical basis for straw-returning practices.
2. Materials and Methods
2.1. Experimental Site Overview
The experiment was conducted at the research experimental base of the Qiqihar Branch of the Heilongjiang Academy of Agricultural Sciences, located in the western part of the Songnen Plain in Heilongjiang Province. The site is in a semi-arid region with a flat terrain at an average elevation of 143 m. It falls under a temperate continental monsoon climate, with an annual average precipitation of 350 mm. The mean annual temperature is 3.2 °C, with an average temperature from April to September of 17.6 °C. The extreme minimum temperature is 4.9 °C and the extreme maximum temperature is 27.1 °C, accumulating 2900 °C as the active temperature. The frost period begins on September 28th, and the frost-free period lasts for 150 days. The soil type is classified as carbonate chernozem.
2.2. Experimental Design
This long-term field experiment commenced in 2016 and included the following seven treatments (Table S1): deep plowing and straw returning (B), rotary tillage and straw returning (RT), crushing and mixing straw returning (TM), pulverized cover straw returning (C), high-stubble-retention straw returning (LHS), strip cover (S), and flat no-tillage without straw returning (CK). Details of the treatments are provided in Table S1. Maize was grown as a single crop, with the entire amount of straw returned to the field at a rate of 10,700 kg/ha. The maize variety used was Nendan 19 (Qiqihar Branch of the Heilongjiang Academy of Agricultural Sciences, Qiqihar, China), and compound fertilizer (N: P2O5: K2O: 26-11-11) was applied at a rate of 400 kg/ha. Mechanical precision-seeding was carried out on 7 May 2018, with row spacing set at 65 cm, plant spacing at 19.5 cm, and a planting density of 65,000 plants per hectare.
2.3. Measurement Items and Methods
Soil samples for this experiment were collected at the maturity stage of the maize in 2018. Five sampling points were selected in an “S” shape in the long-term field-experiment area, and samples from these five points were mixed to form one composite sample, resulting in twenty one samples. After air-drying, the soil samples were sieved to remove residual branches, roots, weeds, and gravel before being used to determine the soil chemical properties. Another portion of the soil samples was stored in sterile centrifuge tubes at −80 °C for the microbial analysis. The pH (soil-to-water ratio of 1:2.5) was determined using the potentiometric method, the alkaline hydrolysis nitrogen (AN) content was determined using the culture diffusion titration method, the soil organic matter (SOM) content was determined using the potassium dichromate heating method, the soil available phosphorus (AP) content was determined using the sodium bicarbonate leaching–molybdenum antimony colorimetric method, the soil available potassium (AK) content was determined using the ammonium acetate–flame photometry method, and the soil organic phosphorus (OP) was determined using the ignition–0.2 N H2SO4 leaching method. The specific methods used to determine the soil chemical properties are referenced in Conventional Analytical Methods for Soil Agricultural Chemistry [22].
2.4. Extraction of Rhizospheric Soil DNA and PCR Amplification
The DNA of all soil samples was extracted using a soil genomic DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China). PCR amplification of the bacterial V3–V4 variable region was performed using specific primers (338F 5′-ACTCCTACGGGAGCAG-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′). All primers were synthesized by Beijing Ovison Gene Technology Co., Ltd. (Beijing, China). The PCR amplification conditions were as follows: the 25 μL PCR reaction contained 12.5 μL 2 × Taq Plus Master Mix, 1 μL forward primer (5 μM), 1 μL reverse primer (5 μM), 3 μL BSA (2 ng/μL), an X (30 ng) DNA sample, and 7.5 - X μL dd H2O. The PCR products were analyzed using 1% agarose gel (Beijing Solaibao Technology Co., Ltd., Beijing, China).
All samples were processed under formal experimental conditions, with three replicates for each sample. The PCR products from the same sample were mixed and detected using 2% agarose gel electrophoresis. The PCR products were recovered using an AxyPrepDNA Gel Recovery Kit (Corning Life Sciences (Wujiang) Co., Ltd., Jiangsu, China), and Tris-HCl elution was performed. A 2% agarose gel was used for the electrophoresis detection. A MiSeq library was constructed and sequenced. The sequencing library was constructed according to the manufacturer’s instructions, and sequencing was performed using the Illumina MiSeq PE250 platform by Beijing Ovison Gene Technology Co., Ltd. (Beijing, China).
After removing the barcode and primer and splicing, high-quality sequences were obtained by further removing the chimera and short sequences. Then, a bioinformatics analysis was performed. The high-quality sequences were classified into operational taxonomic units (OTUs) using the clustering method of the vsearch 2.7.1 software uparse, and the OTUs at a 97% similarity level were clustered [23]. Unoise 3 was used to denoise the samples [24]. The OTU representative sequences were compared and analyzed using the RDP Classifier algorithm, based on the Silva128 database [25,26]. The KEGG (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg/, accessed on 21 December 2022) database and Picrust 2 were used to predict the functions [27].
2.5. Statistical Data Analysis
The experimental data were subjected to a statistical analysis using IBM SPSS Statistics 24 (IBM, Armonk, NY, USA). An analysis of variance (ANOVA) was employed to assess the differences in the mean values, followed by the least significant difference (LSD) test, which was conducted at a 5% probability level. The relative abundance of the soil microorganisms was calculated using the Chao 1 index. The diversity accounting for both the relative abundance and evenness was evaluated using the Shannon index and Simpson index, and was assessed for significance using the t-test method. For other datasets, the LSD method was applied for significance testing. The selection of carbon, nitrogen, and phosphorus functional genes was guided by the research of Tang [28]. Clustering diagrams, box plots, petal plots, heat maps, and correlation analyses were performed using Genescloud Tools (https://www.genescloud.cn, accessed on 1 September 2023).
3. Results
3.1. Impact of Different Straw-Returning Methods on Soil Nutrients
The basic chemical properties of the soil under different straw-returning methods are presented in Table 1. The soil’s chemical properties underwent varying degrees of change after different straw-returning treatments. The SOM content significantly increased in all straw-returning methods compared with the CK treatment, with the highest increase observed in the C treatment at 56.76%. The soil pH values ranged from 5.63 to 6.47, and all straw-returning methods significantly increased the pH compared with CK. The AN content showed a significant increase in all straw-returning methods compared with CK, with the highest increase in the C treatment at 58.80%. The AK content was significantly higher in all straw-returning methods than in the CK treatment, with the C treatment showing the most substantial increase at 137.78%. The AP content exhibited a significant decrease in all straw-returning methods, with the highest decrease in the B treatment at 51.38%. However, OP showed a significant increase, with the RT treatment showing the highest increase.

Table 1.
The chemical characteristics of soil under various returning techniques.
3.2. Impact of Different Straw-Returning Methods on Soil Bacterial Community Composition
Under different straw-returning methods, the composition of the soil bacteria at the phylum level remained consistent, with no specific bacterial phyla detected. In total, 11 bacterial phyla with relative abundances more significant than 1% and 19 bacterial genera with relative abundances more significant than 1% (including other genera and unidentified genera) were detected in the soil samples of the seven treatments. As shown in Figure 1, after various straw-returning treatments, Actinobacteria, Proteobacteria, and Acidobacteria exhibited higher relative abundances than the other phyla, making them the dominant phyla, accounting for 27.42–34.71%, 20.05–29.07%, and 10.82–19.71%, respectively. The relative abundance of Actinobacteria increased in all straw-returning treatments compared with CK, particularly in the C treatment, and showed a significant increase of 14.49%. Proteobacteria increased in all straw-returning treatments, except B and S treatments, with a substantial increase of 23.72% in the C treatment. Acidobacteria increased in all treatments, except RT and C treatments, with a significant increase of 22.04% in the B treatment.
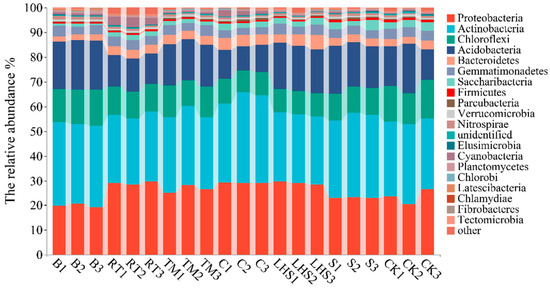
Figure 1.
Impact of various returning techniques on the horizontal-level species composition of soil.
As shown in Figure 2, at the genus level, the unidentified genera exhibited the highest relative abundance, ranging from 51.88% to 63.26%. Following closely was the relative abundance of the others. Furthermore, the top three dominant genera in terms of relative abundance were Sphingomonas, RB41, and Blastococcus, with relative abundances ranging from 1.70% to 4.69%, 1.88% to 3.71%, and 0.97% to 3.31%, respectively. As depicted in the graph, after the straw returning, the relative abundance of Sphingomonas significantly decreased compared with CK, particularly for treatment B, exhibiting a reduction of 63.84%. Conversely, the relative abundance of RB41 increased in all treatments, with treatment B showing a significant rise of 97.68% compared with CK. Similarly, Blastococcus demonstrated an overall increase in relative abundance, with treatment C showing a substantial rise of 241.02% compared with CK. Pseudarthrobacter exhibited a relative abundance in all treatments, with treatments TM and C increasing by 343.30% and 327.03%, respectively. Nocardioides also showed a consistent increase, particularly in treatment C, which increased by 77.79%. On the other hand, the relative abundance of Rhodanobacter decreased in all treatments, with treatment B showing the most significant reduction of 91.51%. Haliangium showed a decreasing trend in relative abundance in all treatments except for RT, with treatment B exhibiting a substantial decrease of 41.05%. Gemmatimonas displayed a significant decline in relative abundance compared with CK, with treatments B and C showing reductions of 71.15% and 62.61%, respectively. Gaiella demonstrated increased relative abundance in all treatments except for treatment C, and treatment B exhibited a significant increase of 67.44%. All treatments of Streptomyces showed a decrease, with treatment B decreasing by 42.01% and treatment C decreasing by 40.78%. Microvirga exhibited an overall increase in relative abundance in all treatments, with treatment C showing a significant increase of 355.08%.
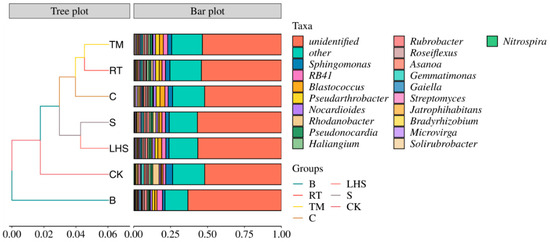
Figure 2.
Clustering bar chart of soil bacterial community samples at a genus level under different returning methods.
3.3. Impact of Different Straw-Returning Methods on Soil Bacterial Diversity in Corn Fields
Figure 3 shows that after straw returning, the Chao1 index of the soil bacteria increased compared with CK. The Chao1 richness index in the TM treatment was 4519.18, showing the highest increase of 9.89% relative to CK. The observed species index in the TM treatment significantly increased by 13.96% compared with CK, indicating a higher microbial community richness in the TM treatment. The PD whole-tree index in the TM treatment showed a significant increase of 12.86% compared with CK. In comparison, the Shannon indices for the TM and LHS treatments significantly increased by 3.71% and 3.50%, respectively, relative to CK. This suggested that the microbial community in the TM treatment exhibited a higher diversity, while the Simpson index showed no significant changes.
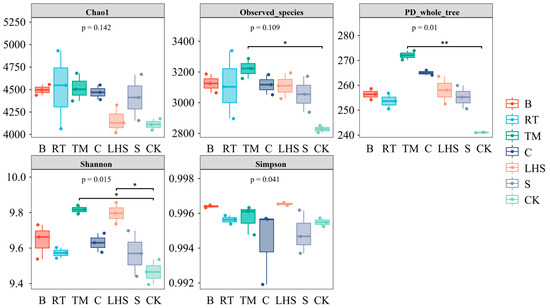
Figure 3.
Alpha analysis of soil bacteria using various returning techniques. * indicates the least significant difference at p < 0.05; ** indicates the least significant difference at p < 0.01.
3.4. Analysis of Beta Diversity of Soil Bacterial Communities under Different Straw-Returning Methods
An integration of the species relative abundance was obtained via a clustering analysis of the operational taxonomic units (OTUs) at the genus level for the bacterial community samples (Figure 2). At a similarity level of 0.05, all samples could be grouped into two major clusters. The B treatment group formed a separate cluster, while the other cluster was divided into two independent subclusters. CK formed a separate cluster in this subcluster, and the other straw-returning treatments formed another cluster. This indicated that straw returning significantly altered the structure of the soil bacterial communities.
To further analyze the impact of soil microorganisms on soil nutrients, we conducted a correlation analysis of the bacterial genera with a relative abundance greater than 1% and the chemical properties. Detailed information is presented in Figure 4. The results indicated that the pH was significantly negatively correlated with Nitrospira (p = 0.048), Gemmatimonas (p = 0.012), and Rhodanobacter (p = 0.025), but significantly positively correlated with Pseudarthrobacter (p = 0.001) and RB41 (p = 0.047). SOM was significantly negatively correlated with Gemmatimonas (p = 0.013), but significantly positively correlated with Microvirga (p = 0.004), Nocardioides (p = 0.008), Pseudarthrobacter (p = 0.001), and Blastococcus (p = 0.007). AN was significantly negatively correlated with Gemmatimonas (p = 0.033), but significantly positively correlated with Microvirga (p = 0.007) and Blastococcus (p = 0.004). AK was significantly negatively correlated with Gemmatimonas (p = 0.049), but significantly positively correlated with Microvirga (p = 0.006) and Blastococcus (p = 0.013). AP was significantly positively correlated with Nitrospira (p = 0.046) and Sphingomonas (p = 0.032), but significantly negatively correlated with Gaiella (p = 0.048). OP was significantly negatively correlated with Nitrospira (p = 0.007), but significantly positively correlated with Pseudarthrobacter (p = 0.001).
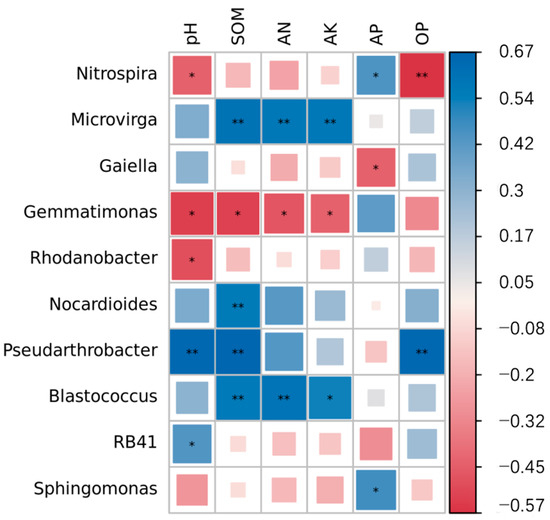
Figure 4.
Heat map of correlation between dominant bacterial genera and environmental factors in soil under different treatments. Blue represents a positive correlation, red represents a negative correlation, and the color shade indicates the strength of the correlation. * p < 0.05; ** p < 0.01.
3.5. Impact of Different Tillage Practices on Microbial Carbon, Nitrogen, and Phosphorus-Cycling Functional Genes
To further elucidate the functional aspects of soil microorganisms, we compared the microbial genes using the KEGG database. The results revealed that the primary function of the microorganisms constituted 81.86–82.40% of the total metabolic pathways, as detailed in Figure S1. Additionally, we performed a variance analysis on the genes involved in soil carbon, nitrogen, and phosphorus cycling under different tillage practices. The findings indicated that compared with the control (CK), 19 significantly different genes were associated with carbon cycling, 14 with nitrogen cycling (including the carbon–nitrogen-cycling genes pmoA-amoA and pmoB-amoB), and 6 with phosphorus cycling. The specific genes that exhibited significant differences are illustrated in Figure 5. After returning crop residue to the field, the relative abundances of the genes related to agricultural-waste decomposition and nutrient cycling significantly decreased. These included the α-amylase gene (amy-F), branching starch hydrolase gene (pulA), β-xylosidase genes (xylA and xynB), α-xylosidase gene (xylS), endo-1,4-β-glucanase gene (xynC), endo-polygalacturonase gene (celA), chitinase gene (chiA), polygalacturonase gene (pgl), methane/monoamine oxidase subunit A gene (pmoA-amoA), methane/monoamine oxidase subunit B gene (pmoB-amoB), hydroxylamine dehydrogenase gene (hao), specific glutamate dehydrogenase gene (gudB), MerR family transcriptional regulator, glutamine synthetase repressor protein gene (glnR), hydroxylamine reductase gene (hcp-A), glycerol kinase gene (glpK), alkaline phosphatase gene (phoA), 4-phytase/acid phosphatase gene (appA), and pyrroloquinoline quinone synthase gene (pqqC). Notably, in treatment B, the relative abundances of the amy-F, xylA, xylS, xynB, xynC, celA, chiA, pgl, pmoA-amoA, pmoB-amoB, glpK, phoA, appA, and pqqC genes decreased by 27.04%, 40.15%, 52.83%, 60.69%, 40.52%, 18.69%, 45.73%, 73.13%, 75.16%, 75.16%, 13.21%, 44.50%, 50.60%, and 27.92%, respectively. For the pulA, hao, gudB, and glnR genes in treatment C, there were significant reductions of 56.21%, 78.75%, 66.46%, and 67.40%, respectively, while the hcp-A gene in the LHS treatment showed a significant decrease of 61.71%. In contrast, the relative abundances of the genes related to low-1,6-glucosidase (IMA), 4-α-glucosyltransferase (malQ), acetyl-CoA carboxylase beta chain gene (pccB-B), acetyl-CoA/propionyl-CoA carboxylase carboxyl transferase subunit gene (pccB-A), fumarate reductase iron-sulfur subunit gene (frdB), fumarate reductase subunit C gene (frdC), fumarate reductase subunit D gene (frdD), methanol dehydrogenase gene (moxF), nitrate reductase (NADH) small subunit gene (nirD), nitrate reductase gene (nrfA), cysteine desulfurase gene (nifS), NADP-specific glutamate dehydrogenase gene (gdh), glutamine synthetase NADPH large-chain gene (gltD), urease gamma/beta subunit gene (ureAB), urease accessory protein gene (ureE), nitrogen regulatory protein gene (ntrY), quinoprotein glucose dehydrogenase gene (gcd), and glycerol-3-phosphate transporter gene (glpT) significantly increased. In treatment C, the IMA, pccB-A, moxF, nirD, gdh, ureAB, ureE, and glpT genes significantly increased by 93.37%, 92.68%, 95.00%, 23.42%, 35.40%, 114.21%, 59.14%, and 75.86%, respectively. For the malQ, frdB, frdC, frdD, nifS, gltD, ntrY, and gcd genes in treatment B, there were significant increases of 23.32%, 123.98%, 124.41%, 153.72%, 6.10%, 3.61%, 28.74%, and 63.37%, respectively. Additionally, the pccB-B gene in the LHS treatment showed a significant increase of 13.22%, and the nrfA gene in the TM treatment significantly increased by 80.27%.
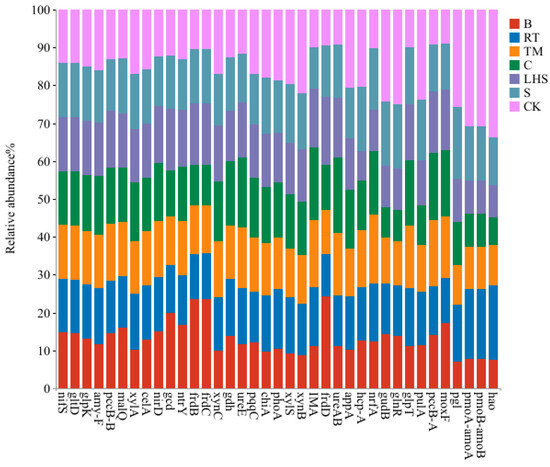
Figure 5.
Relative abundance of distinct gene compositions related to carbon, nitrogen, and phosphorus cycles in soils treated differently.
3.6. Correlation Analysis of the Carbon, Nitrogen, and Phosphorus-Cycling Functional Genes and the Chemical Properties
The correlation analysis of the functional genes involved in carbon, nitrogen, and phosphorus cycling and the chemical properties is detailed in Figure 6. The soil pH was significantly positively correlated with the pccB-A (p = 0.006), pccB-B (p = 0.026), moxF (p = 0.017), and nirD (p = 0.022) genes. It was significantly negatively correlated with the celA (p = 0.010), pgl (p = 0.009), pmoA-amoA (p = 0.007), pmoB-amoB (p = 0.007), hao (p = 0.010), xylS (p = 0.030), xynB (p = 0.012), chiA (p = 0.011), gudB (p = 0.015), and glnR (p = 0.019) genes. SOM was significantly positively correlated with the IMA (p = 0.004), pccB-B (p = 0.002), pccB-A (p = 0.003), nirD (p = 0.003), ureE (p = 0.002), moxF (p = 0.012), gdh (p = 0.042), ureAB (p = 0.011), and glpT (p = 0.020) genes. It was significantly negatively correlated with the celA (p = 0.012), gudB (p = 0.000), glnR (p = 0.000), pulA (p = 0.039), pmoA-amoA (p = 0.040), pmoB-amoB (p = 0.040), and hao (p = 0.024) genes. AN was significantly positively correlated with the IMA (p = 0.017), gdh (p = 0.016), ureAB (p = 0.012), and ureE (p = 0.012) genes, but was significantly negatively correlated with the gudB (p = 0.005), glnR (p = 0.009), and hao (p = 0.050) genes. AK was significantly positively correlated with the ureAB gene (p = 0.019), but significantly negatively correlated with the pmoA-amoA (p = 0.050), pmoB-amoB (p = 0.050), hao (p = 0.031), gudB (p = 0.041), and glnR (p = 0.044) genes. AP was significantly positively correlated with the pqqC (p = 0.000), xylS (p = 0.044), xynB (p = 0.044), xynC (p = 0.044), celA (p = 0.044), and appA (p = 0.044) genes. It was significantly negatively correlated with the frdB (p = 0.040) and frdC (p = 0.044) genes. OP was positively correlated with the IMA (p = 0.046) gene.
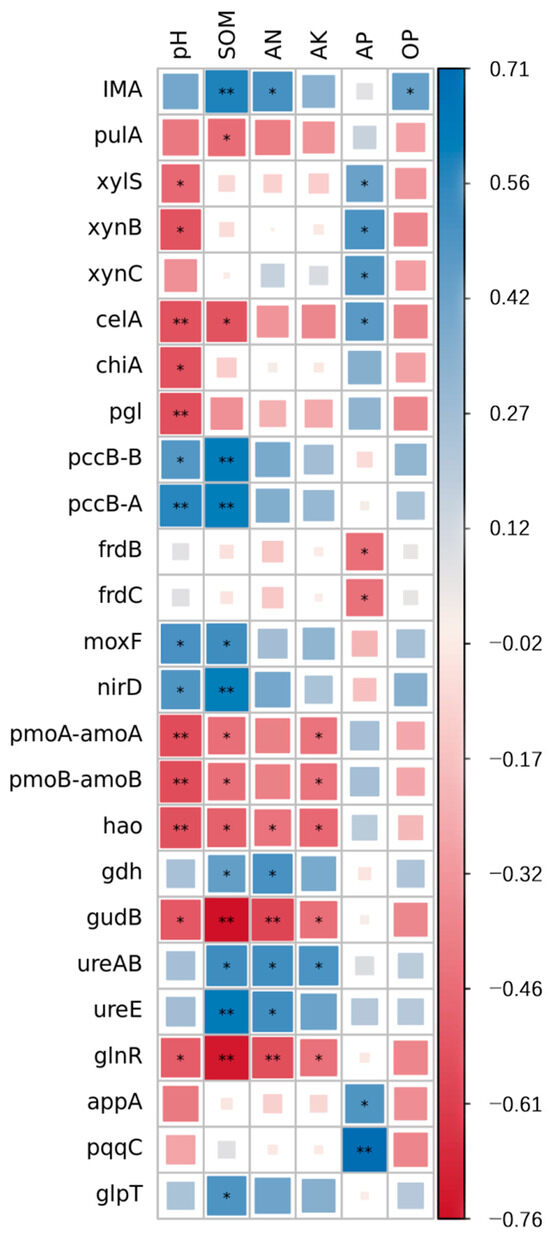
Figure 6.
Correlation heat map of distinct genes and chemical characteristics of soils after several treatments. Blue represents a positive correlation, red represents a negative correlation, and the color shade indicates the strength of the correlation. * p < 0.05; ** p < 0.01.
4. Discussion
The direct incorporation of crop residue into the soil significantly alters the chemical properties of the soil, inducing the release of soil nutrients by the decomposition of the straw [29,30]. Similar conclusions were drawn in this study, where straw returning enhanced the content of SOM, AN, and AK. Notably, there was a decrease in AP, possibly due to most of the phosphorus in the straw existing in an organic form, requiring an extended period for mineralization [31,32]. In the C treatment, the nutrient content of SOM, AN, and AK was significantly higher than in the other treatments. This could be attributed to the finer straw particles from the crushing of the corn straw, facilitating the soil microorganisms’ decomposition of nutrients in the straw. Additionally, the reduced soil disturbance and enhanced nutrient retention on the soil surface, as observed in the no-tillage with straw-cover treatment, aligned with the findings of Dalal et al. [33]. Therefore, an analysis of soil nutrients and straw mulching with crushing was deemed a rational approach.
Studies indicate that straw returning enriches the soil bacterial communities’ abundance and diversity indices [21,34]. In our research, the highest increase in the richness and diversity indices of soil bacterial communities, particularly in the TM treatment, could be attributed to the more uniform incorporation of the straw, providing a stable and moist environment for bacterial growth while reducing the soil bulk density and compactness [35]. Consistent with previous studies, the relative abundance of Actinobacteria and Proteobacteria, especially in the TM and C treatments, was higher [36,37,38]. The correlation analysis used in our research suggested that different straw-returning methods altered the structure of the soil bacterial community, influencing the relative abundance of Nitrospira, Microvirga, Gemmatimonas, Nocardioides, Pseudarthrobacter, and Blastococcus. Among these, beneficial microorganisms such as Blastococcus, Nocardioides, and Microvirga were the most abundant in the C treatment. At the same time, Pseudarthrobacter had the highest relative abundance in the TM treatment, followed by the C treatment. This enrichment of beneficial microorganisms enhanced the degradation of the straw, consistent with previous studies [13,39]. Hence, from the perspective of the microbial community composition, the TM and C treatments were considered to be the optimal straw-returning methods.
Soil microorganisms are susceptible to environmental changes, and the incorporation of straw into fields after different treatments has been found to alter the habitat for soil bacteria [40]. Research indicates that residue cover can directly influence the abundance and diversity of the microorganisms associated with soil organic carbon degradation, subsequently affecting soil carbon–nitrogen cycling genes [11,12,41].
Based on bacterial functional predictions, relative abundances, and the correlation analysis, this study determined that 25 functional genes associated with carbon, nitrogen, and phosphorus cycling exhibited significant or highly significant correlations with soil chemical properties. Following the incorporation of straw into the soil, the pulA gene responsible for soil carbon hydrolysis, the hao gene involved in soil nitrogen nitrification, the gudB gene associated with the degradation and synthesis of soil organic nitrogen, and the glnR gene participating in the soil nitrogen limitation response were significantly reduced by 56.21%, 78.75%, 66.46%, and 67.40%, respectively, under the C treatment. In contrast, the IMA gene involved in soil carbon hydrolysis, the pccB-A gene associated with soil carbon fixation, the moxF gene related to methane metabolism, the nirD gene participating in soil nitrogen nitrate reduction, and the gdh, ureAB, and ureE genes associated with the degradation and synthesis of soil organic nitrogen as well as the glpT gene involved in phosphorus absorption exhibited significant increases of 93.37%, 92.68%, 95.00%, 23.42%, 35.40%, 114.21%, 59.14%, and 75.86%.
Moreover, the nrfA gene involved in nitrate reduction showed a significant increase of 80.27% under the TM treatment. This could be attributed to an alteration to the original habitat for the soil bacteria after incorporating the straw, providing carbon and nutrient sources to the soil bacteria and creating a nutrient-rich environment that stimulated bacterial proliferation. Consequently, this adjustment to the bacterial community structure and composition promoted the enrichment of bacteria containing the aforementioned functional genes in the straw-incorporated soil, leading to the degradation of the straw [42,43,44]. Simultaneously, the straw itself, when incorporated, carried a significant amount of acquired microorganisms, further altering the microbial genomic composition [32]. The C treatment, involving crushed straw-cover incorporation, resulted in finer straw particles, which were more conducive for the soil bacteria in the plow layer to decompose, providing ample nutrients and leading to the highest increase in the relative abundance of the aforementioned functional genes compared with the other treatments.
The changes in the functional genes, in turn, facilitated the accumulation and transformation of the soil nutrients, improving the soil nutrient content. The soil pH significantly influenced functional genes such as celA, pgl, pmoA-amoA, pmoB-amoB, hao, and pccB-A. At the same time, SOM significantly affected celA, gudB, glnR, IMA, pccB-B, pccB-A, nirD, and ureE, and AN affected gudB and glnR, among the other functional genes. Consistent with previous studies, AP was significantly correlated with the pqqC functional gene [45]. Functional genes such as pmoA-amoA, pmoB-amoB, hao, and ureE are associated with organic-matter degradation. Straw returning mainly stimulates the regulation of bacterial communities by altering the relative abundance of these genes in the soil, promoting the degradation of straw to release nutrients, thus improving the soil nutrient status [46]. From the perspective of functional genes, both the C and TM treatments should be considered optimal for straw returning.
5. Conclusions
Based on a long-term field experiment using Illumina high-throughput sequencing technology, this study investigated the effects of different corn-straw-returning methods on the chemical properties of chernozem and bacterial diversity. The results are summarized as follows:
- (1)
- Various straw-returning methods significantly increased the soil SOM, AN, OP, and AK contents while decreasing the AP content. The nutrient content was considerably higher in the C treatment than in the other treatments.
- (2)
- The different methods of straw-residue incorporation into fields resulted in changes to the structure and composition of soil bacterial communities. Some beneficial microorganisms exhibited the highest abundance in both the TM and C treatments. Additionally, alterations were observed in the abundance and diversity of bacterial communities, with the TM treatment showing a significant increase in both richness and diversity compared with CK. At the phylum level, the C treatment significantly increased the relative abundance of Actinobacteria. In contrast, at the genus level, the C treatment significantly increased the relative abundance of Blastococcus, Nocardioides, and Microvirga, but decreased the relative abundance of Gemmatimonas, Gaiella, and Streptomyces. The TM treatment significantly increased the relative abundance of Pseudarthrobacter, promoting soil nutrient cycling and ecological health.
- (3)
- A functional prediction of the bacterial genes involved in carbon, nitrogen, and phosphorus cycling indicated that 25 genes showed significant or highly significant correlations with the soil chemical properties. After straw returning, the pulA, hao, gudB, and glnR genes in the C treatment significantly decreased by 56.21%, 78.75%, 66.46%, and 67.40%, respectively. In contrast, the IMA, pccB-A, moxF, nirD, gdh, ureAB, ureE, and glpT genes significantly increased by 93.37%, 92.68%, 95.00%, 23.42%, 35.40%, 114.21%, 59.14%, and 75.86%, respectively. The nrfA gene in the TM treatment significantly increased by 80.27%. Straw returning mainly stimulated the regulation of bacterial community activity via changes in the relative abundance of these genes, promoting straw degradation and nutrient release, thus improving the soil nutrient content. In summary, the best returning methods were straw mulching and crushing from the perspective of the soil nutrients, microbial community composition, and soil carbon, nitrogen, and phosphorus-cycling functional genes.
The return of straw to farmland positively affects the species balance and ecological functions of the essential nutrients and bacterial communities of soil. This study provides a theoretical basis and scientific reference for the promotion of the soil ecosystem function as well as improving soil health, reducing fertilizer use, and increasing efficiency from the perspective of the basic chemical properties, bacterial diversity, and soil carbon, nitrogen, and phosphorus-cycling functional genes of soil under different straw-returning methods. However, further research is needed on corn plants’ nutrient content and yields under different straw-returning methods.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14102223/s1: Figure S1: Predicted potential functions of bacterial communities in soil under various methods of crop-residue incorporation according to the KEGG database; Table S1: Different specific ways of straw returning to the field.
Author Contributions
Conceptualization, W.H. and S.L.; methodology, C.W.; software, D.W.; validation, D.W. and Y.L.; formal analysis, Q.L.; investigation, Q.L.; resources, S.L.; data curation, C.W.; writing—original draft preparation, W.H.; writing—review and editing, W.H.; visualization, D.W. and Y.L.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2023YFD2300403) and the Key Special Project on Technological Innovation for the Protection and Utilization of Black Soil (2023YFD150110510).
Data Availability Statement
The data used in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Duan, Y.; Wang, G.; Wang, A.; Zhang, D. Straw alters the soil organic carbon composition and microbial community under different tillage practices in a meadow soil in Northeast China. Soil Till. Res. 2021, 208, 104879. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotox. Environ. Safe. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jiang, C.; Li, S.; Yu, W. Maize yield and nitrogen-use characteristics were promoted as consistently improved soil fertility: 6-year straw incorporation in Northeast China. Plant Soil Environ. 2021, 67, 383–389. [Google Scholar] [CrossRef]
- Zhang, M.; Song, D.; Pu, X.; Dang, P.; Qin, X.; Siddique, K.H.M. Effect of different straw returning measures on resource use efficiency and spring maize yield under a plastic film mulch system. Eur. J. Agron. 2022, 134, 126461. [Google Scholar] [CrossRef]
- Huang, T.; Yang, N.; Lu, C.; Qin, X.; Siddique, K.H.M. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Till. Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Zhao, X.; He, L.; Zhang, Z.; Wang, H.; Zhao, L. Simulation of accumulation and mineralization (CO2 release) of organic carbon in chernozem under different straw return ways after corn harvesting. Soil. Till. Res. 2016, 156, 148–154. [Google Scholar] [CrossRef]
- Wen, Y.; Fan, Z.; Hu, F.; Fan, H.; He, W.; Sun, Y.; Wang, F.; Zhao, C.; Yu, A.; Chai, Q. No-tillage with straw mulching boosts wheat grain yield by improving the eco-physiological characteristics in arid regions. J. Integr. Agric. 2023, 22, 3416–3429. [Google Scholar]
- Lou, Y.; Liang, W.; Xu, M.; He, X.; Wang, Y.; Zhao, K. Straw coverage alleviates seasonal variability of the topsoil microbial biomass and activity. Catena 2011, 86, 117–120. [Google Scholar] [CrossRef]
- Wu, G.; Ling, J.; Zhao, D.; Liu, Z.; Xu, Y.; Kuzyakov, Y.; Marsden, K.; Wen, Y.; Zhou, S. Straw return counteracts the negative effects of warming on microbial community and soil multifunctionality. Agric. Ecosyst. Environ. 2023, 352, 108508. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long-term no-tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Till. Res. 2020, 196, 104452. [Google Scholar] [CrossRef]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2006, 38, 247–255. [Google Scholar] [CrossRef]
- Siczek, A.; Frac, M. Soil microbial activity as influenced by compaction and straw mulching. Int. Agrophys. 2012, 26, 65–69. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Till. Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.; Han, B.; Ouyang, Z.; Duan, X.; Zheng, H.; Miao, H. Soil carbon sequestrations by nitrogen fertilizer application, straw return and no-tillage in China’s cropland. Glob. Chang. Biol. 2009, 15, 281–305. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Vinay, N.; Wang, D.; Mo, F.; Liao, Y.; Wen, X. Microbial functional genes within soil aggregates drive organic carbon mineralization under contrasting tillage practices. Land Degrad. Dev. 2023, 34, 3618–3635. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, X.; Guan, S.; Dou, S. Deep incorporation of corn straw benefits soil organic carbon and microbial community composition in a black soil of Northeast China. Soil Use Manag. 2022, 38, 1266–1279. [Google Scholar] [CrossRef]
- Choudhary, M.; Jat, H.S.; Mukhopadhyay, R.; Poonia, T.; Phogat, A.; Dixit, B.; Kumar, R.; Arora, S.; Yadav, R.K. Functional diversity and behavioral changes of microbial communities under salt affected soils. Appl. Soil Ecol. 2023, 190, 105017. [Google Scholar] [CrossRef]
- Gong, S.; Wang, S.; Bai, X.; Luo, G.; Wu, L.; Chen, F.; Qian, Q.; Xiao, J.; Zeng, C. Response of the weathering carbon sink in terrestrial rocks to climate variables and ecological restoration in China. Sci. Total Environ. 2020, 750, 141525. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.F.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Palme, J.B.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Luan, L.; Zheng, J.; Cheng, M.; Hu, K.; Kong, P.; Jiang, Y.; Sun, B. Effects of different types of straw returning on bacterial diversity and community structure in dryland red soil. Soil 2021, 53, 991–997. [Google Scholar]
- Soil Society of China. Conventional Analytical Methods for Soil Agricultural Chemistry; Soil Society of China: Nanjing, China, 1982. [Google Scholar]
- Edgar, C.R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Im-proved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Tang, Z. Impact of Green Manure and Straw Return on Microbial Functional Genes in Rice Rhizosphere; Chinese Academy of Agricultural Sciences: Beijing, China, 2020. [Google Scholar]
- Cheng, M.; Xie, W.; Yang, Z.; Zhou, H. Effects of long-term straw return on corn yield, soil nutrient contents and enzyme activities in dryland of the Loess Plateau, China. Chin. J. Eco-Agric. 2019, 27, 1528–1536. [Google Scholar]
- Lv, G.; Zhang, Y.; Yu, Y.; Wang, X.; Peixoto, L.; Pnag, H. Annual burying of straw after pelletizing: A novel and feasible way to improve soil fertility and productivity in Northeast China. Soil Till. Res. 2023, 230, 105699. [Google Scholar]
- Singh, Y.; Gupta, R.K.; Singh, J.; Singh, G.; Ladha, J.K. Placement effects on rice residue decomposition and nutrient dynamics on two soil types during wheat cropping in rice-wheat system in northwestern India. Nutr. Cycl. Agroecosys. 2010, 88, 471–480. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E.; Wang, W.; Reeves, S.; Gibson, I. Organic carbon and total nitrogen stocks in a Vertisol following 40 years of no-tillage, crop residue retention and nitrogen fertilisation. Soil Till. Res. 2011, 112, 133–139. [Google Scholar] [CrossRef]
- Zhang, M.; Dang, P.; Haegeman, B.; Han, X.; Wang, X.; Pu, X.; Qin, X.; Siddique, K.H.M. The effects of straw return on soil bacterial diversity and functional profiles: A meta-analysis. Soil Biol. Biochem. 2024, 195, 109484. [Google Scholar] [CrossRef]
- Reichert, J.M.; Suzuki, L.E.A.S.; Reinert, D.J.; Horn, R.; Hcekansson, I. Reference bulk density and critical de-gree-of-compactness for no-till crop production in subtropical highly weathered soils. Soil Till. Res. 2009, 102, 242–254. [Google Scholar] [CrossRef]
- Dang, P.; Li, C.; Lu, C.; Zhang, M.; Huang, T.; Wan, C.; Wang, H.; Chen, Y.; Qin, X.; Liao, Y.; et al. Effect of fertilizer management on the soil bacterial community in agroecosystems across the globe. Agric. Ecosyst. Environ. 2022, 326, 107795. [Google Scholar] [CrossRef]
- Breulmann, M.; Masyutenko, N.P.; Kogut, B.M.; Schroll, R.; Dorfler, U.; Buscot, F.; Schulz, E. Short-term bioavailability of carbon in soil organic matter fractions of different particle sizes and densities in grassland ecosystems. Sci. Total Environ. 2014, 497–498, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Lloyd, J.; Herms, D.A.; Hoitink, H.A.J.; Michel, F.C. Effects of mulching and fertilization on soil nutrients, microbial activity and rhizosphere bacterial community structure determined by analysis of TRFLPs of PCR-amplified 16S rRNA genes. Appl. Soil Ecol. 2002, 21, 31–48. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Sun, R.; Ye, X.; Ma, C.; Mao, J.; Zhang, C.; Gao, H.; Zhang, W. Soil microbial communities under wheat and maize straw incorporation are closely associated with soil organic carbon fractions and chemical structure. Appl. Soil Ecol. 2023, 182, 104724. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B.; Shen, Q.; Zhang, R. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2015, 18, 1907–1917. [Google Scholar] [CrossRef]
- Siedt, M.; Schaffer, A.; Smith, K.E.C.; Nabel, M.; Nickoll, M.; Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2020, 751, 141607. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Li, Z.; Liu, K.; Zamanian, K. Tillage practice impacts on the carbon sequestration potential of topsoil microbial communities in an agricultural field. Agronomy 2020, 11, 60. [Google Scholar] [CrossRef]
- Tu, Q.; Lin, L.; Cheng, L.; Deng, Y.; He, Z. NCycDB: A curated integrative database for fast and accurate metagenomic profiling of nitrogen cycling genes. Bioinformatics 2019, 35, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Yuan, J.; Tang, Z.; Wang, J.; Zhang, Y. Long-term organic fertilization strengthens the soil phosphorus cycle and phosphorus availability by regulating the pqqC- and phoD-harboring bacterial communities. Microb. Ecol. 2023, 86, 2716–2732. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qiu, G.; Wei, J.; Guo, Z.; Wang, W.; Liu, X.; Song, Y. Activated carbon enhanced traditional activated sludge process for chemical explosion accident wastewater treatment. Environ. Res. 2023, 225, 115595. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).