Impact of Pulse Electric Field Stimulation on Negative Air Ion Release Capacity of Snake Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Samples
2.2. Experimental Method
2.2.1. Pulsed Electric Field Setup
2.2.2. Measurement of Negative Air Ions
2.2.3. Measurement of Chlorophyll Content and Chlorophyll Fluorescence Parameters
2.3. Data Statistics and Analysis
3. Results
3.1. Changes in Chlorophyll Content in Snake Plant Leaves
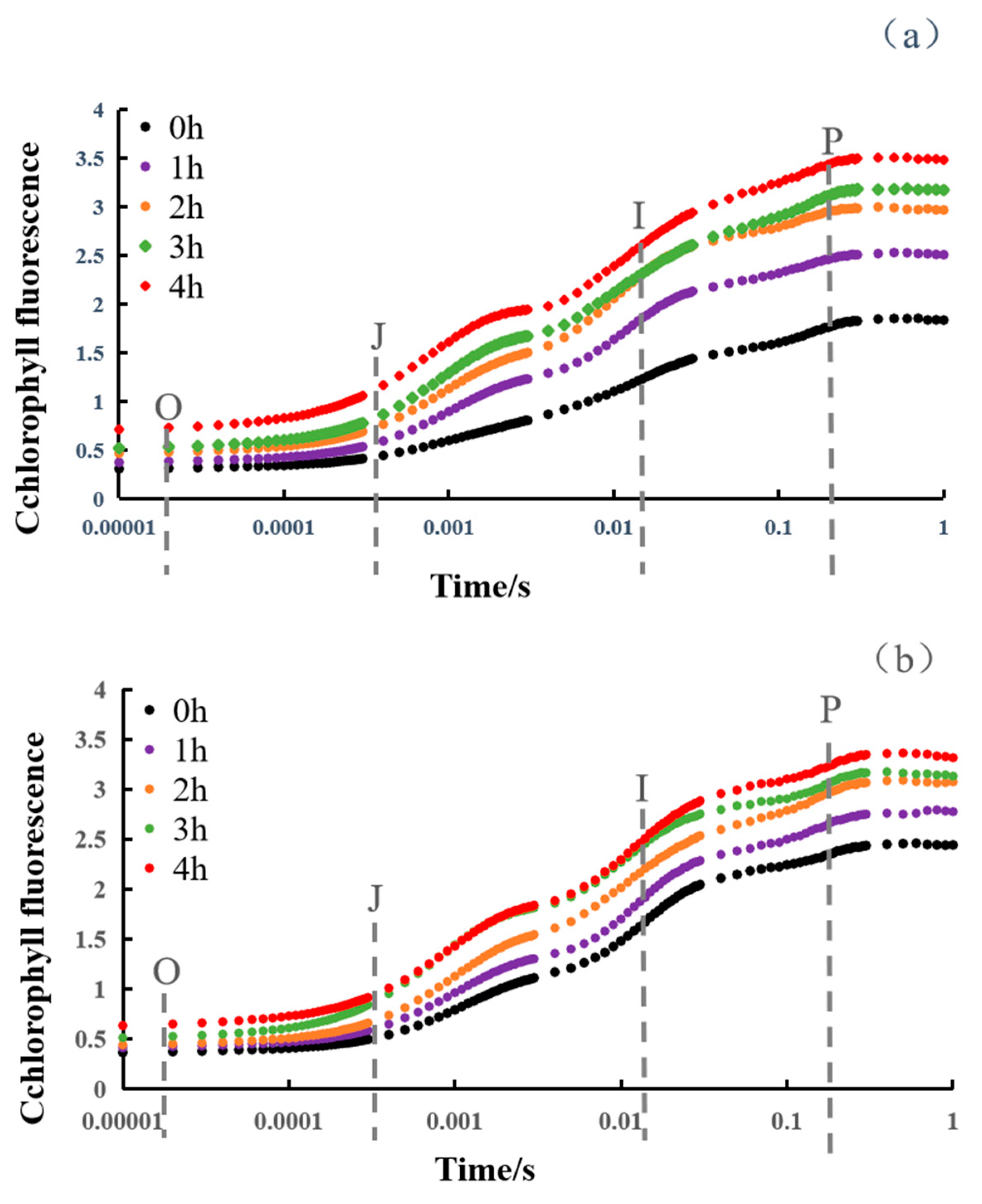
3.2. Dynamic Changes in OJIP Curve of Snake Plant Leaves
3.2.1. ChlF Rise
3.2.2. The Relative Variable Fluorescence Changes in Snake Plant Leaves
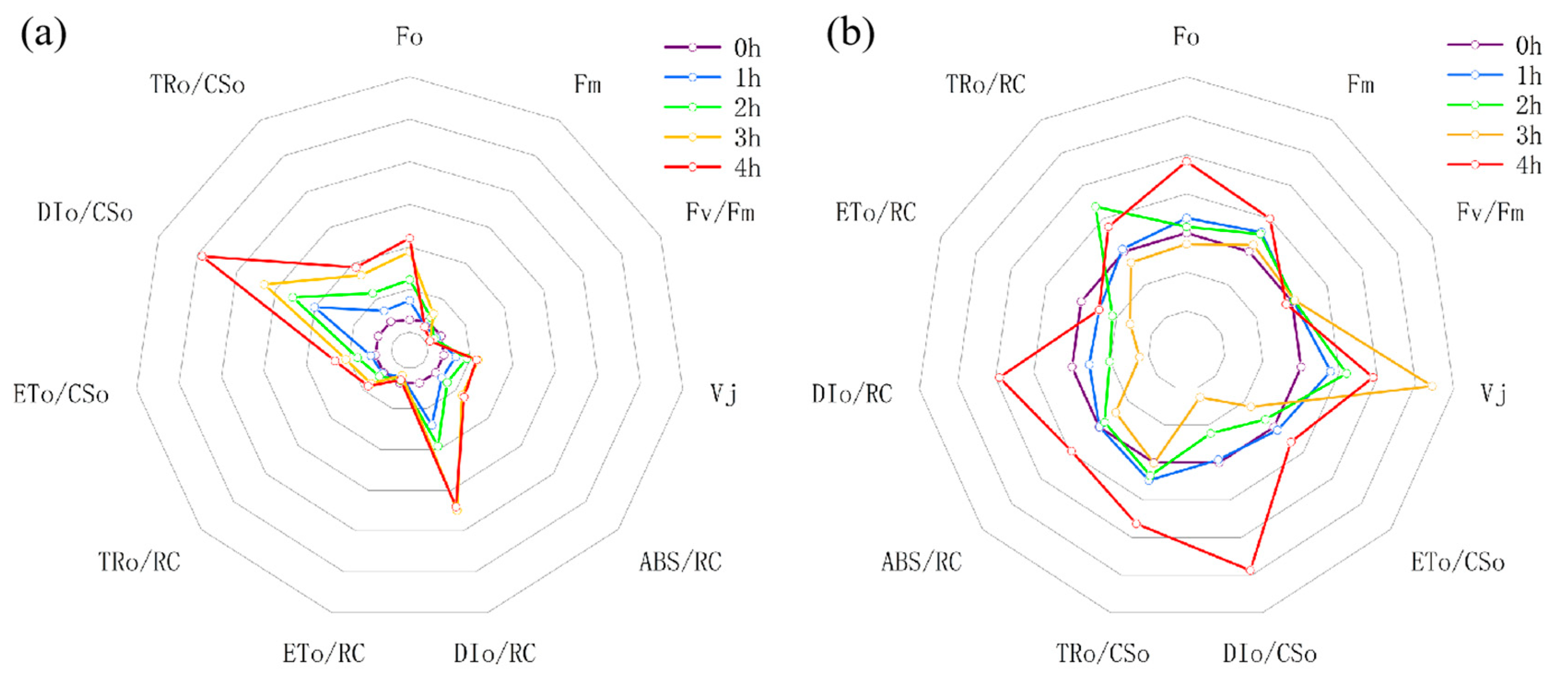
3.3. Dynamic Changes in Chlorophyll Fluorescence Parameters of Snake Plant Leaves
3.3.1. Basic Parameters
3.3.2. Specific Energy Fluxes
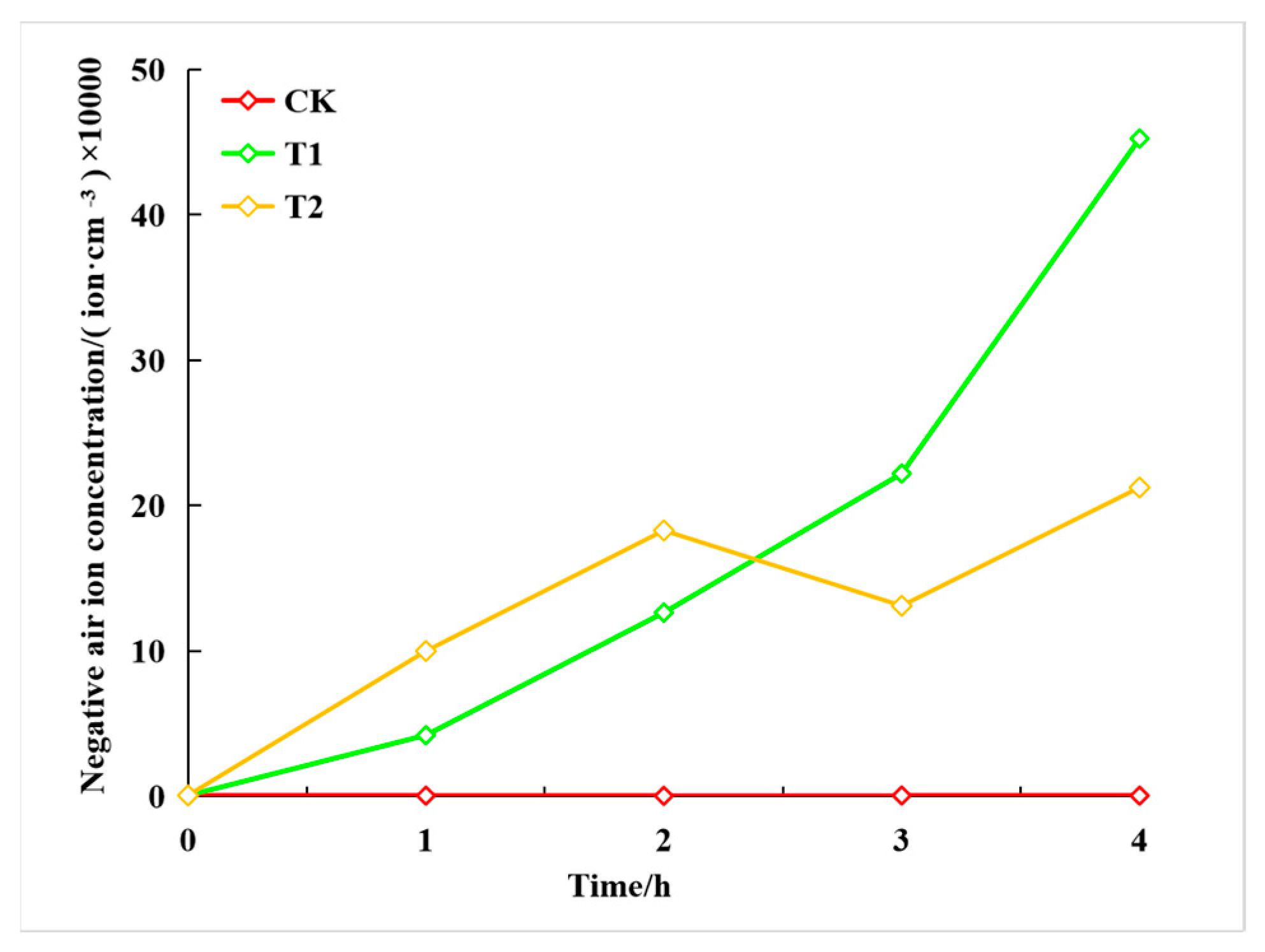
3.4. Dynamic Changes in NAI Concentration Released by Snake Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasmawati, H.; Ruslin, R.; Arfan, A.; Sida, N.A.; Saputra, D.I.; Halimah, E.; Mustarichie, R. Antibacterial potency of an active compound from Sansevieria trifasciata Prain: An integrated in vitro and in silico study. Molecules 2023, 28, 6096. [Google Scholar] [CrossRef]
- Wantahe, E.; Bigambo, P. Review of Sansevieria Ehrenbergii (SE) leaf fibers and their potential applications. Cellulose 2023, 30, 9241–9259. [Google Scholar] [CrossRef]
- Permana, B.H.; Krobthong, S.; Yingchutrakul, Y.; Thiravetyan, P.; Treesubsuntorn, C. Sansevieria trifasciata’s specific metabolite improves tolerance and efficiency for particulate matter and volatile organic compound removal. Environ. Pollut. 2024, 355, 124199. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, Y.H.K.I.; Rathnayake, I.V.N.; Deeyamulla, M.P. The entophytic and potting soil bacteria of the Sansevieria trifasciata plant have a purifying impact on indoor toluene. Int. J. Environ. Res. 2023, 17, 48. [Google Scholar] [CrossRef]
- Tikhonov, V.P.; Tsvetkov, V.D.; Litvinova, E.G.; Sirota, T.V.; Kondrashova, M.N. Generation of negative air ions by plants upon pulsed electrical stimulation applied to soil. Russ. J. Plant Physiol. 2004, 51, 414–419. [Google Scholar] [CrossRef]
- Buckley, R.; Zhong, L.; Yu, H.; Zhu, D.; Cooper, M. Health benefits of airborne terpenoids and aeroanions: Insights from thematic review of Chinese-Language research on forest sensory experiences. Environments 2024, 11, 79. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, J.; Sang, Y.; Du, L.; Ni, X.; Hu, Y. Empirical analysis of the influence of vegetation photosynthetic productivity on negative air ions in forest ecosystems driven by solar radiation. Ecol. Indic. 2024, 166, 112377. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Q.; Wu, Y.; Song, Y.; Dong, W.; Chu, M.; Yang, D.; Zhang, X.; Zhang, J.; Chen, C.; et al. Metabolic linkages between indoor negative air ions, particulate matter and cardiorespiratory function: A randomized, double-blind crossover study among children. Environ. Int. 2020, 138, 105663. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Atmaca, B.; Bulut, N.; Uzuner, S. Development of pulsed electric fields treatment unit to treat wheat grains: Improvement of seed vigour and stress tolerance. Comput. Electron. Agric. 2021, 185, 106129. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Cheng, Y.; Gao, Z.; Qu, K.; Chen, Z.; Yue, L.; Guan, W. Effects of pulsed electric field and high-pressure processing treatments on the juice yield and quality of sea buckthorn. Foods 2024, 13, 1829. [Google Scholar] [CrossRef]
- Wang, D.; Hayashi, Y.; Enoki, T.; Nakahara, K.; Arita, T.; Higashi, Y.; Kuno, Y.; Terazawa, T.; Namihira, T. Influence of pulsed electric fields on photosynthesis in Light/Dark-Acclimated Lettuce. Agronomy 2022, 12, 173. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Atmaca, B.; Uzuner, S. Corn processing by pulsed electric fields with respect to microbial inactivation and improvement of seed vigour. Comput. Electron. Agric. 2024, 219, 108830. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Center, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Sudarić, A.; Matoša Kočar, M.; Duvnjak, T.; Liović, I.; Mijić, A.; Varga, I.; Viljevac Vuletić, M. Detailed insight into the behaviour of Chlorophyll a fluorescence transient curves and parameters during different times of dark adaptation in sunflower leaves. Agronomy 2024, 14, 954. [Google Scholar] [CrossRef]
- Itam, M.; Hall, D.; Kramer, D.; Merewitz, E. Early detection of Kentucky bluegrass and perennial ryegrass responses to drought stress by measuring chlorophyll fluorescence parameters. Crop Sci. 2024, 64, 1015–1026. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Q. Hyperspectral indices developed from fractional-order derivative spectra improved estimation of leaf chlorophyll fluorescence parameters. Plants 2024, 13, 1923. [Google Scholar] [CrossRef]
- Nie, R.; Wei, X.; Jin, N.; Su, S.; Chen, X. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 2024, 103, 675–687. [Google Scholar] [CrossRef]
- Xi, G.; He, R.; Liu, K.; Zhao, Y. Effect of pulsed electric field on spontaneous photon emission of corn seedlings under osmotic stress. Trans. Chin. Soc. Agric. Eng. 2015, 31, 319–324. [Google Scholar]
- Fratianni, A.; Niro, S.; Messia, M.C.; Panfili, G.; Marra, F.; Cinquanta, L. Evaluation of carotenoids and furosine content in air dried carrots and parsnips pre-treated with pulsed electric field (PEF). Eur. Food Res. Technol. 2019, 245, 2529–2537. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Zheng, J.; Xin, G.; Wu, R. Study on the indoor application of the plants-NAI generator. J. Guangzhou Univ. Nat. Sci. Ed. 2014, 13, 29–37. [Google Scholar]
- Wu, R.; Zheng, J.; Sun, Y.; Wang, Q.; Deng, C.; Ye, D. Research on generation of negative air ions by plants and stomatal characteristics under pulsed electrical field stimulation. Int. J. Agric. Biol. 2017, 19, 1235–1245. [Google Scholar] [CrossRef]
- Wang, H. Changes of Anion Concentration and Ultrastructure of Mesophyll Cells Stimulated by Pulsed Electric Field. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- Tikhonov, V.P.; Tsvetkov, V.D.; Litvinova, E.G.; Sirota, T.V.; Kondrashova, M.N. Generation of negative air ions by wheat seedlings in a high voltage electrization of soil. Biofizika 2002, 47, 130–134. [Google Scholar] [PubMed]
- Wang, J.; Li, S. Changes in negative air ions concentration under different light intensities and development of a model to relate light intensity to directional change. J. Environ. Manag. 2009, 90, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Wu, R. The Efficient Release of Anions and Their Generator in Functional Plants. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2011. [Google Scholar]
- Strasserf, R.J.; Srivastava, A. Polyphasic Chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1994, 61, 32–42. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiey, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Shanker, A.K.; Kundu, S.; Reddy, S. Chlorophyll fluorescence induction kinetics and yield responses in rainfed crops with variable potassium nutrition in K deficient semi-arid alfisols. J. Photochem. Photobiol. B Biol. 2016, 160, 86–95. [Google Scholar] [CrossRef]
- Speranza, A.; Crosti, P.; Malerba, M.; Stocchi, O.; Scoccianti, V. The environmental endocrine disruptor, bisphenol A, affects germination, elicits stress response and alters steroid hormone production in kiwifruit pollen. Plant Biol. 2011, 13, 209–217. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhang, C.; Luo, J.; Jiang, N.; Zhang, F.; Zhu, W. Heat stress recovery of chlorophyll fluorescence in tomato (Lycopersicon esculentum Mill.) leaves through nitrogen levels. Agronomy 2023, 13, 2858. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Using of chlorophyll a fluorescence OJIP transients for sensing salt stress in the leaves and fruits of tomato. Sci. Hortic. 2017, 219, 216–221. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, L.; Zhang, S.; Wang, J.; Xie, S.; Zhao, M.; Chen, G.; Sun, R.; Wang, C. Comprehensive evaluation for cold tolerance in Wucai (Brassica campestris L.) by the performance index on an absorption basis (PIabs). Agronomy 2019, 9, 61. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Vilas, J.M.; Sartore, G.D.; Bezus, R.; Colazo, J.; Maiale, S.J. Field and genetic evidence support the photosynthetic performance index (PIABS) as an indicator of rice grain yield. Plant Physiol. Biochem. 2023, 201, 107897. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Stockel, J.; Srivastava, A. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.D.C.V.; Bessa, L.A.; Silva, F.G.; Rosa, M.; Vasconcelos Filho, S.D.C.; Vitorinol, L.C. Morpho-Anatomical and physiological responses can predict the ideal period for the transplantation of hydroponic seedlings of Hymenaea courbaril, a neotropical fruit tree. Plants 2020, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Athar, H.U.; Ambreen, S.; Javed, M.; Hina, M.; Rasul, S.; Zafar, Z.U.; Manzoor, H.; Ogbaga, C.C.; Afzal, M.; Al-Qurainy, F.; et al. Influence of sub-lethal crude oil concentration on growth, water relations and photosynthetic capacity of maize (Zea mays L.) plants. Environ. Sci. Pollut. Res. Int. 2016, 23, 18320–18331. [Google Scholar] [CrossRef] [PubMed]
- Chiango, H.; Figueiredo, A.; Sousa, L.; Sinclair, T.; Da Silva, J.M. Assessing drought tolerance of traditional maize genotypes of Mozambique using chlorophyll fluorescence parameters. S. Afr. J. Bot. 2021, 138, 311–317. [Google Scholar] [CrossRef]
- Snider, J.L.; Thangthong, N.; Pilon, C.; Virk, G.; Tishchenko, V. OJIP-fluorescence parameters as rapid indicators of cotton (Gossypium hirsutum L.) seedling vigor under contrasting growth temperature regimes. Plant Physiol. Biochem. 2018, 132, 249–257. [Google Scholar] [CrossRef]
- Weng, H.; Liu, Y.; Captoline, I.; Li, X.; Ye, D.; Wu, R. Citrus Huanglongbing detection based on polyphasic chlorophyll a fluorescence coupled with machine learning and model transfer in two citrus cultivars. Comput. Electron. Agric. 2021, 187, 106289. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, C.; Pei, W.; Fan, K.; Shen, W. Chlorophyll a fluorescence as a tool to monitor physiological status in the leaves of Artemisia ordosica under root cutting conditions. Front. Plant Sci. 2023, 14, 1308209. [Google Scholar] [CrossRef]
- Weng, H.; Wu, M.; Li, X.; Wu, L.; Li, J.; Atoba, T.O.; Zhao, J.; Wu, R.; Ye, D. High-throughput phenotyping salt tolerance in JUNCAOs by combining prompt chlorophyll a fluorescence with hyperspectral spectroscopy. Plant Sci. 2023, 330, 111660. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Differential heat sensitivity index in barley cultivars (Hordeum vulgare L.) monitored by chlorophyll a fluorescence OKJIP. Plant Physiol. Biochem. 2016, 105, 102–108. [Google Scholar] [CrossRef]

| Treatment Group | SPAD Value of Leaf at Different Times | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| 0 kv (CK) | 71.92 ± 0.79 a | 71.12 ± 1.69 b | 70.05 ± 1.58 b | 70.39 ± 1.16 b | 72.94 ± 0.81 b |
| 5 kv (T1) | 72.54 ± 0.86 a | 75.72 ± 0.74 a | 74.46 ± 2.65 a | 76.50 ± 0.85 a | 77.11 ± 0.69 a |
| 7 kv (T2) | 72.44 ± 0.98 a | 75.73 ± 0.63 a | 75.22 ± 2.30 a | 75.51 ± 0.71 a | 77.28 ± 1.81 a |
| Treatment Group | Change in Chlorophyll Fluorescence Parameter Values of Ga at Different Times 2 | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| 0 kv (CK) Fv/Fo | 5.25 ± 0.14 b | 5.17 ± 0.68 a | 5.41 ± 0.23 a | 5.22 ± 0.27 a | 5.39 ± 0.15 ab |
| 5 kv (T1) Fv/Fo | 5.53 ± 0.09 a | 5.36 ± 0.26 a | 5.26 ± 0.27 a | 5.14 ± 0.28 a | 5.10 ± 0.23 b |
| 7 kv (T2) Fv/Fo | 5.42 ± 0.30 ab | 5.42 ± 0.29 a | 5.40 ± 0.24 a | 5.41 ± 0.19 a | 5.30 ± 0.24 a |
| 0 kv (CK) Sm | 27.38 ± 5.78 a | 27.44 ± 4.37 a | 28.78 ± 4.82 a | 28.56 ± 5.26 a | 28.46 ± 4.91 a |
| 5 kv (T1) Sm | 29.07 ± 5.24 a | 29.73 ± 4.78 a | 29.73 ± 0.01 a | 28.32 ± 4.29 a | 27.72 ± 4.17 a |
| 7 kv (T2) Sm | 27.47 ± 3.09 a | 28.00 ± 3.25 a | 27.12 ± 3.49 a | 27.96 ± 4.90 a | 26.77 ± 3.73 a |
| 0 kv (CK) φEo | 0.52 ± 0.02 a | 0.53 ± 0.01 a | 0.52 ± 0.02 a | 0.50 ± 0.02 b | 0.51 ± 0.02 a |
| 5 kv (T1) φEo | 0.52 ± 0.01 a | 0.51 ± 0.01 a | 0.51 ± 0.01 a | 0.52 ± 0.00 a | 0.50 ± 0.01 a |
| 7 kv (T2) φEo | 0.53 ± 0.02 a | 0.52 ± 0.02 a | 0.52 ± 0.02 a | 0.52 ± 0.02 a | 0.51 ± 0.02 a |
| 0 kv (CK) PIabs | 9.05 ± 1.51 a | 8.89 ± 2.15 a | 9.24 ± 1.65 a | 9.16 ± 1.65 a | 9.47 ± 1.50 a |
| 5 kv (T1) PIabs | 9.77 ± 0.30 a | 9.22 ± 0.64 a | 8.81 ± 0.41 a | 8.42 ± 0.28 a | 8.24 ± 0.53 a |
| 7 kv (T2) PIabs | 10.75 ± 1.4 a | 10.25 ± 11 a | 10.09 ± 1.27 a | 9.83 ± 1.98 a | 9.42 ± 1.51 a |
| 0 kv (CK) PItotal | 3.20 ± 0.70 a | 2.94 ± 0.97 b | 3.12 ± 0.71 a | 2.95 ± 0.68 b | 3.22 ± 0.57 a |
| 5 kv (T1) PItotal | 3.25 ± 1.31 a | 3.23 ± 0.65 a | 3.13 ± 0.58 a | 3.00 ± 0.58 a | 2.87 ± 1.81 a |
| 7 kv (T2) PItotal | 3.29 ± 0.81 a | 3.19 ± 0.72 b | 3.15 ± 0.87 a | 3.10 ± 0.94 a | 2.92 ± 0.83 a |
| Treatment Group | Negative Ion Concentration Changes/(ion·cm−3) | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| 0 V (CK) | 521.36 ± 17.86 a | 406.25 ± 32.60 b | 364.45 ± 12.35 b | 539.33 ± 14.29 b | 400.78 ± 10.25 b |
| 5 kv (T1) | 526.17 ± 19.67 a | 41,986.22 ± 5293.44 a | 126,217.45 ± 119,339.75 a | 222,001.80 ± 256,322.90 a | 416,466.58 ± 328,669.54 a |
| 7 kv (T2) | 592.93 ± 21.17 a | 32,412.12 ± 25,969.57 ab | 182,844.66 ± 93,041.30 a | 131,039.77 ± 44,299.84 a | 212,401.10 ± 91,149.66 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Huang, D.; Cheng, Z.; Wu, R. Impact of Pulse Electric Field Stimulation on Negative Air Ion Release Capacity of Snake Plants. Agronomy 2024, 14, 2248. https://doi.org/10.3390/agronomy14102248
Liu J, Huang D, Cheng Z, Wu R. Impact of Pulse Electric Field Stimulation on Negative Air Ion Release Capacity of Snake Plants. Agronomy. 2024; 14(10):2248. https://doi.org/10.3390/agronomy14102248
Chicago/Turabian StyleLiu, Jin, Deyao Huang, Zhiyuan Cheng, and Renye Wu. 2024. "Impact of Pulse Electric Field Stimulation on Negative Air Ion Release Capacity of Snake Plants" Agronomy 14, no. 10: 2248. https://doi.org/10.3390/agronomy14102248





