Effects of Organic Agricultural Materials and Cultivation Methods on the Control of Ginger Rhizome Rot Disease and Growth in Organic Ginger Farming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Ginger Cultivation Field
2.3. Culture and Treatment of the Microalga and Antimicrobial Microorganisms
2.4. Organic Agricultural Materials
2.4.1. Lime–Sulfur Mixture
2.4.2. Compost Tea
2.4.3. Essential Oils
2.5. Eco-Friendly Disinfection Methods for Ginger Rhizome
2.6. Estimation of Control Effect of Ginger Rhizome Rot
- 0: no disease symptoms;
- 1: less than 5% of the plant affected;
- 3: 6–25% of the plant showing symptoms;
- 5: 26–50% of rhizome rot;
- 7: 51–75% of rhizome rot;
- 9: more than 75% of rhizome rot.
2.7. Experimental Data Collection and Statistical Analysis
3. Results
3.1. Evaluation of Lime–Sulfur Mixture Contents for Disinfection of Ginger Rhizome Seeds
3.2. Comparison of Emergence Rates and Fresh Weight of Organic Ginger Rhizome Seeds Treated with Antifungal Bacterial Isolates and Microalga
3.3. Comparison of Ginger Yield by Ridge Width (Single Row, Flat Row) and Organic Agricultural Material Treatment
3.4. Growth Promotion Effects of Organic Ginger by Soil Treatment with Organic Agricultural Material
3.5. Comparison of Organic Ginger Rhizome Rot Infection Rates by Ginger Variety, Ridge Width, and Organic Agricultural Material Treatment
3.6. Distribution of Pathogens by Ginger Variety, Ridge Width, and Organic Agricultural Material Treatment
3.7. Comparison of Ginger Yield by Variety and Organic Agricultural Material Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzanna, R.; Lindmark, L.; Frondoza, C. Ginger an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food. 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Ko, M.S.; Kim, H.J.; Kwak, I.S.; Kim, D.H.; Chung, B.W. Separation of 6-gingerol from ginger and antioxidative activity. Korean J. Biotechnol. Bioeng. 2006, 21, 484–488. [Google Scholar]
- Jo, M.H.; Ham, I.K.; Lee, G.H.; Lee, J.K.; Lee, G.S.; Park, S.K.; Kim, T.I.; Lee, E.M. Comparison of active ingredients between field grown and in vitro cultured rhizome of Korean native ginger (Zinger officinale Roscoe). Korean J. Plant Res. 2011, 24, 404–4012. (In Korean) [Google Scholar] [CrossRef]
- Kryama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar]
- Pandey, A.; Pandey, S.T.; Yadav, A.; Tiwan, S.K.; Srivastava, N. A Review on Family Zingiberaceae. Int. J. Med. Pharm. Res. 2023, 4, 354–360. [Google Scholar]
- Bhai, R.S.; Kishore, V.K.; Kumar, A.; Anandaraj, M. Screening of rhizobacterial isolates against soft-rot disease of ginger (Zingiber officinale). J. Spices Aromat. Crops 2005, 14, 130–136. [Google Scholar]
- MAFRA. 2022, Agriculture and Food Statistics Yearbook; Ministry of Agriculture, Food, and Rural Affairs: Seojong, Republic of Korea, 2023.
- Choi, J.Y.; Lee, Y.M.; Kim, J.Y.; Kim, J.S.; Jeong, S.E.; Park, S.H.; Kim, M.H.; Moon, K.D. Physicochemical properties and antioxidant activities of ginger (Zingiber officinale Roscoe) slices according to temperature and duration of hot water treatment. Korean J. Food Preserv. 2021, 28, 716–726. [Google Scholar] [CrossRef]
- Dohroo, N.P.; Kansal, S.; Ahluwalia, N. Studies on eco-farmer-friendly practices for management of soft-rot of ginger (Zingiber officinale). Indian Phytopathol. 2015, 68, 93–96. [Google Scholar]
- Kim, D.E. Effect of Artificial Shading and Mulching Methods on the Growth and Yield of Ginger (Zingiber officinale Rosc.). Master’s Thesis, Kongju National University, Kongju, Republic of Korea, 2007. [Google Scholar]
- Lee, W.C. A Fact-Finding Survey on the Farming Status of Ginger (Zingiber officinale Rosc.) Cultivation. Master’s Thesis, Kongju National University, Kongju, Republic of Korea, 2008. [Google Scholar]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Nair, K.P. Production, Marketing, and Economics of Ginger. In Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices; Springer: Cham, Switzerland, 2019. [Google Scholar]
- KSPP. List of Plant Diseases in Korea, 6th ed.; The Korean Society of Plant Pathology: Seoul, Republic of Korea, 2024. [Google Scholar]
- Yadav, D.; Gaurav, H.; Yadav, R.; Waris, R.; Afzal, K.; Shukla, A.C. A comprehensive review on soft rot disease management in ginger (Zingiber officinale) for enhancing its pharmaceutical and industrial values. Heliyon 2023, 4, e18337. [Google Scholar] [CrossRef]
- Jung, Y.J.; Nou, I.S.; Kim, Y.K.; Kang, K.K. Effect of green manure crops incorporation for reduction of Pythium zingiberum in ginger continuous cultivation. Korean J. Plant Res. 2015, 28, 271–278. (In Korean) [Google Scholar] [CrossRef]
- Mahesha, H.S. Integrated Management of Rhizome Rot and Wilt Disease Complex of Ginger. Ph.D. Thesis, University of Agricultural Sciences, Dharwad, India, 2020. [Google Scholar]
- Yang, K.D.; Kim, H.M.; Lee, W.H.; So, I.Y. Studies on rhizome rot of ginger caused by Fusarium oxysporum f. sp. zingiberi and Pythium zingiberum. Plant Pathology J. 1988, 4, 271–277. [Google Scholar]
- Ishii, M.; Aragaki, M. Ginger wilt caused by Pseudomonas solanacearum E.F. Smith. Plant Dis. Rep. 1963, 47, 710–713. [Google Scholar]
- Choi, I.Y.; Lee, W.H.; So, I.Y. Effects of chemicals on growth of Pythium zingiberum causing rhizome rot of ginger and Inhibition of the disease development. Plant Pathol. J. 1996, 12, 331–335. [Google Scholar]
- Maisuria, V.; Nerurkar, A. Characterization and differentiation of soft rot causing Pectobacterium carotovorum of Indian origin, European. J. Plant Pathol. 2013, 136, 87–102. [Google Scholar] [CrossRef]
- Prameela, T.P.; Bhai, R.S. Bacterial wilt of ginger (Zingiber officinale Rosc.) incited by Ralstonia pseudosolanacearum—A review based on pathogen diversity, diagnostics and management. J. Plant Pathol. 2020, 102, 709–719. [Google Scholar] [CrossRef]
- Kim, B.R.; Kwon, M.K.; Hahm, S.S.; Kim, Y.J.; Lee, S.G.; Lee, S.B. Determination of economic thresholds for rhizome diseases of ginger (Zingiber officinale). Korean J. Pest. Sci. 2019, 23, 251–256. [Google Scholar] [CrossRef]
- Stirling, G.R.; Eden, L.M.; Ashley, M.G. Sudden wilt of capsicum in tropical and subtropical Australia: A severe form of Pythium root rot exacerbated by high soil temperatures. Australas. Plant Pathol. 2004, 33, 357–366. [Google Scholar] [CrossRef]
- Stirling, G.R.; Turaganivalu, U.; Stirling, A.M.; Lomavatu, M.F.; Smith, M.K. Rhizome rot of ginger (Zingiber officinale) caused by Pythium myriotylum in Fiji and Australia. Australas. Plant Pathol. 2009, 38, 453–460. [Google Scholar] [CrossRef]
- Choi, J.E. Chemical control of rhizome rot of ginger by seed-rhizome and soil treatment. Korean J. Agric. Sci. 1999, 26, 1–5. [Google Scholar]
- Gangawane, L.V.; Shaikh, S.A. Management of resistance in Pythium aphanidermatum to aluminium ethyl phosphite. Curr. Sci. 1988, 56, 905–906. [Google Scholar]
- Pattnaik, P.K.; Kar, D.; Kuanar, A.; Mishra, B. Screening of ginger germplasm for resistance to rhizome rot. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 85, 303–308. [Google Scholar] [CrossRef]
- Daguerre, Y.; Siege, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Xie, K.; Sun, M.; Shi, A.; Di, Q.; Chen, R.; Jin, D.; Li, Y.; Yu, X.; Chen, S.; He, C. The application of tomato plant residue compost and plant growth-promoting rhizobacteria improves soil quality and enhances the ginger field soil bacterial community. Agronomy 2022, 12, 1741. [Google Scholar] [CrossRef]
- Maewan, H.; Hayati, I.; Mulyati, S. Effectiveness of biofungicide formula on rhizome rot disease of red ginger and its plant growth. Biodiversitas 2023, 24, 2143–2148. [Google Scholar]
- Rajan, P.P.; Gupta, S.R.; Sarma, Y.R.; Jackson, G.V.H. Diseases of ginger and their control with Trichoderma harzianum. Indian Phytopathol. 2002, 55, 173–177. [Google Scholar]
- Gupta, M.; Dohroo, N.P.; Gangta, V.; Shanmugam, V. Effect of microbial inoculants on rhizome disease and growth parameters of ginger. Indian Phytopathol. 2010, 63, 438–441. [Google Scholar]
- Dohroo, N.P.; Kansal, S.; Mehta, P. Evaluation of eco-friendly disease management practices against soft rot of ginger caused by Pythium aphanidermatum. Plant Dis. Res. 2012, 27, 1–5. [Google Scholar]
- Kumar, A.; Anandraj, M.; Sharma, Y.R. Rhizome solarization and microwave treatment: Ecofriendly methods for disinfecting ginger seed rhizomes. In Bacterial Wilt and Ralstonia solanacearum Species Complex, 1st ed.; Allen, C., Hayward, A.C., Prion, P., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2003; pp. 185–195. [Google Scholar]
- Horita, M.; Kobara, Y.; Yano, K.; Hayashi, K.; Nakamura, Y.; Iiyama, K.; Oki, T. Comprehensive control System for ginger bacterial wilt disease based on anaerobic soil disinfestation. Agronomy 2023, 13, 1791. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Fisher, P.R.; Gómez, C. Modeling the effect of temperature on ginger and turmeric rhizome sprouting. Agronomy 2021, 11, 1931. [Google Scholar] [CrossRef]
- Kumar, P.; Verma, S.; Sharma, A. Impact of ridge and furrow system on growth and yield of ginger (Zingiber officinale). Soil Crop Sci. 2019, 35, 225–234. [Google Scholar]
- Monnaf, M.A.; Rahim, M.A.; Hossain, M.M.A.; Alam, M.S. Effect of planting method and rhizome size on the growth and yield of ginger. J. Agrofor. Environ. 2010, 4, 73–76. [Google Scholar]
- Hosain, K.; Hossain, M.; Hassan, S.M.E.; Islam, M.; Rahman, A.; Rahman, S. Application of organic soil amendments in controlling rhizome rot of ginger. Int. J. Plant Soil Sci. 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Islam, A.; Mostarina, T.; Naher, S.; Naher, S.; Kakon, A. Effect of different management practices on the control of rhizome rot, yield and best economic return of ginger. Int. J. Agric. Pap. 2017, 2, 1–5. [Google Scholar]
- Moreno, D.A.; Villora, G.; Soriano, M.T.; Castila, N.; Romero, L. Sulfur, chromium, and selenium accumulated in Chinese cabbage under direct covers. J. Environ. Manag. 2005, 74, 89–96. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, H.S.; Jung, C.E. Toxicity of the lime sulfur as a flower thinner of apple to the honey bee, Apis mellifera L. and other pollinators. Korean J. Apic. 2008, 23, 43–50. [Google Scholar]
- Lee, J.E.; Seo, D.H.; Eo, H.M.; Yun, S.I. Yield response of Chinese cabbage to compost, gypsum, and phosphate treatments under the saline-sodic soil conditions of reclaimed tidal land. Korean J. Hortic. Sci. Technol. 2016, 34, 587–595. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, K.W.; Lee, C.G.; Choi, J.J.; Lee, H.D.; Yoon, W.M.; Kyung, K.C. Effects of sulfur spray in northern-type garlic (Allium sativum L.). Korean J. Hort. Sci. Technol. 2011, 29, 65. [Google Scholar]
- Shim, C.K.; Kim, M.J.; Kim, Y.K.; Hong, S.J.; Kim, S.C. Reducing phytotoxicity by adjusted pH and control effect of loess-sulfur complex as organic farming material against powdery mildew in tomato. Korean J. Pestic. Sci. 2014, 18, 376–382. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, Y.K.; Shim, C.K.; Kim, M.J.; Park, J.H.; Han, E.J.; Jee, H.J.; Kim, S.C. Suppressive effect of organic farming materials on the development of tomato gray mold. Korean J. Org. Agric. 2015, 23, 567–582. [Google Scholar]
- Park, J.S.; Cho, W.J.; Kim, W.S. Selection and control effect of environment friendly organic materials for controlling the main disease of Yuzu. Korean J. Org. Agric. 2014, 22, 115–127. [Google Scholar] [CrossRef]
- Yoon, D.H.; Park, H.J.; Nam, K.W. Control effect of environmental-friendly organic materials against major pear diseases. Korean J. Pestic. Sci. 2010, 14, 401–406. [Google Scholar]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Park, J.H.; Hong, S.J.; Jee, H.J.; Han, E.J.; Yoon, J.C. Effect of Chlorella vulgaris CHK0008 fertilization on enhanced of storage and freshness in organic strawberry and leaf vegetables. Hort. Sci. Technol. 2014, 32, 872–878. [Google Scholar]
- Wu, Q.; Ma, Y.; Zhang, L.; Han, J.; Lei, Y.; Le, L.; Huang, C.; Kan, J.; Fu, C. Extraction, functionality, and application of Chlorella pyrenoidosa protein/peptide. Curr. Res. Food Sci. 2023, 7, 100621. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Mutum, L.; Janda, T.; Molnár, Z. Potential benefit of microalgae and their interaction with bacteria to sustainable crop production. Plant Growth Regul. 2017, 82, 341–356. [Google Scholar] [CrossRef]
- Futó, Z.; Maróti, G. Strain-specific biostimulant effects of Chlorella and Chlamydomonas green microalgae on Medicago truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef]
- Mostafa, S.S.M.; El-Hassanin, A.S.; Soliman, A.S.; El-Changhaby, G.A.; Rasha, S.; Elgaml, N.M.M.; Awad, A.A. Phycoremediation of potato industry wastewater for nutrient recovery, pollution reduction, and biofertilizer production for greenhouse cultivation of lettuce and celery in sandy soils. Int. J. Plant Biol. 2024, 15, 652–672. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, M.J.; Shim, C.K.; Bae, S.Y.; Jang, S.H. Potential of algae–bacteria synergistic effects on vegetable production. Front. Plant Sci. 2021, 12, 656662. [Google Scholar] [CrossRef]
- Scheuerell, S.; Mahaffee, W. Compost Tea: Principles and prospects for plant disease control. Compost. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- St. Martin, C.C.G.; Brathwaite, R.A.I. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Biol. Agric. Hort. 2012, 28, 1–33. [Google Scholar] [CrossRef]
- Ingham, E.R.; Alms, M. Compost Tea Manual; Soil Food Web, Inc.: Corvallis, OR, USA, 1999. [Google Scholar]
- Touart, A.P. Time for compost tea in the Northwest. BioCycle 2000, 41, 74–77. [Google Scholar]
- Kim, M.J.; Shim, C.K.; Ko, B.G.; Kim, J. Effect of the microalga Chlorella fusca CHK0059 on strawberry PGPR and biological control of Fusarium Wilt disease in non-pesticide hydroponic strawberry cultivation. J. Microbiol. Biotechnol. 2020, 30, 708–716. [Google Scholar] [CrossRef] [PubMed]
- NAAS (National Institute of Agricultural Sciences). Easy to Follow Organic Farming Techniques; NAAS: Suwon, Republic of Korea, 2010; 83p. [Google Scholar]
- Kwon, J.H.; Shim, C.K.; Jee, H.J.; Park, C.S. Control of powdery mildew on solanaceous crops by using COY (Cooking Oil and Yolk Mixture) in the greenhouse. Plant Dis. Res. 2009, 15, 23–29. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, O.S.; Shim, C.K.; Lee, J.H. Assessment of hot water treatment and lime sulfur mixture on germination and disinfection efficacy of organic wheat seeds. Korean J. Crop Sci. 2023, 68, 371–382. [Google Scholar]
- Wang, Y.; Li, H.; Zhang, Z.; Chen, J. Effect of lime sulfur mixture on the emergence and growth of ginger rhizomes in organic farming. J. Org. Agric. 2020, 12, 456–467. [Google Scholar]
- Zhang, X.; Liu, Q.; Yang, Y.; Zhao, H. Impact of sulfur-based treatments on crop health and productivity in organic and conventional systems. Agric. Sci. Technol. 2018, 10, 123–134. [Google Scholar]
- Kopta, T.; Pavlíková, M.; Sękara, A.; Pokluda, R.; Maršálek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of Lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Horti Agrobot. 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Gonmei, C.; Simon, S. Effect of Bacillus subtilis, soil amendments and microalgae treatment on Fusarium equiseta of turmeric (Curcuma longa L.) Prayagraj, India. Int. J. Plant Soil Sci. 2024, 36, 323–336. [Google Scholar] [CrossRef]
- Johnson, T.; Williams, D.; Thompson, R. Evaluating the impact of biostimulants and biocontrol agents on ginger. Crop Sci. J. 2018, 48, 315–328. [Google Scholar]
- Zhang, W.; Liu, Y.; Zhao, H. Soil type influence on the efficacy of microbial treatments in ginger cultivation. Soil Biol. Biochem. 2017, 60, 78–85. [Google Scholar]
- Sharma, R.; Gupta, V.; Singh, J. Effect of ridge width and mulching on yield and quality of ginger (Zingiber officinale Roscoe). J. Agric. Sci. 2017, 45, 123–130. [Google Scholar]
- Patil, R.; Desai, S.; Kadam, S. Influence of bed configuration and organic amendments on growth, yield, and quality of ginger. Org. Agric. J. 2018, 20, 145–156. [Google Scholar]
- Shalini, K.; Prabhu, T.; Shenbagavalli, S.; Rajangam, J. Effect of various organic amendments on growth of ginger (Zingiber officinale Rosc.) under coconut cropping system. Int. J. Plant Soil Sci. 2023, 35, 842–849. [Google Scholar] [CrossRef]
- Singh, A.K. Management of rhizome rot caused by Pythium, Fusarium and Ralstonia sppin ginger (Ginger officinale) under natural field condition. Indian J. Agric. Sci. 2011, 81, 268–270. [Google Scholar]
- Behera, S.; Sial, P.; Das, H.; Pradhan, K. Pythium soft rot management in ginger (Zingiber officinale Roscoe)—A review. Curr. J. Appl. Sci. Technol. 2020, 39, 106–115. [Google Scholar] [CrossRef]
- Islam, M.; Khatun, F.; Faruk, I.; Rahman, M. Incidence of rhizome rot of ginger in some selected areas of Bangladesh and the causal pathogens associated with the disease. Bangladesh J. Agric. Res. 2019, 44, 569–576. [Google Scholar] [CrossRef]
- Shanmugam, V.; Thakur, H.; Kaur, J. Genetic diversity of Fusarium spp. inciting rhizome rot of ginger and its management by PGPR consortium in the western Himalayas. Biol. Control 2013, 66, 1–7. [Google Scholar] [CrossRef]
- Metthews, A.; Muthukumer, S.T.; Hamill, S.; Aiken, E.A.; Chen, A. Impact of inoculum density of Fusarium oxysporum f. sp. zingiberi on symptomatic appearances and yield of ginger (Zingiber officinale Roscoe). Acc. Microbiol. 2023, 5, 000605.v3. [Google Scholar] [CrossRef]
- Kifelow, H.; Kassa, B.; Sadessa, K.; Hunduma, T. Prevalence of bacterial wilt of ginger (Z. officinale) caused by Ralstonia solansearum (Smith) in Ethiopia. Int. J. Res. Stud. Agric. Sci. 2015, 1, 14–22. [Google Scholar]
- Chang, K.J.; Sung, I.J.; Lee, S.S.; Ahn, C.H.; Byun, J.M.; Pak, C.H. Studies on the environmentally-friendly production of ginseng (Panaxs ginseng C.A. Mayer) by lime sulfur treatment. Prac. Agric. Fish. Res. 2013, 15, 183–202. [Google Scholar]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health andenvironment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef]
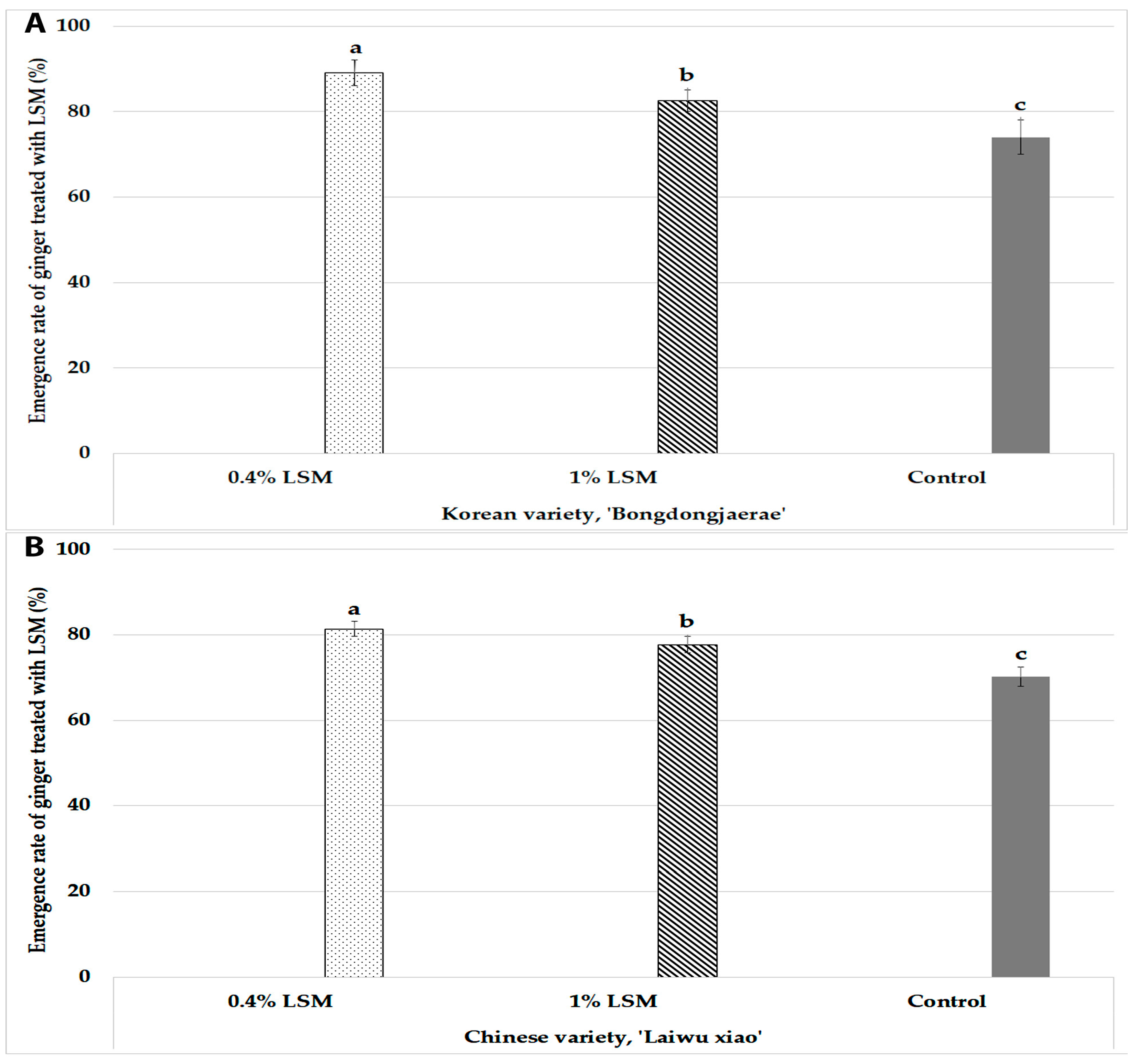
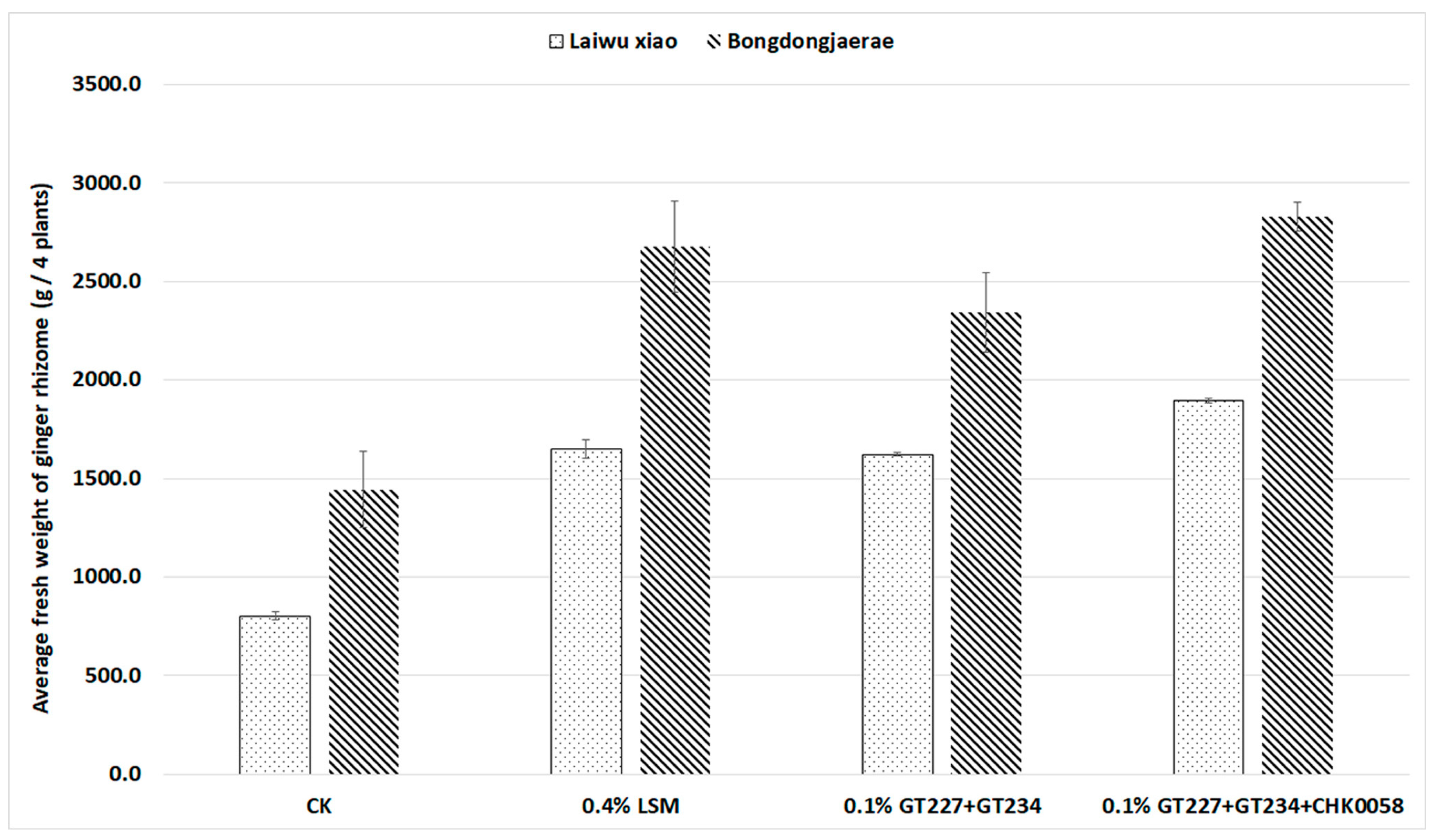
| Treatment (a) | Korean Ginger Variety, Bongdongjaerae | |
|---|---|---|
| Emergence Rate (% ± SD) | Fresh Weight of Rhizome (g/Plant ± SD) | |
| CK | 85.6 ± 15.2 e | 310.0 ± 8.4 f |
| 0.1% GT234 | 90.2 ± 4.2 d | 350.1 ± 6.2 e |
| 0.1% GT227 | 91.2 ± 3.6 c | 363.2 ± 5.3 d |
| 0.2% CHK0058 | 100 ± 0 a | 388.2 ± 4.2 c |
| 0.1% GT234 + 0.2% CHK0058 | 92.2 ± 2.3 c | 394.8 ± 4.8 b |
| 0.1% GT227+ CHK0058 | 95.2 ± 5.4 b | 405.2 ± 5.2 a |
| Treatment (a) | Average Fresh Weight of Ginger (g/10 Plants) | |
|---|---|---|
| Flat–Bed | Single–Bed | |
| 0.1% GT234 | 250.7 ± 15.5 c | 270.0 ± 14.2 d |
| 0.1% GT227 | 283.3 ± 18.2 b | 263.3 ± 13.2 e |
| 0.2% CHK0058 | 290.2 ± 10.2 a | 363.3 ± 9.5 a |
| 4% CT | 286.7 ± 10.4 b | 337.0 ± 8.5 b |
| 0.4% LSM | 232.4 ± 11.2 d | 287.7 ± 7.4 c |
| CK | 210.0 ± 14.2 e | 256.7 ± 13.2 f |
| Max | 290.2 | 363.3 |
| Min | 232.4 | 263.3 |
| Mean | 258.9 ± 30.3 | 296.3 ± 39.9 |
| Treatments (a) | Plant Height of Ginger (cm) | |||
|---|---|---|---|---|
| Flat Ridge | Single Ridge | |||
| Chinese Variety, Laiwu Xiao | Korean Variety, Bongdongjaerae | Chinese Variety, Laiwu Xiao | Korean Variety, Bongdongjaerae | |
| 0.4% LSM | 29 | 31 | 24 | 29 |
| 0.1% GT227 + 0.1% GT234 | 26 | 30 | 23 | 29 |
| 0.1% GT227 + 0.1% GT234 + 0.2% CF | 30 | 31 | 25 | 31 |
| CK | 17 | 27 | 20 | 25 |
| Treatments (a) | Ridge Type (b) | Disease Incidence of Rhizome Rot (%) | Control Value (%) | ||
|---|---|---|---|---|---|
| Chinese Variety, Laiwu Xiao | Korean Variety, Bongdongjaerae | Chinese Variety, Laiwu Xiao | Korean Variety, Bongdongjaerae | ||
| 0.4% LSM | Flat | 24.4 | 15.0 | 53.1 | 50.3 |
| Single | 19.0 | 0 | 55.2 | 100.0 | |
| 0.1% GT227 + 0.1% GT234 | Flat | 30.0 | 22.4 | 42.3 | 25.8 |
| Single | 25.0 | 12.5 | 41.0 | 32.4 | |
| 0.1% GT227 + 0.1% GT234 + 0.2% CHK0058 | Flat | 20.0 | 10.0 | 61.5 | 66.9 |
| Single | 15.0 | 0 | 64.6 | 100.0 | |
| CK | Flat | 52.0 | 30.2 | - | - |
| Single | 42.4 | 18.5 | - | - | |
| Mean | Flat | 31.6 | 19.4 | 52.3 | 47.7 |
| Single | 25.4 | 7.8 | 53.6 | 77.5 | |
| LSD 0.05 | Flat | 0.04 | 0.04 | 0.03 | 0.02 |
| Single | 0.03 | 0.02 | 0.02 | 0.01 | |
| Type of Ridge (a) | Variety | Disease Incidence (0–9) (b) | Percentage of Isolated Pathogens (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pythium sp. | Fusarium sp. | Ralstonia sp. | |||||||
| Early | Harvest | Early | Harvest | Early | Harvest | Early | Harvest | ||
| Flat | Chinese variety, ‘Laiwu xiao’ | 4.3 ± 0.5 | 8.2 ± 0.8 | 70 ± 9.4 | 65 ± 7.1 | 20 ± 8.2 | 19.5 ± 8.6 | 10 ± 3.3 | 15 ± 4.7 |
| Korean variety, ‘Bongdongjaerae’ | 0 ± 0 | 3.8 ± 1.1 | 0 ± 0 | 70 ± 8.2 | 0 ± 0 | 25 ± 4.7 | 0 ± 0 | 5.0 ± 0.8 | |
| Single | Chinese variety, ‘Laiwu xiao’ | 2.2 ± 0.6 | 4.5 ± 0.7 | 80 ± 8.2 | 50 ± 9.4 | 20.0 ± 8.2 | 32 ± 7.9 | 0 ± 0 | 18 ± 9.5 |
| Korean variety, ‘Bongdongjaerae’ | 0 ± 0 | 2.2 ± 0.4 | 0 ± 0 | 7512.7± | 0 ± 0 | 19.5 ± 6.0 | 0 ± 0 | 5 ± 1.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Shim, C.; Lee, J. Effects of Organic Agricultural Materials and Cultivation Methods on the Control of Ginger Rhizome Rot Disease and Growth in Organic Ginger Farming. Agronomy 2024, 14, 2285. https://doi.org/10.3390/agronomy14102285
Kim M, Shim C, Lee J. Effects of Organic Agricultural Materials and Cultivation Methods on the Control of Ginger Rhizome Rot Disease and Growth in Organic Ginger Farming. Agronomy. 2024; 14(10):2285. https://doi.org/10.3390/agronomy14102285
Chicago/Turabian StyleKim, Minjeong, Changki Shim, and Jaehyeong Lee. 2024. "Effects of Organic Agricultural Materials and Cultivation Methods on the Control of Ginger Rhizome Rot Disease and Growth in Organic Ginger Farming" Agronomy 14, no. 10: 2285. https://doi.org/10.3390/agronomy14102285






