Screening Low-Cadmium and High-Mineral Nutrient Rapeseed (Brassica napus L.) Cultivars According to the Uptake and Transport Characteristics of Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement Items and Methods
2.2.1. Determination of Dry Matter Accumulation in Rapeseed
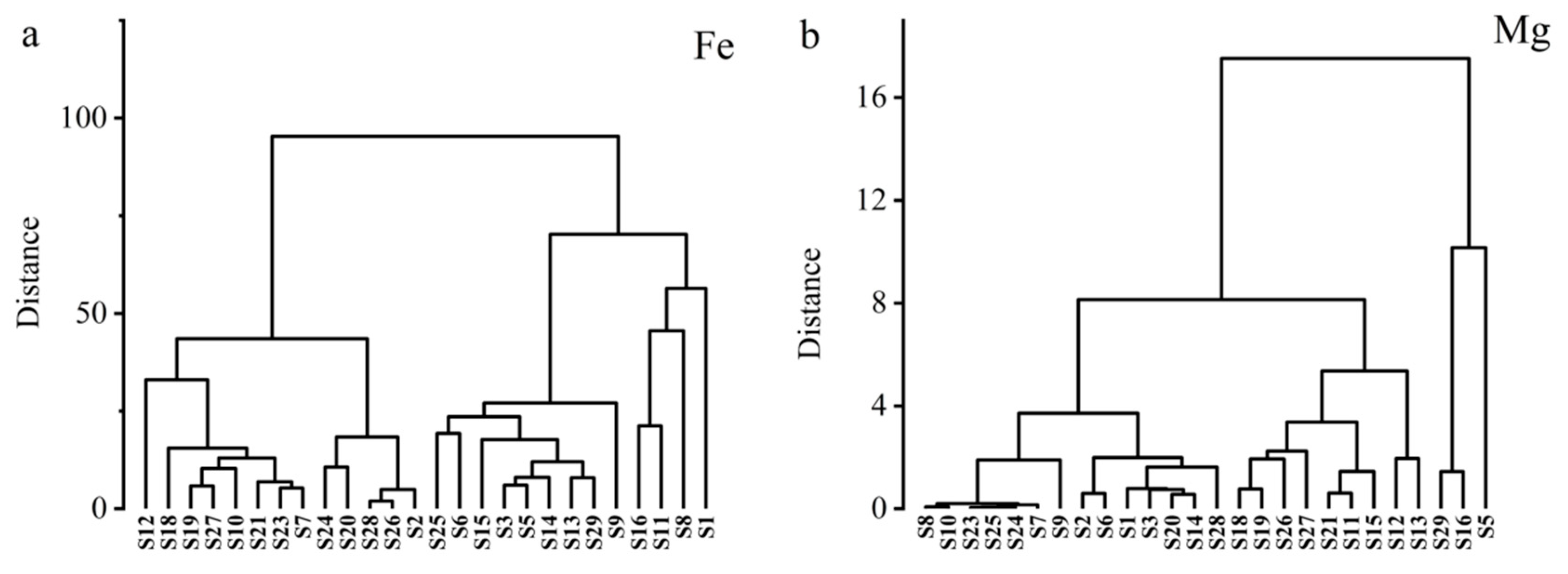
2.2.2. Determination of Nutrient Element Content in Different Parts of Rapeseed
2.2.3. Absorption and Translocation Capacity to Cd of Rapeseed
2.3. Data Analysis
3. Results
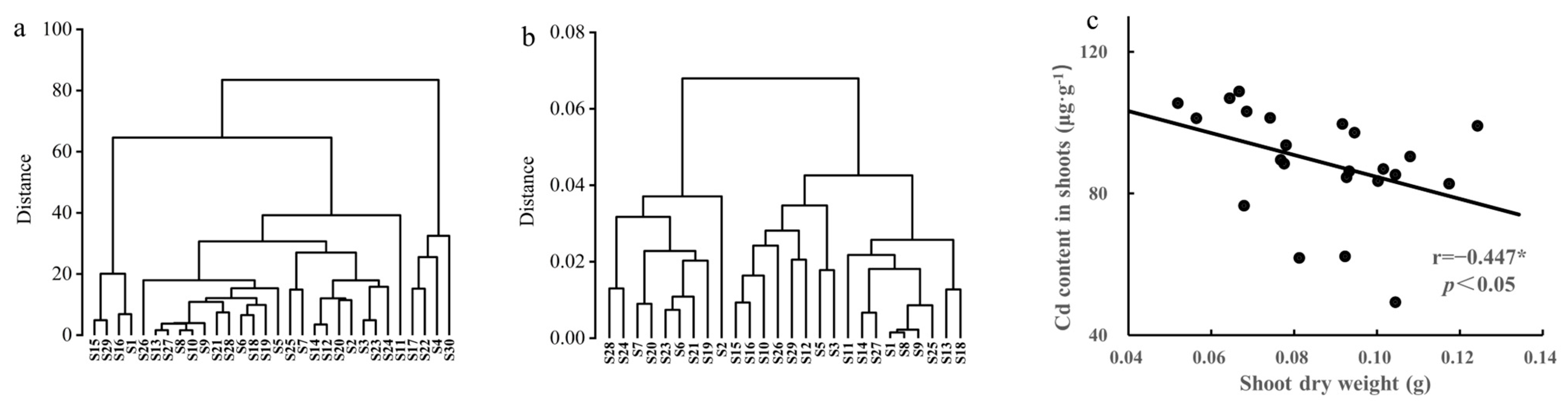
3.1. Differences in Plant Biomass among Different Rapeseed Cultivars
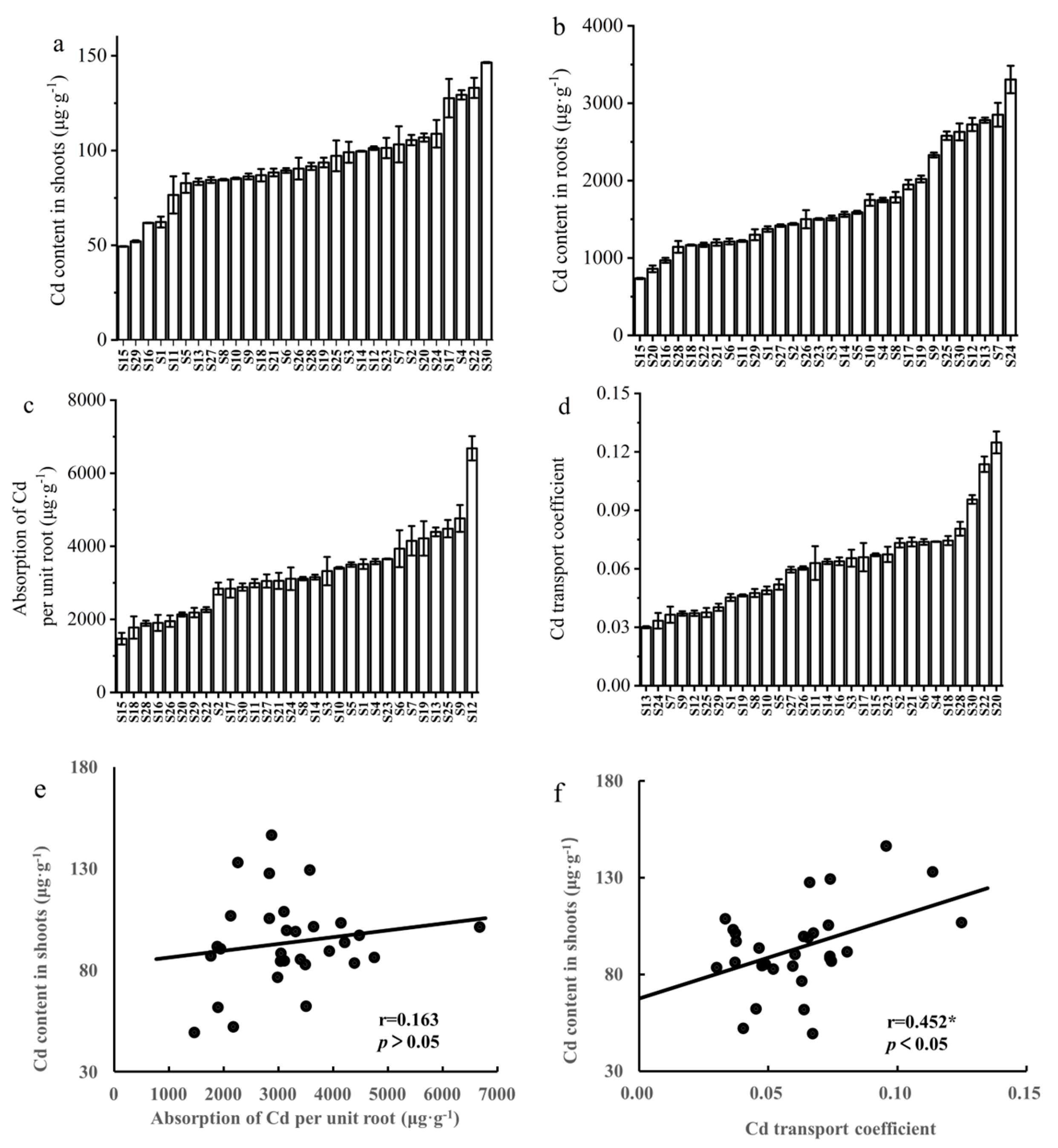
3.2. Variations in Cd Uptake and Transportation among Different Rapeseed Cultivars
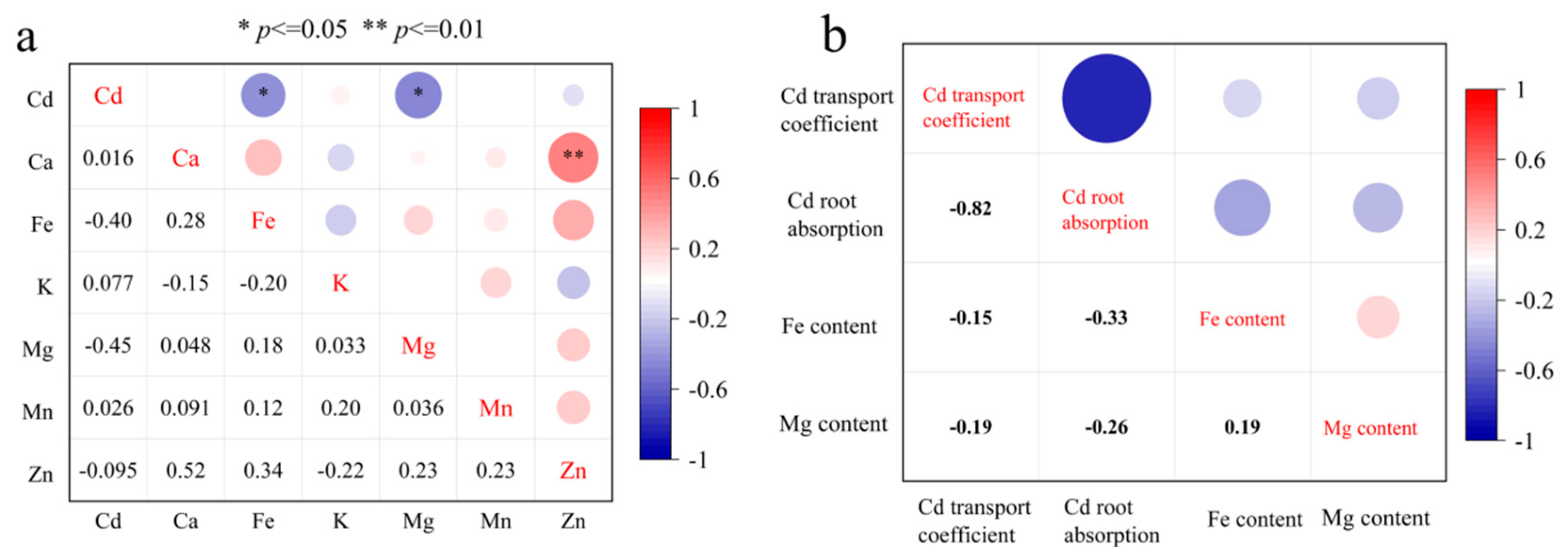
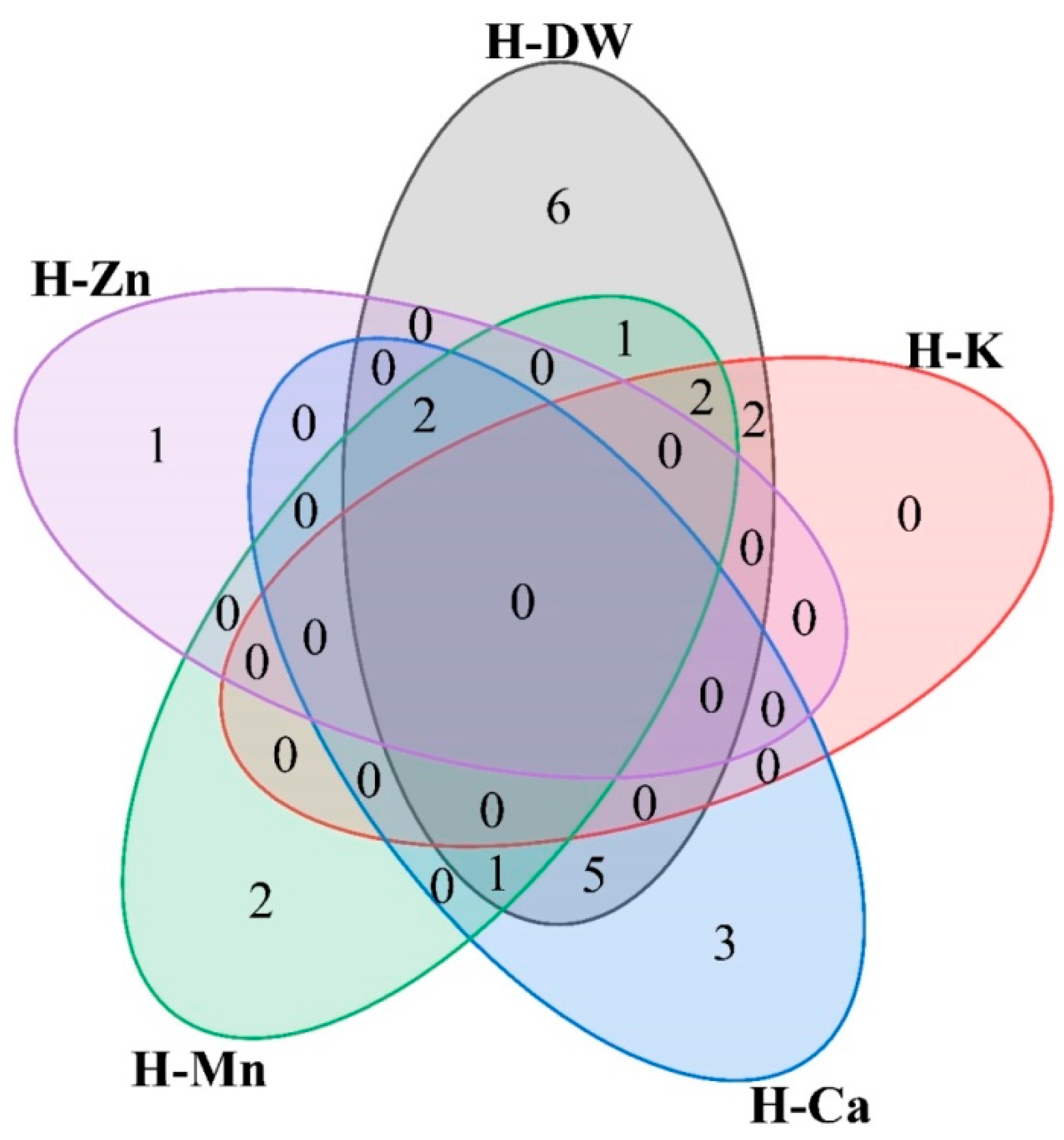
3.3. Selection of Rapeseed Cultivars with Low Cd and High Nutritional Element Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peralta-Videa, J.; Zhao, L.; Lopez-Moreno, M.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.; Antunes, M. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. Vol. 2017, 241, 73–137. [Google Scholar]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Della Rovere, F.; Eiche, E.; Riemann, M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar] [CrossRef]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron mitigates cadmium toxicity to rapeseed (Brassica napus) shoots by relieving oxidative stress and enhancing cadmium chelation onto cell walls. Environ. Pollut. 2020, 263, 114546. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, H.; Tang, X.; Li, J.; Zhang, Z.; Chai, G.; Wu, X. NPs-Ca promotes Cd accumulation and enhances Cd tolerance of rapeseed shoots by affecting Cd transfer and Cd fixation in pectin. Chemosphere 2023, 341, 140001. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Peng, S.; Cai, L.; Li, S. Advances in soil cadmium pollution remediation methods and bioremediation research. Environ. Dev. 2014, 3, 86–90. [Google Scholar]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef]
- Liu, S.; Fan, C.; Li, X.; Huang, J. Breeding strategies for improving the yield and quality of oilseed crops: A review. Int. J. Mol. Sci. 2019, 20, 4482. [Google Scholar]
- Zhang, Z.; Zhou, T.; Tang, T.; Song, H.; Guan, C.; Huang, J.; Hua, Y. A multiomics approach reveals the pivotal role of subcellular reallocation in determining rapeseed resistance to cadmium toxicity. J. Exp. Bot. 2019, 70, 5437–5455. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Broadley, M. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, H.; An, D. Screening and identification of wild diploid wheat and its progeny with enriched micronutrient germplasm resources. Chin. J. Eco-Agric. 2011, 19, 1205–1209. [Google Scholar] [CrossRef]
- Fan, Y.; Zhuang, Z.; Zhao, L.; Zhang, C.; Wang, Q.; Li, H.; Wan, Y. Uptake and transport characteristics of cadmium and nutrients in different cultivars of maize (Zea mays) at seedling stage. J. Agro-Environ. Sci. 2023, 42, 744–753. [Google Scholar]
- Wu, J.; Li, R.; Lu, Y.; Bai, Z. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J. Environ. Manag. 2021, 295, 113081–113095. [Google Scholar] [CrossRef]
- Peng, T.; Yu, X.; Cheng, Z.; Ping, Z.; Xiao, Z. Transcriptional analysis of heavy metal P1B-ATPases (HMAs) elucidates competitive interaction in metal transport between cadmium and mineral elements in rice plants. Environ. Sci. Pollut. Res. 2023, 30, 287–297. [Google Scholar]
- Wang, R. China’s grain and oil production, sales, and import and export situation. China Oils Fats 2022, 48, 1–7. [Google Scholar]
- Lu, G.; Zhang, F.; Zheng, P.; Cheng, Y.; Feng, L.; Fu, G.; Zhang, X. Relationship among yield components and selection criteria for yield improvement in early rapeseed (Brassica napus L.). Agric. Sci. China 2011, 10, 997–1003. [Google Scholar] [CrossRef]
- Khan, M.; Dar, Z.; Dar, S. Breeding strategies for improving rice yield—A review. Agric. Sci. 2015, 6, 467. [Google Scholar] [CrossRef][Green Version]
- Schiessl, S.; Quezada-Martinez, D.; Orantes-Bonilla, M.; Snowdon, R. Transcriptomics reveal high regulatory diversity of drought tolerance strategies in a biennial oil crop. Plant Sci. 2020, 297, 110515. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.; Pogoda, C.; Reinert, S.; Kane, N.; Hulke, B. Breeding for sustainable oilseed crop yield and quality in a changing climate. Theor. Appl. Genet. 2021, 134, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, X. Differences in plant growth, Cd and nutrient uptake, Cd translocation between two tobacco cultivars under Cd stress. J. Soil Water Conserv. 2009, 117–131. [Google Scholar]
- Hoagland, D.; Arnon, D. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 357–359. [Google Scholar]
- Wu, X.; Tian, H.; Li, L.; Guan, C.; Zhang, Z. Higher Cd-accumulating oilseed rape has stronger Cd tolerance due to stronger Cd fixation in pectin and hemicellulose and higher Cd chelation. Environ. Pollut. 2021, 285, 117218. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Guo, Z.; Shi, L.; Feng, W.; Xiao, X.; Peng, C. Effects of mixed amendments on the phytoavailability of cd in contaminated paddy soil under a rice-rape rotation system. Environ. Sci. Pollut. Res. 2019, 26, 14128–14136. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 267, 447–455. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Rengel, Z.; Gao, W.; Nie, Z.; Li, C.; Hou, M.; Cheng, J.; Zhao, P. Boron inhibits cadmium uptake in wheat (Triticum aestivum) by regulating gene expression. Plant Sci. 2020, 297, 110522. [Google Scholar] [CrossRef]
- Deng, L.; Li, M.; Liu, X.; Liu, G.; Zhou, X.; Fan, L.; Qu, L. Screening and evaluation of rapeseed cultivars suitable for planting in cadmium-contaminated farmland. Mol. Plant Breed. 2023, 1–10. [Google Scholar]
- He, X.; Wang, J.; Li, W.; Wu, C.; Long, H. Effects of cadmium stress on growth and cadmium accumulation characteristics of Brassica napus L. seedings. Heilongjiang Agric. Sci. 2023, 2, 59–65. [Google Scholar]
- Zou, J.; Lu, J.W.; Chen, F.; Li, Y. Application of ICP-MS to detection of mineral elements in double-low and double-high rapeseed. Spectrosc. Spectr. Anal. 2009, 9, 2571–2573. [Google Scholar]
- Caldelas, C.; Weiss, D. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Sadana, U.; Samal, D.; Claassen, N. Differences in manganese efficiency of wheat (Triticum aestivum L.) and raya (Brassica juncea L.) as related to root-shoot relations and manganese influx. J. Plant Nutr. Soil Sci. 2003, 166, 385–389. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Wang, F.; Pan, X.; Zhu, C.; Jiang, D. Characterization of cadmium uptake and translocation in a cadmium-sensitive mutant of rice (Oryza sativa L. ssp. japonica). Arch. Environ. Contam. Toxicol. 2009, 57, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R. How Does Contamination of Rice Soils with Cd and Zn Cause High Incidence of Human Cd Disease in Subsistence Rice Farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Li, W.; Chen, R.; Zhao, Y.; Tang, Q.; Liu, Z. Effects of manganese concentrations and transporters on uptake and translocation of cadmium in rice seedlings. J. Agro-Environ. Sci. 2016, 35, 1429–1435. [Google Scholar]
- Zou, R.; Wang, L.; Li, Y.C.; Tong, Z.; Huo, W.; Chi, K.; Fan, H. Cadmium absorption and translocation of amaranth (Amaranthus mangostanus L.) affected by iron deficiency. Environ. Pollut. 2020, 256, 113410. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Huang, X.; Chen, Y.; Lv, C.; Bai, L.; Dai, J. Cultivar-specific response of rhizosphere bacterial community to uptake of cadmium and mineral elements in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2023, 249, 114403. [Google Scholar] [CrossRef]
- Luo, Q.; Bai, B.; Xie, Y.; Yao, D.; Chen, Z.; Zhang, D.; Wu, J. Spatial ionomics provides new insights into the accumulation and transport of mineral ions in rice (Oryza sativa L.) under Cadmium stress. Environ. Exp. Bot. 2023, 208, 105267. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M. Iron-and manganese-assisted cadmium tolerance in Oryza sativa L.: Lowering of rhizotoxicity next to functional photosynthesis. Planta 2015, 241, 1519–1528. [Google Scholar] [CrossRef]
- Yang, C.; Juang, K. Alleviation effects of calcium and potassium on cadmium rhizotoxicity and absorption by soybean and wheat roots. J. Plant Nutr. Soil Sci. 2015, 178, 748–754. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, Y.; Yin, C.; Liu, X. Effects of nitrogen reduction at different growth stages on maize water and nitrogen utilization under shallow buried drip fertigated irrigation. Agronomy 2024, 14, 63. [Google Scholar] [CrossRef]
- Wu, X.; Riaz, M.; Yan, L.; Jiang, C. Distribution and mobility of foliar-applied boron (10B) in citrange rootstock under different boron conditions. J. Plant Growth Regul. 2020, 39, 575–582. [Google Scholar] [CrossRef]
- Liu, L.; Cui, K.; Qi, X.; Wu, Y.; Huang, J.; Peng, S. Varietal responses of root characteristics to low nitrogen application explain the differing nitrogen uptake and grain yield in two rice varieties. Front. Plant Sci. 2023, 14, 1244281. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhu, S.; Bai, S.; Xia, Y.; Luo, L.; Cai, Q. The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. J. Hazard. Mater. 2015, 299, 94–102. [Google Scholar] [CrossRef]
- Deng, T.; Wu, J.; Lu, W.; Guan, Y.; Li, G.; Zhang, Q.; Zeng, Z. Differences in cadmium accumulation and translocation in different Zea mays cultivars. J. Agro-Environ. Sci. 2019, 38, 1265–1271. [Google Scholar]


| Number | Cultivar | Source |
|---|---|---|
| S1 | BN365 | Hunan Agricultural University |
| S2 | BN170 | |
| S3 | BN275 | |
| S4 | BN328 | |
| S5 | BN269 | |
| S6 | BN141 | |
| S7 | BN219 | |
| S8 | BN048 | |
| S9 | BN017 | |
| S10 | BN086 | |
| S11 | BN085 | |
| S12 | BN129 | |
| S13 | BN112 | |
| S14 | BN123 | |
| S15 | BN067 | |
| S16 | BN121 | |
| S17 | OQ232 | |
| S18 | QW343 | |
| S19 | QW303 | |
| S20 | QW330 | |
| S21 | QW269 | |
| S22 | QW305 | |
| S23 | ZQ071 | |
| S24 | ZQ484 | |
| S25 | ZQ175 | |
| S26 | OJ114 | |
| S27 | OJ125 | |
| S28 | OJ117 | |
| S29 | HUA42 | |
| S30 | LV35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Tian, H.; Zhang, H.; Chai, G.; Wu, X. Screening Low-Cadmium and High-Mineral Nutrient Rapeseed (Brassica napus L.) Cultivars According to the Uptake and Transport Characteristics of Elements. Agronomy 2024, 14, 2258. https://doi.org/10.3390/agronomy14102258
Tang X, Tian H, Zhang H, Chai G, Wu X. Screening Low-Cadmium and High-Mineral Nutrient Rapeseed (Brassica napus L.) Cultivars According to the Uptake and Transport Characteristics of Elements. Agronomy. 2024; 14(10):2258. https://doi.org/10.3390/agronomy14102258
Chicago/Turabian StyleTang, Xu, Hui Tian, Haoran Zhang, Guohua Chai, and Xiuwen Wu. 2024. "Screening Low-Cadmium and High-Mineral Nutrient Rapeseed (Brassica napus L.) Cultivars According to the Uptake and Transport Characteristics of Elements" Agronomy 14, no. 10: 2258. https://doi.org/10.3390/agronomy14102258
APA StyleTang, X., Tian, H., Zhang, H., Chai, G., & Wu, X. (2024). Screening Low-Cadmium and High-Mineral Nutrient Rapeseed (Brassica napus L.) Cultivars According to the Uptake and Transport Characteristics of Elements. Agronomy, 14(10), 2258. https://doi.org/10.3390/agronomy14102258






