Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece
Abstract
1. Introduction
2. Materials and Methods
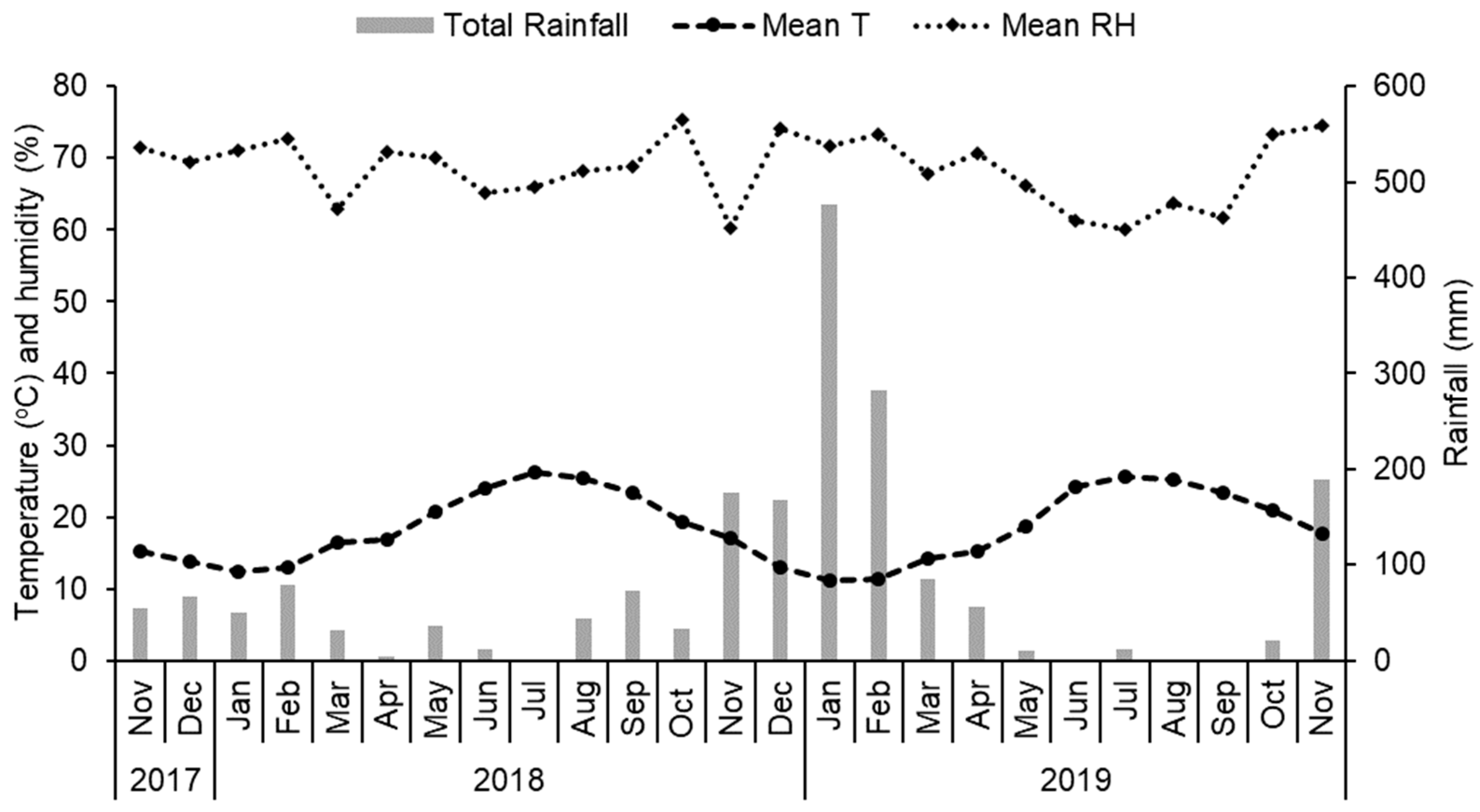
2.1. Study Site
2.2. Sampling of Insects
2.2.1. Malaise Trap
2.2.2. Sweep Net
2.3. Auchenorrhyncha Identification
2.4. Data Analysis
3. Results
3.1. Identification and Abundance of Insects
3.2. Sampling Methods
3.3. Auchenorrhyncha Abundance and Altitude
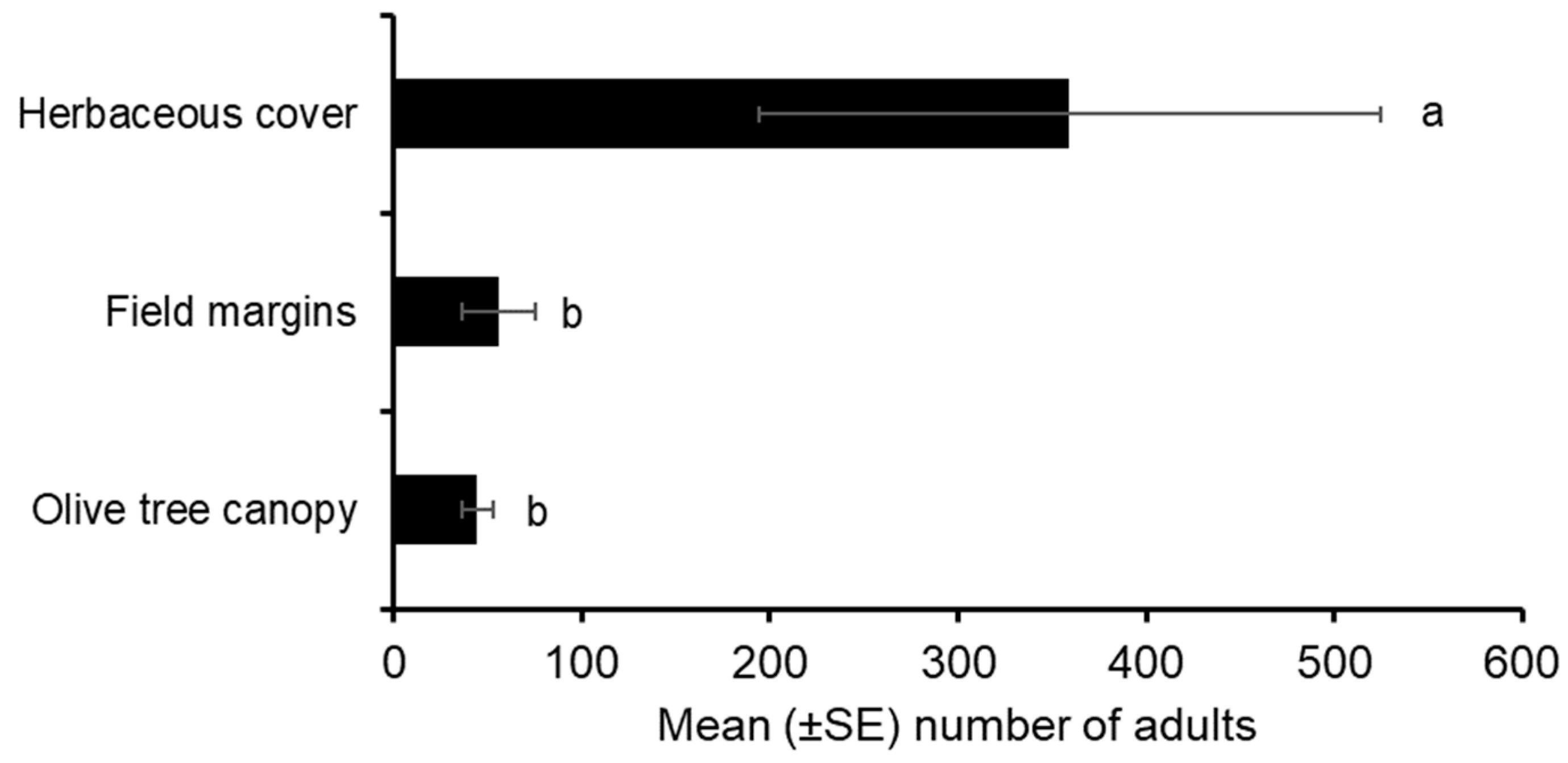
3.4. Habitat Preference
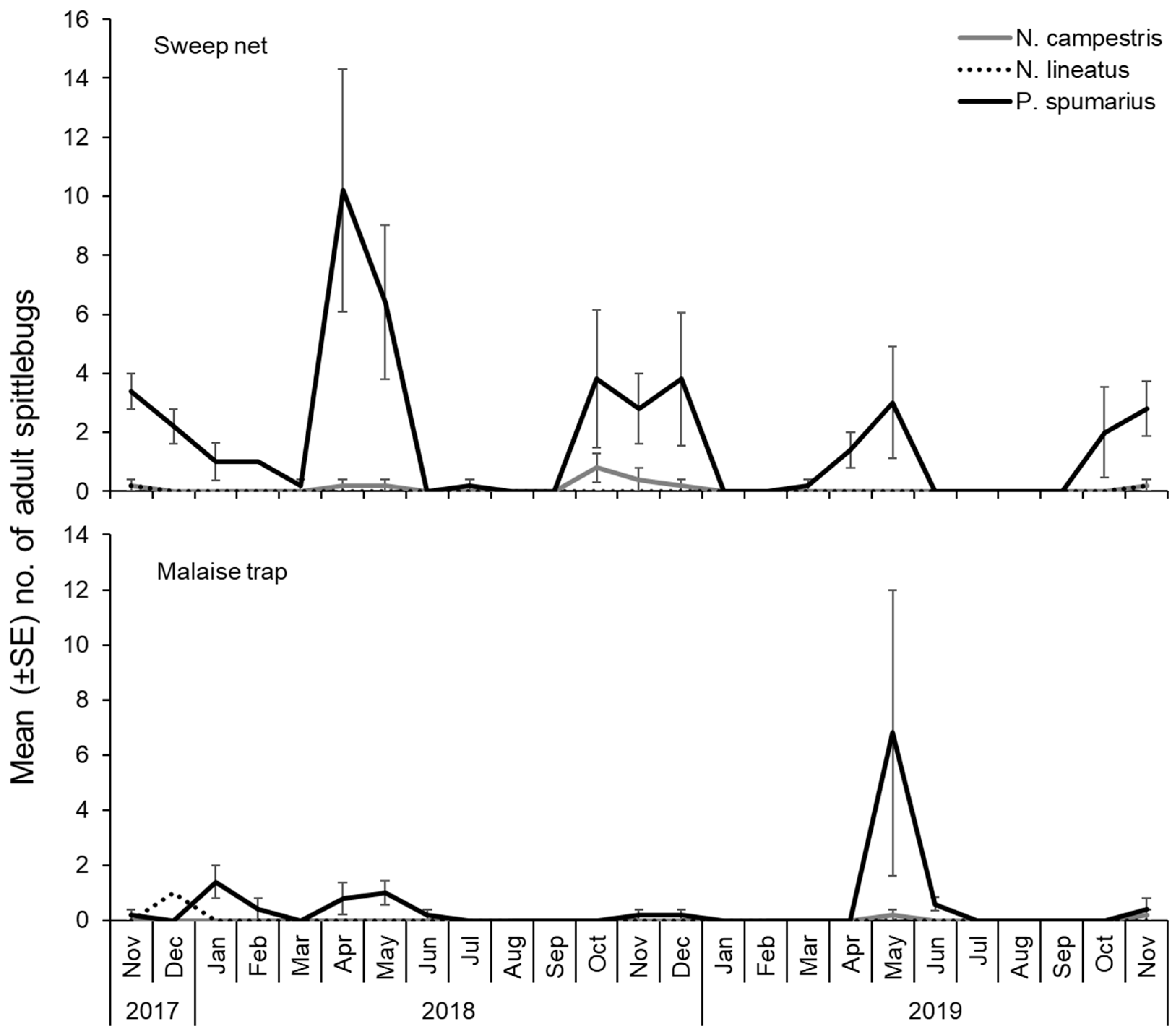
3.5. Seasonal Fluctuation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nault, L.R.; Ammar, E.D. Leafhopper and planthopper transmission of plant viruses. Annu. Rev. Entomol. 1989, 34, 503–529. [Google Scholar] [CrossRef]
- Orlovskis, Z.; Canale, M.C.; Thole, V.; Pecher, P.; Lopes, J.R.; Hogenhout, S.A. Insect-borne plant pathogenic bacteria: Getting a ride goes beyond physical contact. Curr. Opin. Insect. Sci. 2015, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Perilla-Henao, L.M.; Casteel, C.L. Vector-borne bacterial plant pathogens: Interactions with hemipteran insects and plants. Front. Plant Sci. 2016, 7, 1163. [Google Scholar] [CrossRef] [PubMed]
- Dimou, D.; Drossopoulou, J.; Moschos, E.; Varveri, C.; Bem, F. First report of Citrus tristeza virus in Greece. Plant Dis. 2002, 86, 329. [Google Scholar] [CrossRef]
- Orfanidou, C.; Pappi, P.G.; Efthimiou, K.E.; Katis, N.I.; Maliogka, V.I. Transmission of Tomato chlorosis virus (ToCV) by Bemisia tabaci biotype Q and evaluation of four weed species as viral sources. Plant Dis. 2016, 100, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Holeva, M.C.; Glynos, P.E.; Karafla, C.D. First report of ‘Candidatus liberibacter solanacearum’ on Carrot in Greece. Plant Dis. 2017, 101, 1819. [Google Scholar] [CrossRef]
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. EVolume Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- EPPO. EPPO Global Database. Available online: https://gd.eppo.int (accessed on 10 July 2022).
- Lopes, J.R.; Landa, B.B.; Fereres, A. A survey of potential insect vectors of the plant pathogenic bacterium Xylella fastidiosa in three regions of Spain. Span. J. Agric. Res. 2014, 12, 795–800. [Google Scholar] [CrossRef]
- Godefroid, M.; Cruaud, A.; Streito, J.C.; Rasplus, J.Y.; Rossi, J.P. Xylella fastidiosa: Climate suitability of European continent. Sci. Rep. 2019, 9, 8844. [Google Scholar] [CrossRef]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific opinion on the risk to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 262. [Google Scholar] [CrossRef]
- European Food Safety Authority. Statement of EFSA on host plants, entry and spread pathways and risk reduction options for Xylella fastidiosa Wells et al. EFSA J. 2013, 11, 3468. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Delbianco, A.; Gibin, D.; Pasinato, L.; Boscia, D.; Morelli, M. Scientific Report on the update of the Xylella spp. host plant database—Systematic literature search up to 31 December 2021. EFSA J. 2022, 20, 7356. [Google Scholar] [CrossRef]
- Dellapé, G.; Paradell, S.; Semorile, L.; Delfederico, L. Potential vectors of Xylella fastidiosa: A study of leafhoppers and treehoppers in citrus agroecosystems affected by Citrus Variegated Chlorosis. Entomol. Exp. Appl. 2016, 161, 92–103. [Google Scholar] [CrossRef]
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Ringenberg, R.; Lopes, J.R.S.; Müller, C.; Azevedo-Filho, W.S.D.; Paranhos, B.A.J.; Botton, M. Survey of potential sharpshooter and spittlebug vectors of Xylella fastidiosa to grapevines at the São Francisco River Valley, Brazil. Rev. Bras. Entomol. 2014, 58, 212–218. [Google Scholar] [CrossRef]
- Serio, F.D.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Carolo, M.D.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U.; et al. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA Support. Publ. 2019, 16, 1628E. [Google Scholar] [CrossRef]
- Cornara, D.; Marra, M.; Tedone, B.; Cavalieri, V.; Porcelli, F.; Fereres, A.; Purcell, A.; Saponari, M. No evidence for cicadas’ implication in Xylella fastidiosa epidemiology. Entomologia Generalis 2020, 40, 125–132. [Google Scholar] [CrossRef]
- Loconsole, G.; Potere, O.; Boscia, D.; Altamura, G.; Djelouah, K.; Elbeaino, T.; Frasheri, D.; Lorusso, D.; Palmisano, F.; Pollastro, P.; et al. Detection of Xylella fastidiosa in olive trees by molecular and serological methods. J. Plant Pathol. 2014, 96, 7–14. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond, and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Scortichini, M.; Loreti, S.; Pucci, N.; Scala, V.; Tatulli, G.; Verweire, D.; Oehl, M.; Widmer, U.; Codina, J.M.; Hertl, P.; et al. Progress towards sustainable control of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Scholten, R.; Sanchez, L.M.; Hornero, A.; Navas-Cortes, J.A.; Zarco-Tejada, P.J.; Beck, P.S. Monitoring the impact of Xylella on Apulia’s olive orchards using Sentinel-2 satellite data and aerial photographs. In Proceedings of the Second European Conference on Xylella fastidiosa, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, 200. [Google Scholar] [CrossRef]
- Denancé, N.; Legendre, B.; Briand, M.; Olivier, V.; De Boisseson, C.; Poliakoff, F.; Jacques, M.A. Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathol. 2017, 66, 1054–1064. [Google Scholar] [CrossRef]
- Saponari, M.; D’Attoma, G.; Abou Kubaa, R.; Loconsole, G.; Altamura, G.; Zicca, S.; Rizzo, D.; Boscia, D. A new variant of Xylella fastidiosa subspecies multiplex detected in different host plants in the recently emerged outbreak in the region of Tuscany, Italy. Eur. J. Plant Pathol. 2019, 154, 1195–1200. [Google Scholar] [CrossRef]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gener. 2019, 39, 158. [Google Scholar] [CrossRef]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef]
- Cruaud, A.; Gonzalez, A.A.; Godefroid, M.; Nidelet, S.; Streito, J.C.; Thuillier, J.M.; Rossi, J.P.; Santoni, S.; Rasplus, J.Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef]
- Drosopoulos, S. New data on the nature and origin of colour polymorphism in the spittlebug genus Philaenus (Hemiptera: Aphorophoridae). Ann. Soc. Entomol. Fr. 2003, 39, 31–42. [Google Scholar] [CrossRef]
- Halkka, O.; Halkka, L. Population genetics of the polymorphic meadow spittlebug, Philaenus spumarius (L.). EVolume Biol. 1989, 24, 149–191. [Google Scholar]
- Stewart, A.J.; Lees, D.R. The colour/pattern polymorphism of Philaenus spumarius (L.) (Homoptera: Cercopidae) in England and Wales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 69–89. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Asche, M. Biosystematic studies on the spittlebug genus Philaenus with the description of a new species. Zool. J. Linn. Soc. 1991, 101, 169–177. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. The Mediterranean: Area of origin of polymorphism and speciation in the spittlebug Philaenus (Hemiptera, Aphrophoridae). Zoosyst. EVolume 2010, 86, 125–128. [Google Scholar] [CrossRef]
- Thanou, Z.N.; Afentoulis, D.G.; Koufopoulou, P.; Ampatzi, A.P.; Lekkou, S.D.; Koutsogiannopoulou, A.; Bravou, A.A.; Stamatakou, G.D.; Voulgaraki, K.N.; Piperkas, A.; et al. New records and updated checklist of Cicadomorpha (Hemiptera: Auchenorrhyncha) species from Greece. Zootaxa 2018, 4413, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Drosopoulos, S. Hemipterological studies in Greece. Part II. Homoptera—Auchenorrhyncha. A catalogue of the reported species. Biol. Gallo-Hell. 1980, 9, 187–194. [Google Scholar]
- Drosopoulos, S.; Asche, M.; Hoch, H. A preliminary list and some notes on the Cicadomorpha (Homoptera-Auchenorrhyncha) collected in Greece. In Proceedings of the 2nd International Congress Concerning the Rhynchota Fauna of Balkan and Adjacent Regions, Mikrolimni, Greece, 18–22 August 1986; Volume 1986, pp. 8–13. [Google Scholar]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Tzobanoglou, D.K.; Goumas, D.; Trantas, E.; Zarboutis, I.; et al. Identification and seasonal abundance of Auchenorrhyncha with a focus on potential insect vectors of Xylella fastidiosa in olive orchards in three regions of Greece. J. Econ. Entomol. 2018, 111, 2536–2545. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in olive orchards of Greece with special reference to Xylella fastidiosa vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Lolas, P.C. Weed Science, Weeds, Herbicides. Environment, Behavior, and Fate; Synxroni Paideia: Thessaloniki, Greece, 2007. [Google Scholar]
- Eleutherochorinos, H.G. Weed Science—Weeds—Herbicides—Environment; Agrotypos: Athens, Greece, 2002. [Google Scholar]
- Naidu, K.R.K.; Ramana, A.V.; De, B. Bio-efficacy and economics of herbicides against weeds of black gram [Vigna mungo (L.) Hepper] grown in rice-fallow. J. Crop Weed 2012, 8, 133–136. [Google Scholar] [CrossRef]
- Matthews, R.W.; Matthews, J.R. The Malaise trap: Its utility and potential for sampling insect populations. Great Lakes Entomol. 2017, 4, 117–122. [Google Scholar] [CrossRef]
- Malaise, R. A new insect-trap. Entomol. Tidskr. 1937, 58, 148–160. [Google Scholar]
- Elbeaino, T.; Yaseen, T.; Valentini, F.; Moussa, I.E.B.; Mazzoni, V.; D’Onghia, A.M. Identification of three potential insect vectors of Xylella fastidiosa in southern Italy. Phytopathol. Mediterr. 2014, 53, 328–332. [Google Scholar] [CrossRef]
- EFSA. Xylella Tutorial | How to Collect Data on Philaenus spumarius (Spittlebug). Available online: https://www.youtube.com/watch?v=Rjh7FFQCtg8 (accessed on 1 August 2024).
- Nickel, H. Leafhoppers and Planthoppers of Germany (Hemiptera, Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects; Pensoft: Sofia, Bulgaria; Moscow, Russia, 2003. [Google Scholar]
- Ribaut, H. Homoptères Auchenorrhynques I: Typhlocybidae; Faune de France; Lechevalier: Paris, France, 1936. [Google Scholar]
- Ribaut, H. Homoptères Auchénorhynques. II: (Jassidae); Lechevalier: Paris, France, 1952. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 1: Introduction, Infraorder Fulgoromorpha; Scandinavian Science Press Ltd.: Klampenborg, Denmark, 1978; Volume 7, No. 1; pp. 1–222. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 2: The Families Cicadidae, Cercopidae, Membracidae, and Cicadellidae (excl. Deltocephalinae); Scandinavian Science Press Ltd.: Klampenborg, Denmark, 1981; Volume 7, No. 2; pp. 223–593. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. Part 3: The Family Cicadellidae: Deltocephalinae, Catalogue, Literature, and Index; Fauna Entomologica Scandinavica; Brill: Leiden, The Netherlands, 1983. [Google Scholar]
- Biedermann, R.; Niedringhaus, R. The Plant- and Leafhoppers of Germany: Identification Key to All Species; Wabv Fründ: Scheeßel, Germany, 2009. [Google Scholar]
- Gnezdilov, V.M.; Holzinger, W.E.; Wilson, M.R. The Western Palaearctic Issidae (Hemiptera, Fulgoroidea): An Illustrated Checklist and Key to Genera and Subgenera. Zool. Inst. RAS 2014, 318 (Suppl. 1), 1–124. [Google Scholar]
- Holzinger, W.E.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe; Brill: Leiden, The Netherlands, 2003; Volume 1. [Google Scholar]
- Le Quesne, W.J.; Payne, K. Hemiptera, Cicadellidae (Typhlocybinae). In Handbooks for the Identification of British Insects; Royal Entomological Society: St Albans, UK, 1981; Volume 2. [Google Scholar]
- Anufriev, G.A.; Danzig, E.M.; Emeljanov, A.F.; Golub, V.B.; Kanyukova, E.V.; Kerzhner, I.M.; Konovalova, Z.A.; Pashchenko, N.F.; Tshernova, G.P.; Vinokurov, N.N. Suborder Cicadinea (Auchenorrhyncha). In Keys to the Insects of the Far East of the USSR; Nauka Publishing House: Leningrad, Russia, 1988; Volume 2, pp. 12–495. [Google Scholar]
- Curry, J.P. The arthropods associated with the decomposition of some common grass and weed species in the soil. Soil Biol. Biochem. 1973, 55, 645–657. [Google Scholar] [CrossRef]
- Cusack, P.D.; Evans, G.O.; Brennan, P.A. A survey of the mites of stored grain and grain products in the Republic of Ireland. Sci. Proc. R. Dublin Soc. 1975, 3, 273–329. [Google Scholar]
- Emmanouel, N.G. Aspects of the Biology of Mites Associated with Cereals during Growth and Storage. Ph.D. Dissertation, National University of Ireland, Dublin, Ireland, 1977. [Google Scholar]
- Dellapé, G.; Bouvet, J.P.; Paradell, S.L. Diversity of cicadomorpha (Hemiptera: Auchenorrhyncha) in citrus orchards in Northeastern Argentina. Fla. Entomol. 2013, 96, 1125–1134. [Google Scholar] [CrossRef]
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population dynamics of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) and its parasitoids in northwestern Argentina. Fla. Entomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Paradell, S.L.; Virla, E.G.; Toledo, A. Leafhoppers species richness and abundance on corn crops in Argentina (Insecta-Hemiptera-Cicadellidae). Bol. San. Veg. Plagas 2001, 27, 465–474. [Google Scholar]
- SAS Institute. JMP: A User’s Guide to Statistical and Data Analysis, 7th ed.; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Theodorou, D.; Koufakis, I.; Thanou, Z.; Kalaitzaki, A.; Chaldeou, E.; Afentoulis, D.; Tsagkarakis, A. Management system affects the occurrence, diversity and seasonal fluctuation of Auchenorrhyncha, potential vectors of Xylella fastidiosa, in the olive agroecosystem. Bull. Insectol. 2021, 74, 27–40. [Google Scholar]
- Ben Moussa, I.E.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D’Onghia, A.M. Seasonal fluctuations of sap-feeding insect species infected by Xylella fastidiosa in Apulian olive groves of southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Simões, P.C.; Quartau, J.A. Distribution of cicadas of the genus Lyristes (Hemiptera: Cicadidae) in the eastern Mediterranean area. Biologia 2013, 68, 961–965. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Varikou, K.; Vahamidis, P.; Kapranas, A.; Milonas, P. Seasonal appearance, abundance, and host preference of Philaenus spumarius and Neophilaenus campestris (Hemiptera: Aphrophoridae) in olive groves in Greece. Environ. Entomol. 2021, 50, 1474–1482. [Google Scholar] [CrossRef]
- Godefroid, M.; Morente, M.; Schartel, T.; Cornara, D.; Purcell, A.; Gallego, D.; Moreno, A.; Pereira, J.A.; Fereres, A. Climate tolerances of Philaenus spumarius should be considered in risk assessment of disease outbreaks related to Xylella fastidiosa. J. Pest Sci. 2021, 95, 855–868. [Google Scholar] [CrossRef]
- Karban, R.; Strauss, S.Y. Physiological tolerance, climate change, and a northward range shift in the spittlebug, Philaenus spumarius. Ecol. Entomol. 2004, 29, 251–254. [Google Scholar] [CrossRef]
- Pérez-Otero, R.; Pérez-Turco, R.; Neto, J.; Fereres, A. The African Psyllid Trioza erytreae Del Guercio (1918) Is Very Sensitive to Low Relative Humidity and High Temperatures. Insects 2024, 15, 62. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2019, 112, 67–74. [Google Scholar] [CrossRef]
- Thanou, Z.N.; Kontogiannis, E.G.; Tsagkarakis, A.E. Impact of weeds on Auchenorrhyncha incidence and species richness in citrus orchards. Phytoparasitica 2021, 49, 333–347. [Google Scholar] [CrossRef]
- Purcell, A.H.; Gravena, S.; Donadio, L.C. Sharpshooter in citrus crops. In Citrus-Integrated Management of Insect and Mite Pests; Estaçao Experimental de Citricultura: Bebedouro, Brazil, 1994; pp. 213–229. [Google Scholar]
- Weaver, C.R.; King, D.R. Meadow spittlebug, Philaenus leucophthalmus (L.). Res. Bull. 1954, 741, 1–99. [Google Scholar]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef]
- Le Cesne, M.; Wilson, S.W.; Soulier-Perkins, A. Elevational gradient of Hemiptera (Heteroptera, Auchenorrhyncha) on a tropical mountain in Papua New Guinea. PeerJ 2015, 3, e978. [Google Scholar] [CrossRef] [PubMed]
- Therios, I. Olive Culture; Gartaganis Publications: Thessaloniki, Greece, 2005. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]





| Area | Location | Coordinates | Alt. * | Crop | Sampling Method | Field Margins Vegetation |
|---|---|---|---|---|---|---|
| Site 1 | Chania | 35°29′12.8″ N 24°01′28.0″ E | 10 | Olea europea ‘Koroneiki’ | MT, SN | Citrus, Avocado, Grasses |
| Site 2 | Zounaki | 35°28′54.3″ N 23°49′48.4″ E | 100 | Olea europea ‘Koroneiki’ | MT, SN | Pistacia lentiscus, Erica sp., Cistus spp., Ilex sp. |
| Site 3 | Zymbragou | 35°26′27.1″ N 23°45′25.0″ E | 300 | Olea europea ‘Koroneiki’ | MT, SN | Arbutus sp., olive trees, Juglans sp. |
| Site 4 | Kasteli | 35°29′31.7″ N 23°38′42.0″ E | 30 | Olea europea ‘Koroneiki’ | MT, SN | Vitex sp., Olive trees |
| Site 5 | Lousakies | 35°28′34.3″ N 23°38′02.7″ E | 180 | Olea europea ‘Koroneiki’ | MT, SN | Pistacia lentiscus, grasses, herbal plants |
| Site 6 | Chania | 35°29′31.9″ N 24°03′00.1″ E | 10 | Citrus spp. | MT | Grasses, Anthemis spp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koufakis, I.E.; Kalaitzaki, A.P.; Pappas, M.L.; Tsagkarakis, A.E.; Tzobanoglou, D.K.; Broufas, G.D. Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece. Agronomy 2024, 14, 2243. https://doi.org/10.3390/agronomy14102243
Koufakis IE, Kalaitzaki AP, Pappas ML, Tsagkarakis AE, Tzobanoglou DK, Broufas GD. Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece. Agronomy. 2024; 14(10):2243. https://doi.org/10.3390/agronomy14102243
Chicago/Turabian StyleKoufakis, Ioannis E., Argyro P. Kalaitzaki, Maria L. Pappas, Antonios E. Tsagkarakis, Despina K. Tzobanoglou, and George D. Broufas. 2024. "Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece" Agronomy 14, no. 10: 2243. https://doi.org/10.3390/agronomy14102243
APA StyleKoufakis, I. E., Kalaitzaki, A. P., Pappas, M. L., Tsagkarakis, A. E., Tzobanoglou, D. K., & Broufas, G. D. (2024). Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece. Agronomy, 14(10), 2243. https://doi.org/10.3390/agronomy14102243











