Impact of Organic Fertilization Strategies on Soil Bacterial Community and Honey Pomelo (Citrus maxima) Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Fertilization Strategies
2.3. Soil Sample Collection
2.4. Honey Pomelo and Soil Properties Determination
2.5. Soil DNA Extraction and Sequencing
2.6. Sequenced Data Processing
2.7. Statistical Analyses
3. Results
3.1. Honey Pomelo and Soil Properties
3.2. Soil Bacterial Diversities
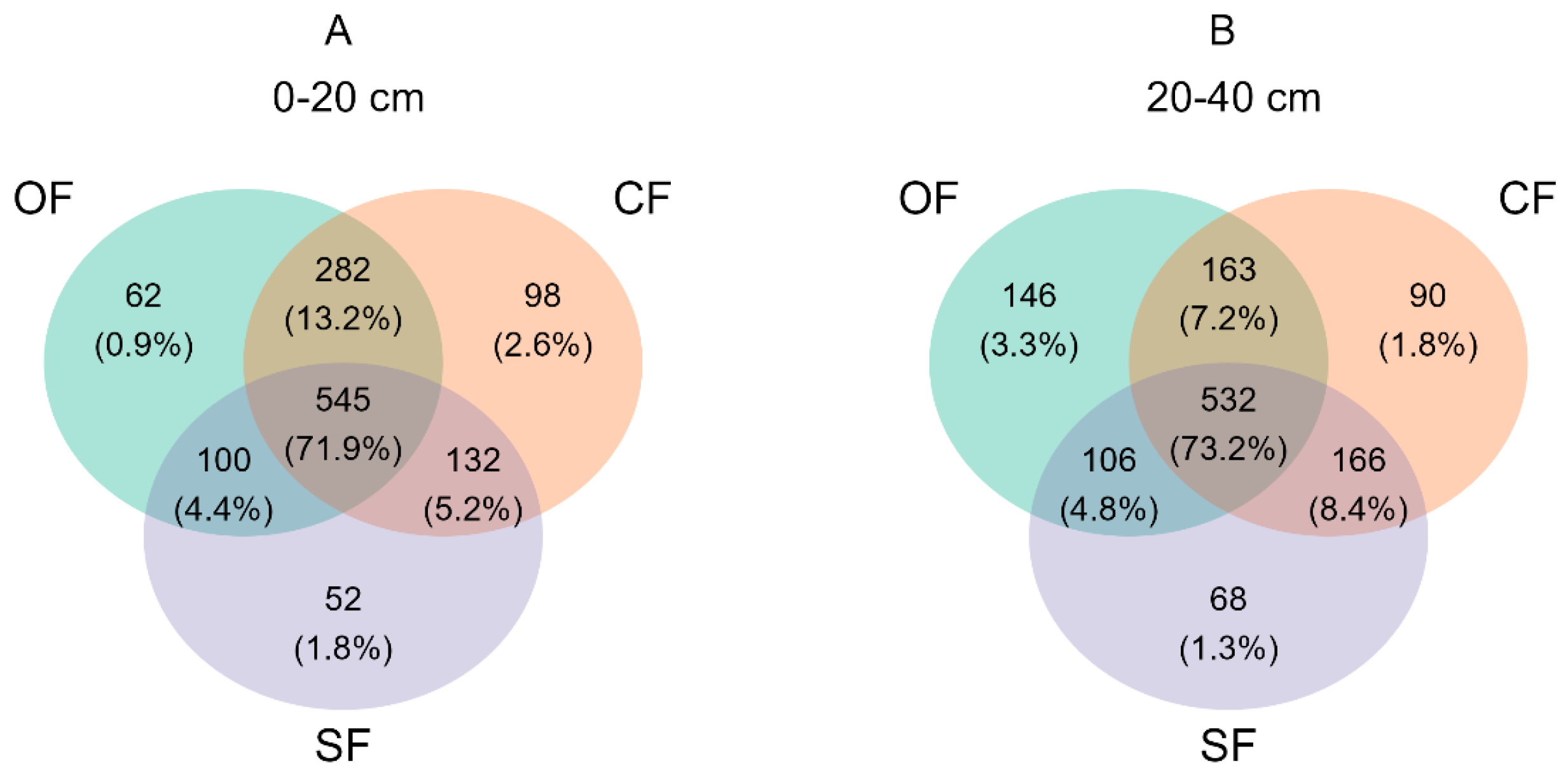
3.2.1. ASV Distribution
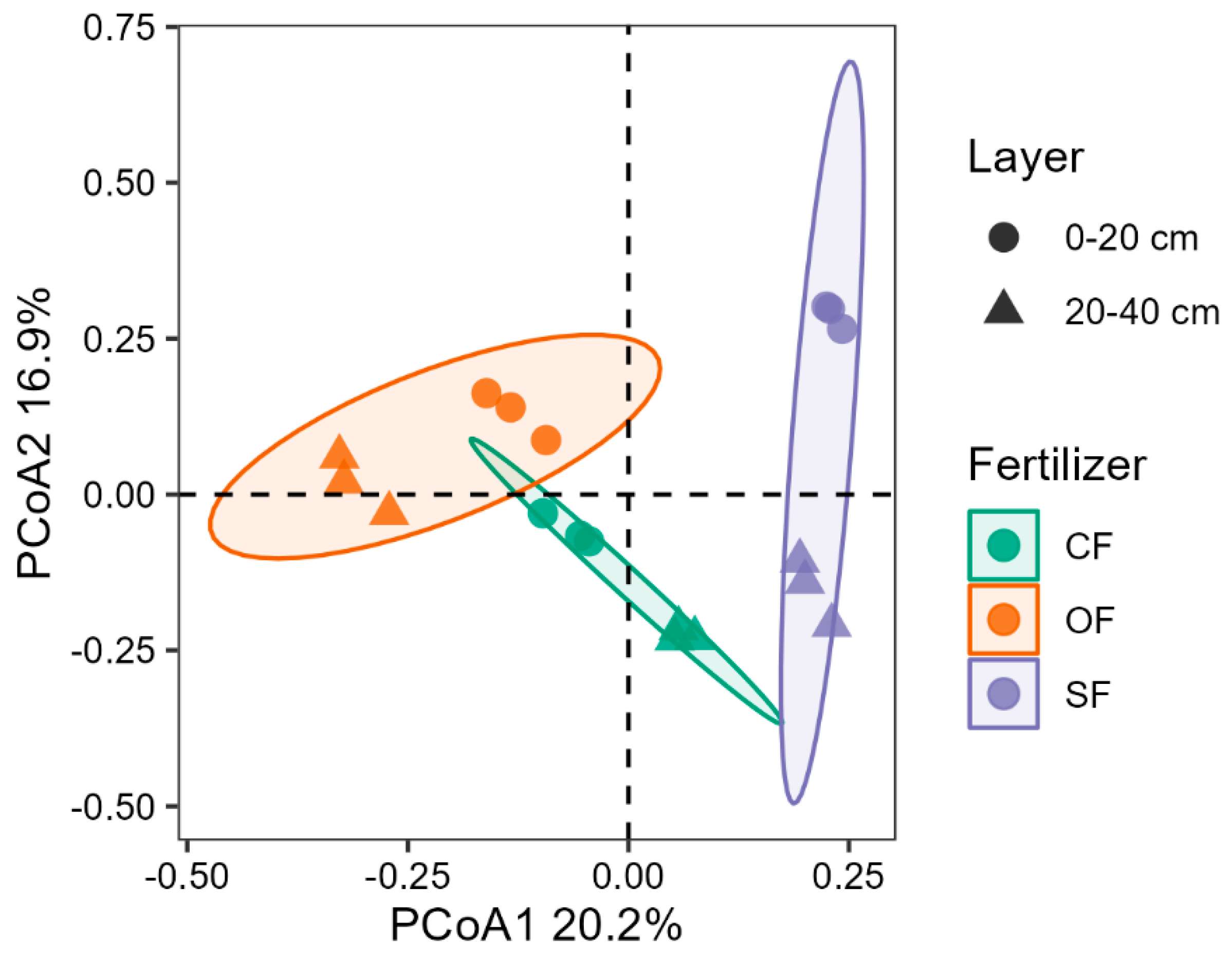
3.2.2. Soil Bacterial Community Structure
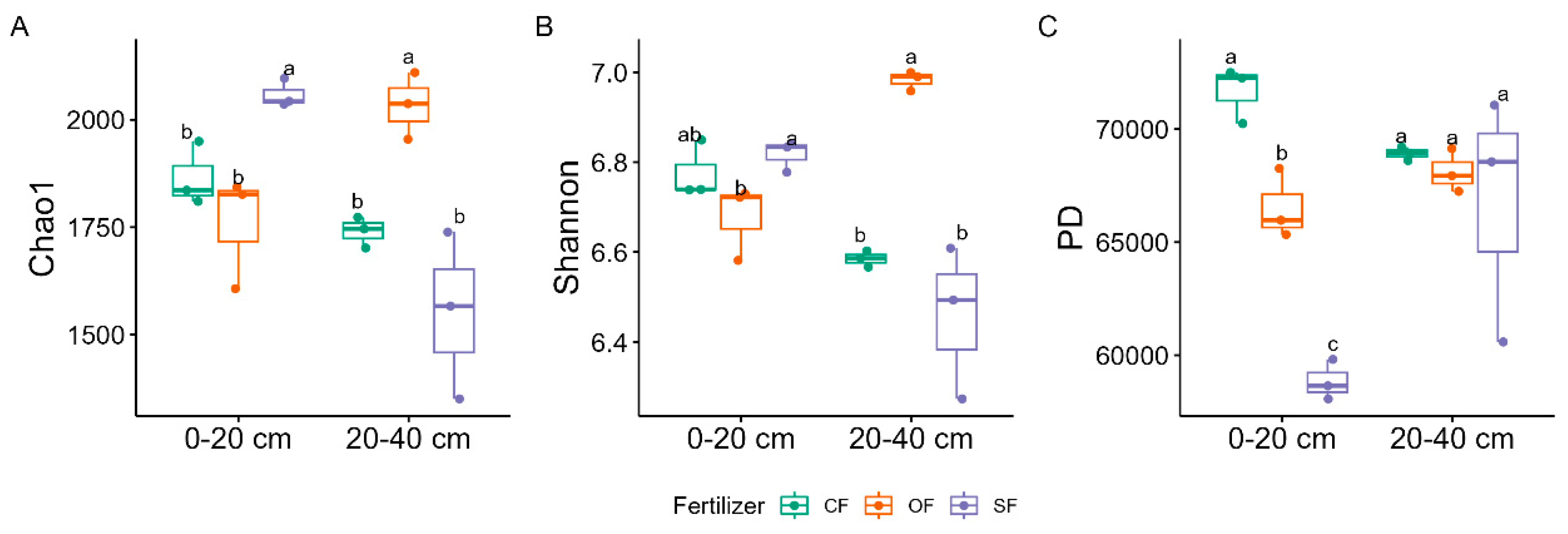
3.2.3. Soil Bacterial Alpha and Beta Diversities
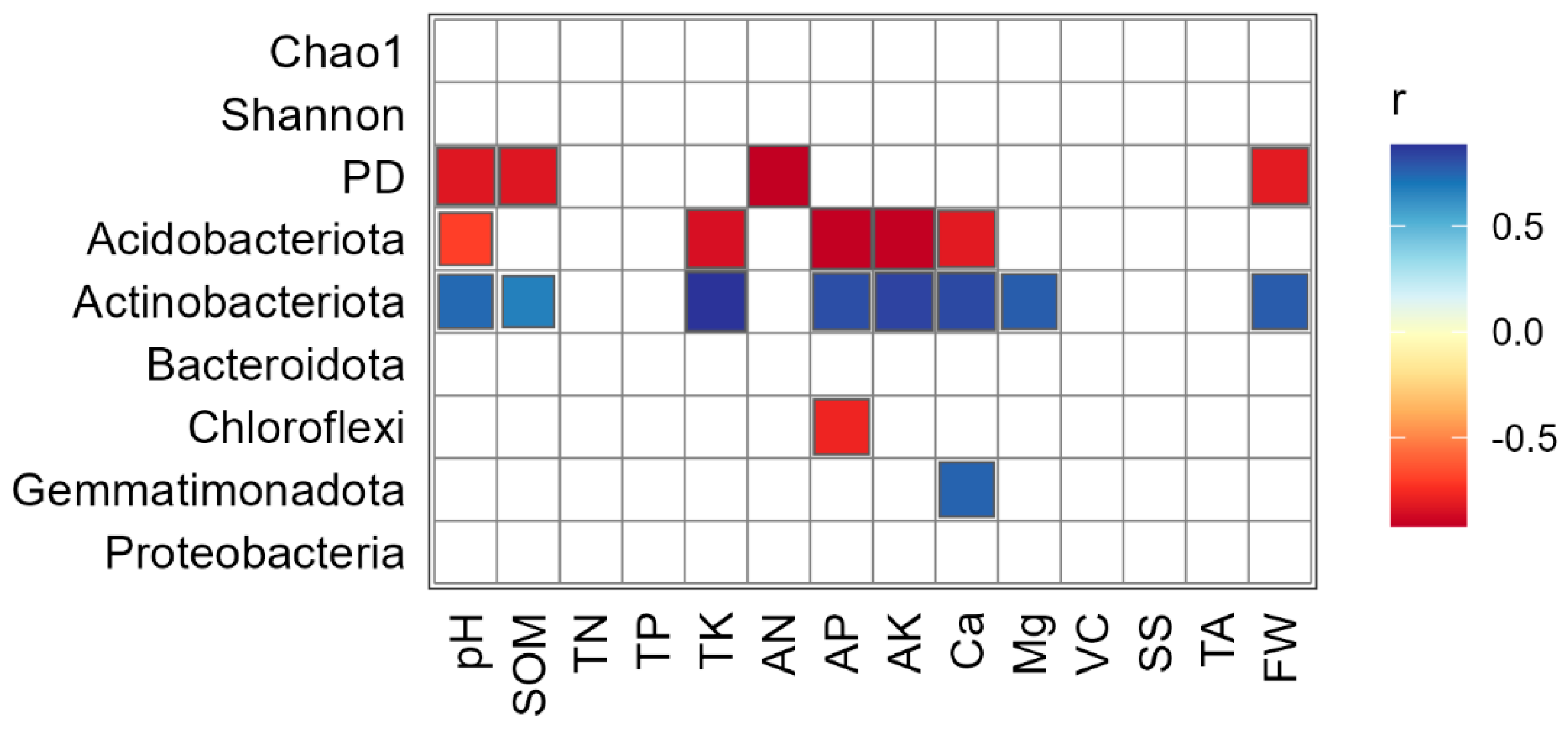
3.3. Relationship between Soil Bacterial Communities, Diversities, Soil and Honey Pomelo Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Huang, X.; Chen, T.; Zhou, P.; Huang, X.; Jin, W.; Liu, D.; Zhang, H.; Zhou, J.; Wang, Z.; et al. Fruit Quality Prediction Based on Soil Mineral Element Content in Peach Orchard. Food Sci. Nutr. 2022, 10, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil Amendments for Sustainable Agriculture: Microbial Organic Fertilizers. Soil Use Manag. 2022, 38, 94–120. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic Fertilizer Activates Soil Beneficial Microorganisms to Promote Strawberry Growth and Soil Health after Fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Fucile, M.; Mattii, G.B. A Review: Soil Management, Sustainable Strategies and Approaches to Improve the Quality of Modern Viticulture. Agronomy 2021, 11, 2359. [Google Scholar] [CrossRef]
- Powlson, D.S.; Gregory, P.J.; Whalley, W.R.; Quinton, J.N.; Hopkins, D.W.; Whitmore, A.P.; Hirsch, P.R.; Goulding, K.W.T. Soil Management in Relation to Sustainable Agriculture and Ecosystem Services. Food Policy 2011, 36, S72–S87. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological Practices for Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Gruhn, P.; Goletti, F.; Yudelman, M. Integrated Nutrient Management, Soil Fertility, and Sustainable Agriculture: Current Issues and Future Challenges; International Food Policy Research Institute: Washington, DC, USA, 2000; ISBN 978-0-89629-637-4. [Google Scholar]
- Makkumrai, W.; Huang, Y.; Xu, Q. Comparison of Pomelo (Citrus maxima) Grown in China and Thailand. Front. Agric. Sci. Eng. 2021, 8, 335–352. [Google Scholar] [CrossRef]
- Yan, X.; Ma, Y.; Kong, K.; Muneer, M.A.; Zhang, L.; Zhang, Y.; Cheng, Z.; Luo, Z.; Ma, C.; Zheng, C.; et al. Mitigating Life-Cycle Environmental Impacts and Increasing Net Ecosystem Economic Benefits via Optimized Fertilization Combined with Lime in Pomelo Production in Southeast China. Sci. Total Environ. 2024, 912, 169007. [Google Scholar] [CrossRef]
- Awadelkareem, W.; Haroun, M.; Wang, J.; Qian, X. Nitrogen Interactions Cause Soil Degradation in Greenhouses: Their Relationship to Soil Preservation in China. Horticulturae 2023, 9, 340. [Google Scholar] [CrossRef]
- Jote, C.A. The Impacts of Using Inorganic Chemical Fertilizers on the Environment and Human Health. Org. Med. Chem. Int. J. 2023, 13, 555864. [Google Scholar]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial Fertilizers Improve Soil Quality and Crop Yield in Coastal Saline Soils by Regulating Soil Bacterial and Fungal Community Structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Abbas, S.A.A.A.; Sharif, L.; Shafiq, M.; Kamran, Z.; Masah; Haseeb, M.; Shahid, M.A. Chapter 13—Microbial Extracellular Polymeric Substance and Impacts on Soil Aggregation. In Bacterial Secondary Metabolites; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 221–237. ISBN 978-0-323-95251-4. [Google Scholar]
- Bharadwaj, A. Role of Microbial Extracellular Polymeric Substances in Soil Fertility. In Microbial Polymers: Applications and Ecological Perspectives; Vaishnav, A., Choudhary, D.K., Eds.; Springer: Singapore, 2021; pp. 341–354. ISBN 9789811600456. [Google Scholar]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Zaib, S. Mighty Microbes: Plant Growth Promoting Microbes in Soil Health and Sustainable Agriculture. In Soil Health; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 243–264. ISBN 978-3-030-44364-1. [Google Scholar]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.; Song, S. Important Soil Microbiota’s Effects on Plants and Soils: A Comprehensive 30-Year Systematic Literature Review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar] [CrossRef] [PubMed]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Shen, J.-P.; Zhang, L.-M.; Guo, J.-F.; Ray, J.L.; He, J.-Z. Impact of Long-Term Fertilization Practices on the Abundance and Composition of Soil Bacterial Communities in Northeast China. Appl. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Kang, T.; Xie, K.; Xie, Z.; Wu, Q.; Hou, S.; Yuan, Y.; Sun, Y.; Song, K.; Zhou, C. Investigation and Analysis on Production Status and Peasant Houshold Characteristic Factors of Jinggang Honey Pomelo. China Fruits 2022, 64, 81–88. (In Chinese) [Google Scholar]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on Carbon Components and Microbial Functional Metabolism during Cow Manure–Straw Composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, L.; Liao, Y.; Lu, D.; Zhang, H.; Liao, X.; Liang, J.B.; Wu, Y. Influence and Characteristics of Bacillus stearothermophilus in Ammonia Reduction during Layer Manure Composting. Ecotoxicol. Environ. Saf. 2019, 180, 80–87. [Google Scholar] [CrossRef]
- Sieuwerts, S.; De Bok, F.A.M.; Mols, E.; De Vos, W.M.; Van Hylckama Vlieg, J.E.T. A Simple and Fast Method for Determining Colony Forming Units. Lett. Appl. Microbiol. 2008, 47, 275–278. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1982; pp. 539–579. ISBN 978-0-89118-977-0. [Google Scholar]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods of Soil and Agricultural Chemistry; Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Stubner, S. Enumeration of 16S rDNA of Desulfotomaculum Lineage 1 in Rice Field Soil by Real-Time PCR with SybrGreenTM Detection. J. Microbiol. Methods 2002, 50, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate High-Throughput Multiple Sequence Alignment of Ribosomal RNA Genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Xue, P.; Chen, Y.; Zhu, Z.; Hao, D.; Song, M. Effects of Duck Manure Replacing Chemical Fertilizer on Soil Nutrient Characteristics and Pear Quality in Pear Planting. Chin. J. Agrometeorol. 2022, 43, 1015. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.; Lv, X.; Qin, Y.; Zhang, D.; Zhang, C.; Song, Z.; Liu, D.; Jiang, L.; Huang, B.; Wang, J. Effects of Different Botanical Oil Meal Mixed with Cow Manure Organic Fertilizers on Soil Microbial Community and Function and Tobacco Yield and Quality. Front. Microbiol. 2023, 14, 1191059. [Google Scholar] [CrossRef]
- Smoliak, S. Effects of Manure, Straw and Inorganic Fertilizers on Northern Great Plains Ranges. J. Range Manag. 1965, 18, 11. [Google Scholar] [CrossRef]
- Chang, E.-H.; Wang, C.-H.; Chen, C.-L.; Chung, R.-S. Effects of Long-Term Treatments of Different Organic Fertilizers Complemented with Chemical N Fertilizer on the Chemical and Biological Properties of Soils. Soil Sci. Plant Nutr. 2014, 60, 499–511. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The Role of Microbial Communities in the Formation and Decomposition of Soil Organic Matter. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 81–118. ISBN 978-90-481-9479-7. [Google Scholar]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Associations between Soil Bacterial Community Structure and Nutrient Cycling Functions in Long-Term Organic Farm Soils Following Cover Crop and Organic Fertilizer Amendment. Sci. Total Environ. 2016, 566–567, 949–959. [Google Scholar] [CrossRef]
- Wu, C.; Yan, B.; Wei, F.; Wang, H.; Gao, L.; Ma, H.; Liu, Q.; Liu, Y.; Liu, G.; Wang, G. Long-Term Application of Nitrogen and Phosphorus Fertilizers Changes the Process of Community Construction by Affecting Keystone Species of Crop Rhizosphere Microorganisms. Sci. Total Environ. 2023, 897, 165239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pokharel, P.; Liu, G.; Chen, J. Reclamation of Desert Land to Different Land-use Types Changes Soil Bacterial Community Composition in a Desert-oasis Ecotone. Land Degrad. Dev. 2021, 32, 1389–1399. [Google Scholar] [CrossRef]
- Lan, J.; Wang, S.; Wang, J.; Qi, X.; Long, Q.; Huang, M. The Shift of Soil Bacterial Community After Afforestation Influence Soil Organic Carbon and Aggregate Stability in Karst Region. Front. Microbiol. 2022, 13, 901126. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A.; Feng, Y.; Prior, S.A. Soil Microbial Community Dynamics as Influenced by Composted Dairy Manure, Soil Properties, and Landscape Position. Soil Sci. 2010, 175, 474. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting Life Strategy Concepts in Environmental Microbial Ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Lewin, G.R.; Carlos, C.; Chevrette, M.G.; Horn, H.A.; McDonald, B.R.; Stankey, R.J.; Fox, B.G.; Currie, C.R. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annu. Rev. Microbiol. 2016, 70, 235–254. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Lu, S.; Xiang, Q.; Yu, X.; Zhao, K.; Zou, L.; Chen, Q.; Tu, S.; Zhang, X. Long-Term Fertilization Structures Bacterial and Archaeal Communities along Soil Depth Gradient in a Paddy Soil. Front. Microbiol. 2017, 8, 1516. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The Effects of Soil Depth on the Structure of Microbial Communities in Agricultural Soils in Iowa (United States). Appl. Environ. Microbiol. 2021, 87, e02673-20. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial Diversity in Soils Subjected to Long-Term Chemical Fertilization Can Be More Stably Maintained with the Addition of Livestock Manure than Wheat Straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J.; Peng, F.; Gao, P. Long-Term Combined Application of Manure and Chemical Fertilizer Sustained Higher Nutrient Status and Rhizospheric Bacterial Diversity in Reddish Paddy Soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-Term Fertilization Changes Bacterial Diversity and Bacterial Communities in the Maize Rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The Effects of Mineral Fertilizer and Organic Manure on Soil Microbial Community and Diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Delitte, M.; Caulier, S.; Bragard, C.; Desoignies, N. Plant Microbiota Beyond Farming Practices: A Review. Front. Sustain. Food Syst. 2021, 5, 624203. [Google Scholar] [CrossRef]


| Treatments | Fertilizers | January | March | May | July | Total N:P:K (kg tree−1) |
|---|---|---|---|---|---|---|
| CF | Commercial organic + compound chemical fertilizer (kg tree−1) | 10 + 1 | 0 + 0.5 | 0 + 0.5 | 0 + 0.5 | 0.44:0.18:0.39 |
| OF | Organic fermented + compound chemical fertilizer (kg tree−1) | 10 + 1 | 0 + 0.5 | 0 + 0.5 | 0 + 0.5 | 0.32:0.49:0.38 |
| SF | Organic fermented fertilizer (kg tree−1) | 10 | 5 | 5 | 5 | 0.34:0.92:0.50 |
| Soil Layer | 0–20 cm | 20–40 cm | ||||
|---|---|---|---|---|---|---|
| Soil Properties | CF | OF | SF | CF | OF | SF |
| pH | 4.66 + 0.06 b | 5.09 + 0.03 a | 5.16 + 0.05 a | 4.32 + 0.09 b | 4.75 + 0.08 a | 4.74 + 0.07 a |
| SOM (g kg−1) | 10.65 + 0.28 b | 12.27 + 0.08 a | 12.65 + 0.47 a | 10.05 + 0.13 b | 10.79 + 0.48 a | 10.17 + 0.12 ab |
| TN (g kg−1) | 0.71 + 0.02 a | 0.76 + 0.06 a | 0.74 + 0.05 a | 0.63 + 0.02 a | 0.66 + 0.04 a | 0.69 + 0.01 a |
| TP (g kg−1) | 0.75 + 0.09 a | 0.80 + 0.03 a | 0.83 + 0.05 a | 0.58 + 0.04 a | 0.62 + 0.04 a | 0.55 + 0.08 a |
| TK (g kg−1) | 21.53 + 1.04 b | 26.09 + 1.41 a | 24.31 + 0.45 a | 21.20 + 1.02 b | 26.22 + 0.80 a | 23.11 + 1.56 b |
| AN (mg kg−1) | 93.94 + 8.41 b | 121.53 + 3.69 a | 144.04 + 14.29 a | 62.23 + 2.66 b | 67.08 + 3.84 b | 90.71 + 6.32 a |
| AP (mg kg−1) | 118.60 + 3.81 c | 204.21 + 4.97 a | 145.04 + 3.08 c | 48.41 + 1.71 b | 109.18 + 1.42 a | 38.33 + 1.17 c |
| AK (mg kg−1) | 378.41 + 10.70 c | 656.57 + 13.86 a | 540.30 + 10.88 b | 364.96 + 6.03 c | 626.55 + 11.57 a | 411.8 + 10.98 b |
| Ca2+ (mg kg−1) | 1.38 + 0.10 b | 2.05 + 0.07 a | 1.85 + 0.12 a | 1.42 + 0.14 b | 1.86 + 0.10 a | 1.90 + 0.20 a |
| Mg2+ (mg kg−1) | 2.90 + 0.13 a | 3.36 + 0.23 a | 3.36 + 0.28 a | 3.39 + 0.26 a | 3.22 + 0.17 a | 3.35 + 0.17 a |
| Fruit properties | CF | OF | SF | |||
| VC (mg 100 g−1) | 39.71 + 6.43 a | 34.05 + 3.87 a | 35.03 + 6.05 a | |||
| SS (mg g−1) | 67.70 + 3.14 a | 60.26 + 6.61 a | 65.47 + 8.49 a | |||
| TA (%) | 0.25 + 0.07 a | 0.30 + 0.09 a | 0.25 + 0.09 a | |||
| FW (kg) | 44.19 + 1.17 b | 50.21 + 1.24 a | 51.54 + 0.67 a | |||
| Variables | Coefficient | p Value | p. Adjusted |
|---|---|---|---|
| pH | 0.169 | 0.027 | 0.045 * |
| SOM | 0.427 | 0.001 | 0.002 ** |
| TN | 0.103 | 0.178 | 0.223 |
| TP | 0.347 | 0.001 | 0.002 ** |
| TK | 0.191 | 0.037 | 0.053 |
| AN | 0.448 | 0.001 | 0.002 ** |
| AP | 0.428 | 0.001 | 0.002 ** |
| AK | 0.437 | 0.001 | 0.002 ** |
| Ca2+ | 0.046 | 0.296 | 0.329 |
| Mg2+ | −0.086 | 0.801 | 0.801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wei, Z.; Tao, L.; Zhong, J.; Liu, X.; Ji, J.; Lan, X.; Hou, H.; Feng, Z.; Xiao, J.; et al. Impact of Organic Fertilization Strategies on Soil Bacterial Community and Honey Pomelo (Citrus maxima) Properties. Agronomy 2024, 14, 2244. https://doi.org/10.3390/agronomy14102244
Li J, Wei Z, Tao L, Zhong J, Liu X, Ji J, Lan X, Hou H, Feng Z, Xiao J, et al. Impact of Organic Fertilization Strategies on Soil Bacterial Community and Honey Pomelo (Citrus maxima) Properties. Agronomy. 2024; 14(10):2244. https://doi.org/10.3390/agronomy14102244
Chicago/Turabian StyleLi, Jinbiao, Zhike Wei, Lin Tao, Jingqi Zhong, Xiumei Liu, Jianhua Ji, Xianjin Lan, Hongqian Hou, Zhaobin Feng, Jingshang Xiao, and et al. 2024. "Impact of Organic Fertilization Strategies on Soil Bacterial Community and Honey Pomelo (Citrus maxima) Properties" Agronomy 14, no. 10: 2244. https://doi.org/10.3390/agronomy14102244






