An Excessive K/Na Ratio in Soil Solutions Impairs the Seedling Establishment of Sunflower (Helianthus annuus L.) through Reducing the Leaf Mg Concentration and Photosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Data Collection
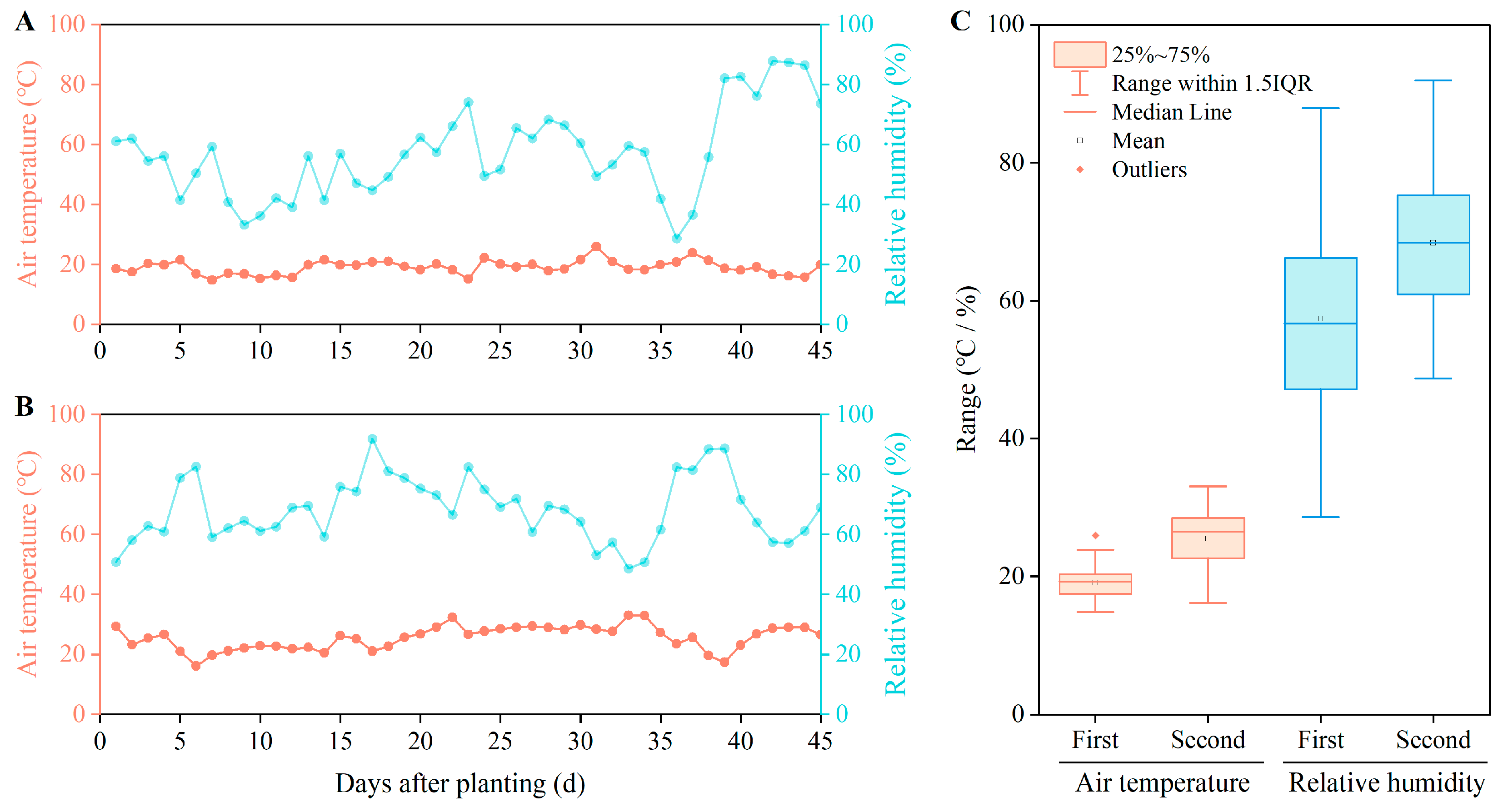
2.2.1. Study Soil Physicochemical Properties
2.2.2. Plant Height, Leaf Area, and Dry Mass of Sunflower
2.2.3. Ion Absorption from Soil and Ion Transport in Different Sunflower Organs
2.2.4. Gas Exchange Parameters and Chlorophyll Concentration
2.3. Data Analysis
3. Results
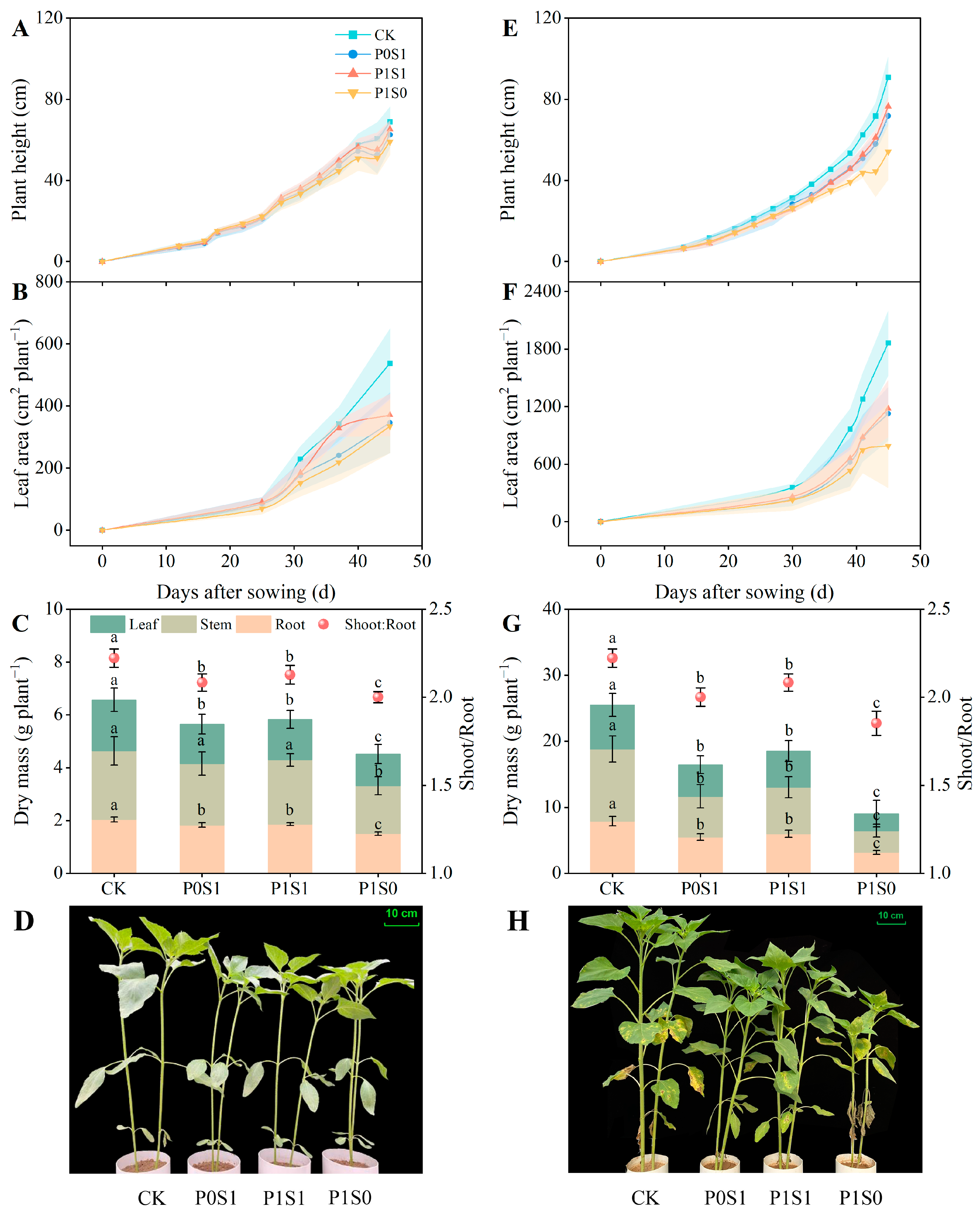
3.1. Plant Height, Leaf Area, and Dry Matter of Sunflower Seedling
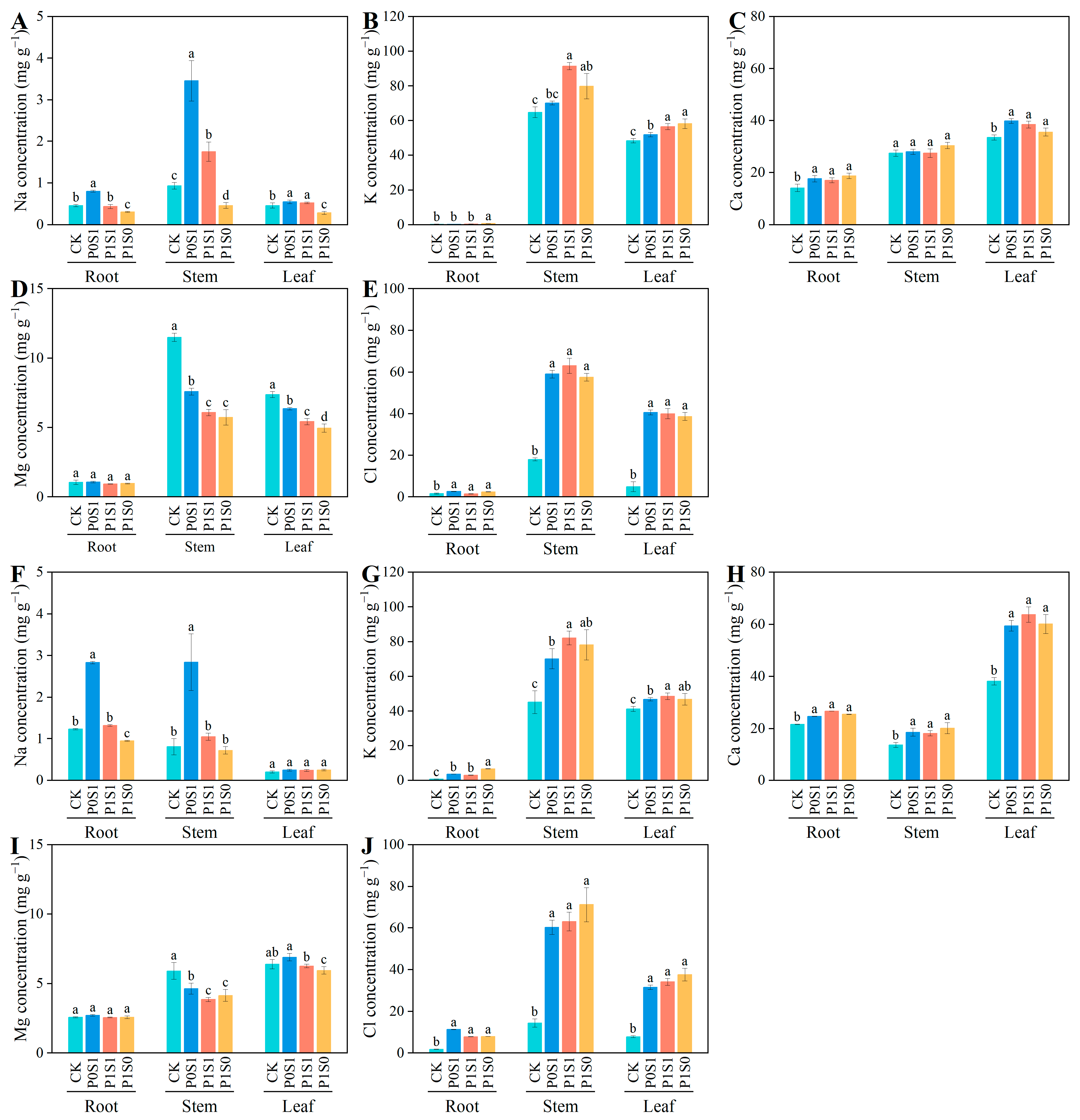
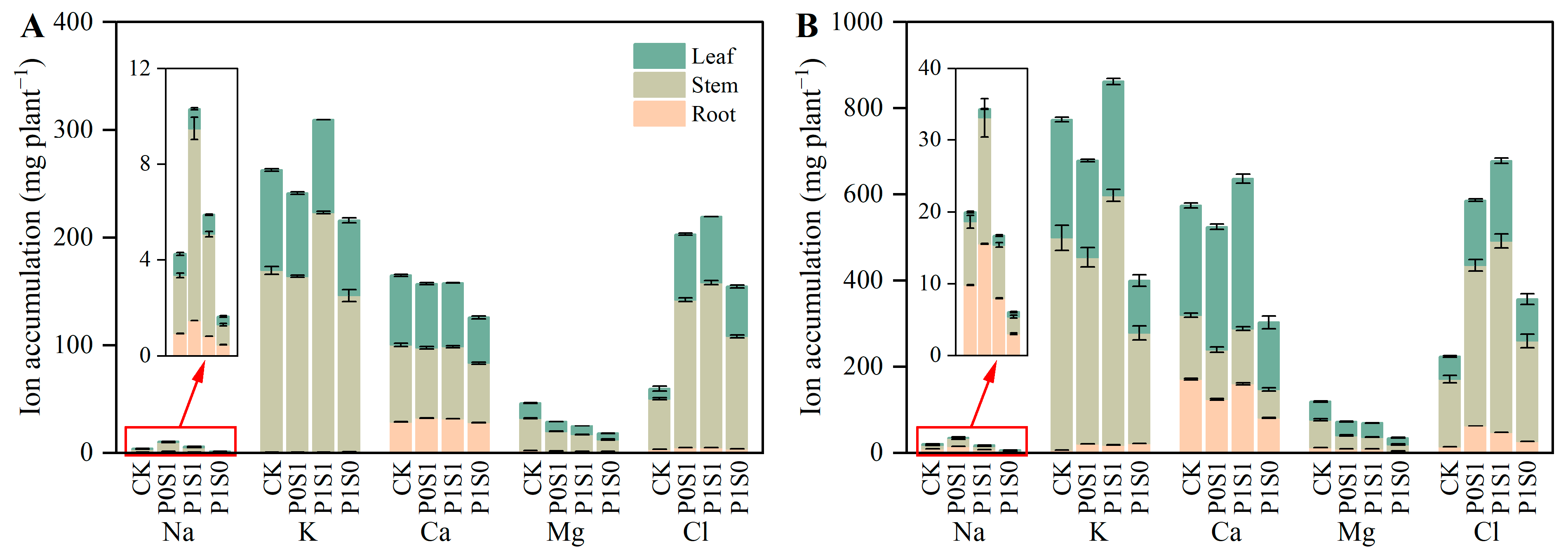
3.2. Ion Concentration and Accumulation in Sunflower Seedling
3.3. Cation Selective Absorption and Transport in Sunflower Seedling
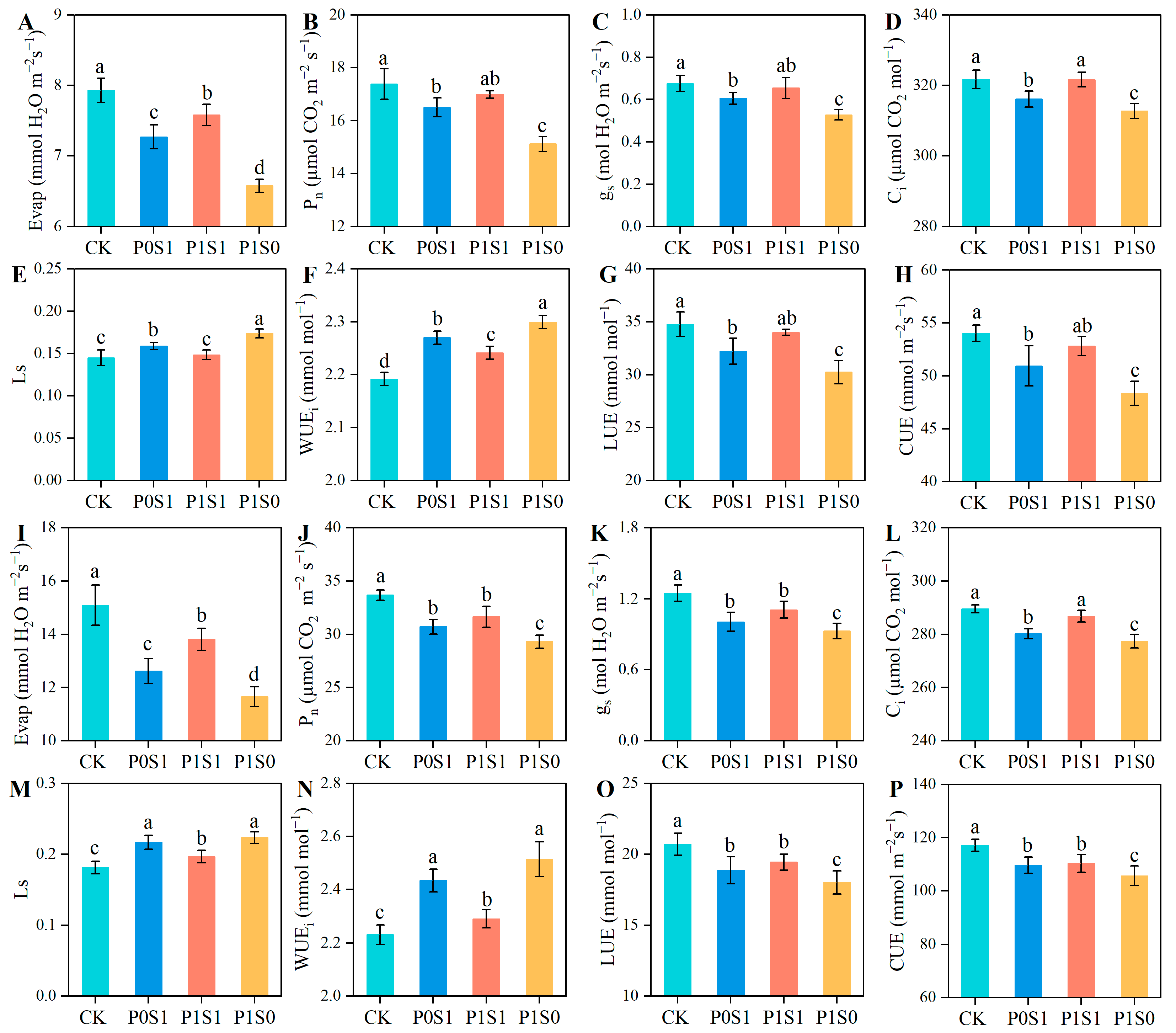
3.4. Gas Exchange Parameters and Chlorophyll Concentration in Sunflower Leaves
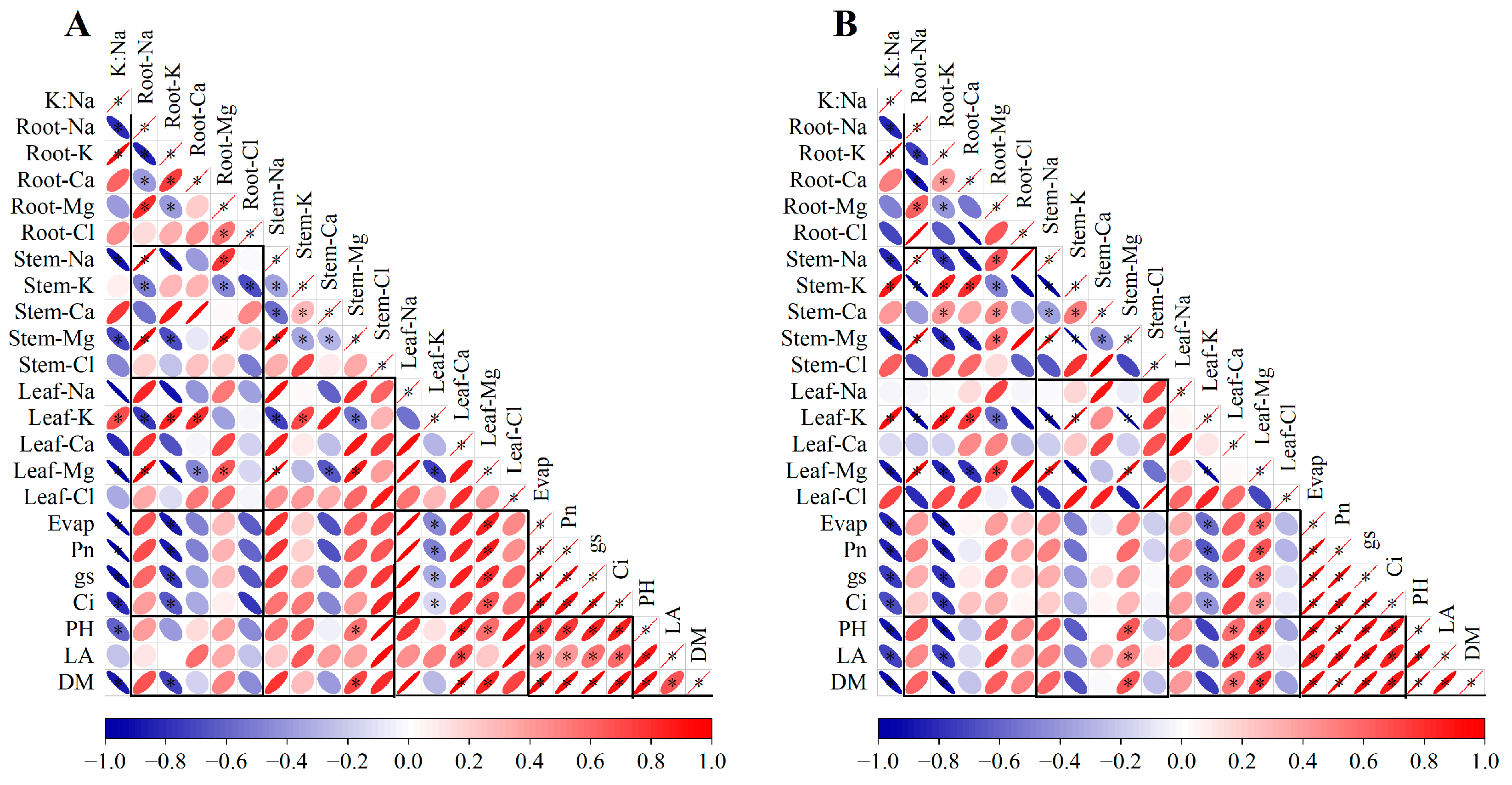
3.5. Correlation of K/Na Ratio, Ions Concentration, Gas Exchange Parameters, and Growth Indicators
4. Discussion
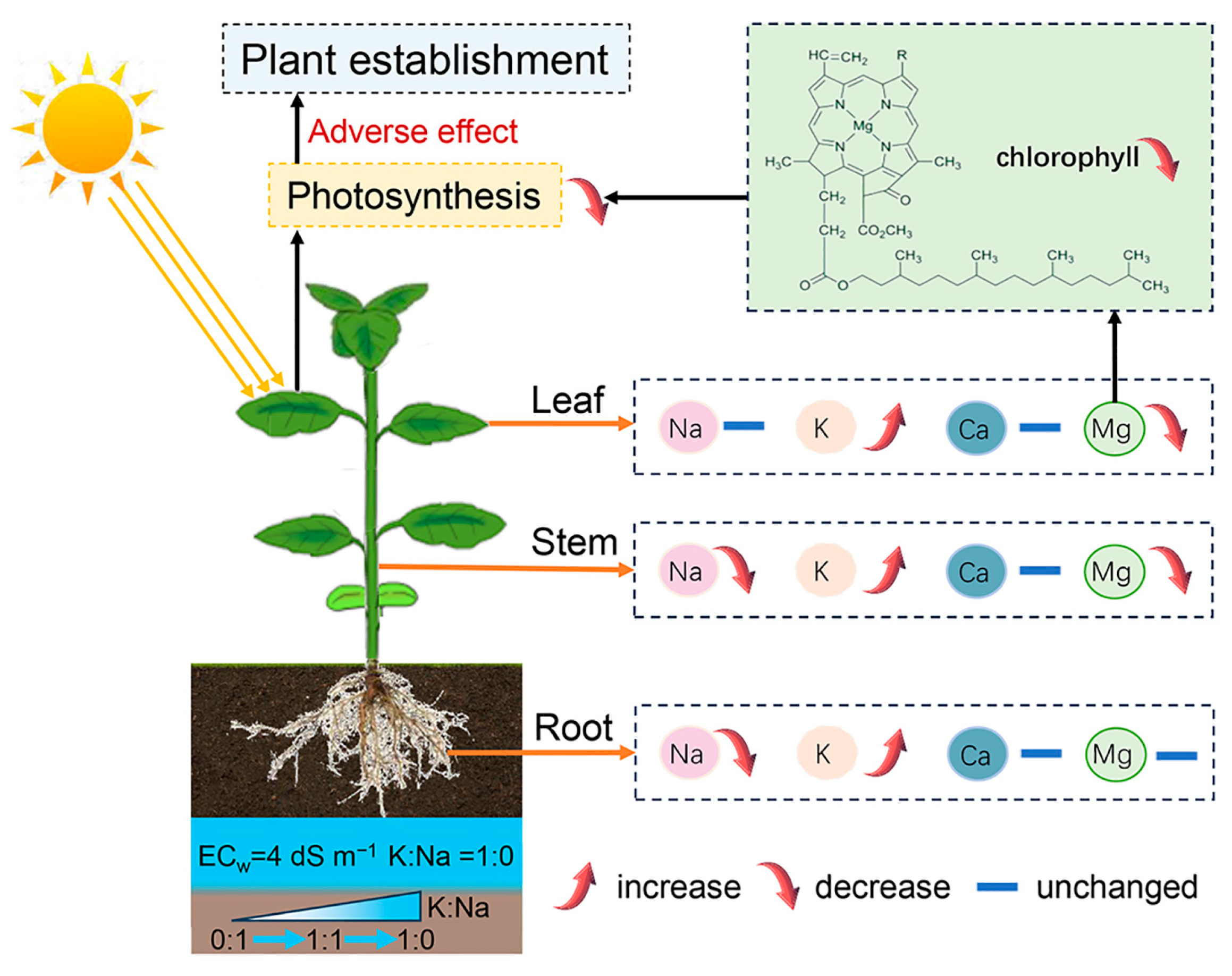
4.1. Effects of K/Na Ratios on Ion Absorption and Transport
4.2. Effects of K/Na Ratios on Crop Photosynthesis and Growth
4.3. Optimal K/Na Ratio to Promote Plant Establishment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalifani, S.; Darvishzadeh, R.; Azad, N.; Seyed Rahmani, R. Prediction of sunflower grain yield under normal and salinity stress by RBF, MLP and, CNN models. Ind. Crops Prod. 2022, 189, 115762. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, K. Talc based exopolysaccharides formulation enhancing growth and production of Hellianthus annuus under saline conditions. Cell. Mol. Biol. 2014, 60, 73–81. [Google Scholar] [CrossRef] [PubMed]
- FAO. Halt soil salinization, boost soil productivity. In Proceedings of the Global Symposium on Salt-Affected Soils, Rome, Italy, 20–22 October 2022. [Google Scholar]
- Schubert, S. Sodium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 697–710. [Google Scholar]
- Pantha, P.; Oh, D.H.; Longstreth, D.; Dassanayake, M. Living with high potassium: Balance between nutrient acquisition and K-induced salt stress signaling. Plant Physiol. 2023, 191, 1102–1121. [Google Scholar] [CrossRef]
- Fageria, N.K. Nutrient Uptake in Crop Plants; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Fageria, N.K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.K.; Appel, T. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Physiology of Crop Production; Haworth: New York, NY, USA, 2006. [Google Scholar]
- Orris, G.J.; Cocker, M.D.; Dunlap, P.; Wynn, J.; Spanski, G.T.; Briggs, D.A.; Gass, L. Potash—A Global Overview of Evaporite-Related Potash Resources, including Spatial Database of Deposits, Occurrences, and Permissive Tracts; Scientific Investigations Report 2010-5090-S; U.S. Geological Survey: Reston, VA, USA, 2014.
- Warren, J.K. Potash Resources: Occurrences and Controls; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Rengasamy, P.; Marchuk, A. Cation ratio of soil structural stability (CROSS). Soil Res. 2011, 49, 280–285. [Google Scholar] [CrossRef]
- Smiles, D.E.; Smith, C.J. A survey of the cation content of piggery effluents and some consequences of their use to irrigate soils. Aust. J. Soil Res. 2004, 42, 231–246. [Google Scholar] [CrossRef]
- Smith, C.J.; Sposito, G.; Oster, J.D. Accounting for potassium and magnesium in irrigation water quality assessment. Calif. Agric. 2016, 70, 71–76. [Google Scholar]
- Wang, Y.; Zhang, T.; Li, B.; Feng, G.; Yan, L.; Gao, Q. Effect of long-term potassium fertilizer levels on the yield of rainfed corn in Northeast China. Appl. Ecol. Environ. Res. 2020, 18, 597–607. [Google Scholar] [CrossRef]
- Wang, M.; Ye, Y.; Chu, X.; Zhao, Y.; Zhang, S.; Chen, H.; Qin, W.; Wang, Y. Responses of garlic quality and yields to various types and rates of potassium fertilizer applications. Hortscience 2022, 57, 72–80. [Google Scholar] [CrossRef]
- Fageria, N.K. Ionic interactions in rice plants from dilute solutions. Plant Soil 1983, 70, 309–316. [Google Scholar] [CrossRef]
- Ding, Y.; Chang, C.; Luo, W.; Wu, Y.; Ren, X.; Wang, P.; Xu, G. High potassium aggravates the oxidative stress inducedy by magnesium deflciency in rice leaves. Pedosphere 2008, 18, 316–327. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium-sodium interactions in soil and plant under saline-sodic conditions. J. Soil Sci. Plant Nutr. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Adiloglu, S.; Adiloglu, A.; Ozkil, M. Effect of different levels of NaCl and KCl on growth and some biological indexes of wheat plant. Pak. J. Biol. Sci. 2007, 10, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Aghajanzadeh, T.; Helm, J.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 2017, 411, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.; Emami, H.; Fotovat, A.; Khorassani, R.; Keller, T. Soil available water and plant growth in relation to K:Na ratio. Geoderma 2020, 363, 114173. [Google Scholar] [CrossRef]
- Zhao, W.; Faust, F.; Schubert, S. Potassium is a potential toxicant for Arabidopsis thaliana under saline conditions. J. Soil Sci. Plant Nutr. 2020, 183, 455–467. [Google Scholar] [CrossRef]
- Richter, J.A.; Behr, J.H.; Erban, A.; Kopka, J.; Zörb, C. Ion-dependent metabolic responses of Vicia faba L. to salt stress. Plant Cell Environ. 2019, 42, 295–309. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salt tolerance classification of crops according to soil salinity and to water stress day index. Agric. Water Manag. 2000, 43, 99–109. [Google Scholar] [CrossRef]
- Francois, L.E. Salinity effects on four sunflower hybrids. Agron. J. 1996, 88, 215–219. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Kong, D.; Shi, H.; Huo, Z.; Yan, Y.; Li, Y.; Zhang, Y. Effects on growth of sunflower under different saline soils in the Hetao Irrigation Area. J. Shenyang Agric. Univ. 2004, 35, 414–416. (In Chinese) [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO: Rome, Italy, 1985. [Google Scholar]
- Quirk, J.P.; Schofield, R.K. The effect of electrolyte concentration on soil permeability. Eur. J. Soil Sci. 1955, 6, 163–178. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Department of Agriculture: Washington DC, USA, 1954.
- Chen, Y.; Banin, A.; Borochovitch, A. Effect of potassium on soil structure in relation to hydraulic conductivity. Geoderma 1983, 30, 135–147. [Google Scholar] [CrossRef]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Biol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Pitman, M.G. Transport across the root and shoot/root interactions. In Salinity Tolerance in Plants: Strategies for Crop Improvement; Staples, R.C., Toenniessen, G.H., Eds.; John Wiley: New York, NY, USA, 1984; pp. 93–123. [Google Scholar]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Stomatal limitations to photosynthesis and their critical Water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Chen, L.; Jia, G. Whole-plant instantaneous and short-term water-use efficiency in response to soil water content and CO2 concentration. Plant Soil 2019, 444, 281–298. [Google Scholar] [CrossRef]
- Medlyn, B.E. Physiological basis of the light use efficiency model. Tree Physiol. 1998, 18, 167–176. [Google Scholar] [CrossRef]
- He, W.; Ma, F. Effects of water gradient on fluorescence characteristics and gas exchange in Sabina Vulgaris seedlings. Acta Phytoecol. Sin. 2000, 24, 630–634. (In Chinese) [Google Scholar]
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix. R Package Version 0.77. (Verified June 2017). 2016. Available online: https://github.com/taiyun/corrplot (accessed on 6 June 2024).
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Bai, X.; Gao, J.; Wang, S.; Cai, H.; Chen, Z.; Zhou, J. Excessive nutrient balance surpluses in newly built solar greenhouses over five years leads to high nutrient accumulations in soil. Agric. Ecosyst. Environ. 2020, 288, 106717. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Schmidhalter, U. Interactive effects of salinity and macronutrient level on wheat. II. Composition. J. Plant Nutr. 1997, 20, 1169–1182. [Google Scholar] [CrossRef]
- Horie, T.; Brodsky, D.E.; Costa, A.; Kaneko, T.; Lo Schiavo, F.; Katsuhara, M.; Schroeder, J.I. K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol. 2011, 156, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Tester, M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000, 122, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.B.; Zeng, W.; Canty, J.; Lam, Y.; Jiang, Y. Sodium and potassium competition in potassium-selective and non-selective channels. Nat. Commun. 2013, 4, 2721. [Google Scholar] [CrossRef]
- Hryvusevich, P.; Navaselsky, I.; Talkachova, Y.; Straltsova, D.; Keisham, M.; Viatoshkin, A.; Samokhina, V.; Smolich, I.; Sokolik, A.; Huang, X.; et al. Sodium influx and potassium efflux currents in sunflower root cells under high salinity. Front. Plant Sci. 2020, 11, 613936. [Google Scholar] [CrossRef]
- Grzebisz, W. Magnesium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 199–259. [Google Scholar]
- Ding, Y.; Luo, W.; Xu, G. Characterization of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhou, T.; Liu, Y.; Raza, S.; Zhou, J. Effects of high potassium and low temperature on the growth and magnesium nutrition of different tomato cultivars. Hortscience 2018, 53, 710–714. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular response to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–90. [Google Scholar] [CrossRef]
- Xu, G.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in chloride nutrition of plants. Adv. Agron. 1999, 68, 97–150. [Google Scholar]
- Schulte, E.E. Soil and Applied Chlorine (A3556); University of Wisconsin Cooperative Extension Service: Madison, WI, USA, 1999. [Google Scholar]
- Kopsell, D.E.; Kopsell, D.A. Chlorine. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 347–366. [Google Scholar]
- Munns, R.; Schachtman, D.P.; Condon, A.G. The Significance of a Two-Phase Growth Response to Salinity in Wheat and Barley. Aust. J. Plant Physiol. 1995, 22, 561–569. [Google Scholar]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Jayaganesh, S.V.; Senthurpandian, V.K. Impact of different sources and doses of magnesium fertilizer on biochemical constituents and quality parameters of black tea. Asian J. Biochem. 2011, 6, 273–281. [Google Scholar] [CrossRef]
- Xu, D. Some problems in stomatal limitation analysis of photosynthesis. Plant Physiol. Commun. 1997, 33, 241–244. (In Chinese) [Google Scholar]
- Liu, J.; Shi, D.C. Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 2010, 48, 127–134. [Google Scholar] [CrossRef]
- Shingles, R.; McCarty, R.E. Direct measurement of ATP-dependent proton concentration changes and characterization of a K-stimulated ATPase in pea chloroplast inner envelope vesicles. Plant Physiol. 1994, 106, 731–737. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhou, T.; Liu, Y.; Zhou, J. High potassium to magnesium ratio affected the growth and magnesium uptake of three tomato (Solanum lycopersicum L.) cultivars. J. Integr. Agric. 2018, 17, 2813–2821. [Google Scholar] [CrossRef]
- Carvalho, P.; Foulkes, M.J. Roots and Uptake of Water and Nutrients. In Encyclopedia of Sustainability Science and Technology; Meyers, R., Ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Smith, C.J.; Oster, J.D.; Sposito, G. Potassium and magnesium in irrigation water quality assessment. Agric. Water Manag. 2015, 157, 59–64. [Google Scholar] [CrossRef]
- Buelow, M.C.; Steenwerth, K.; Parikh, S.J. The effect of mineral-ion interactions on soil hydraulic conductivity. Agric. Water Manag. 2015, 152, 277–285. [Google Scholar] [CrossRef]
- Liang, X.; Rengasamy, P.; Smernik, R.; Mosley, L.M. Does the high potassium content in recycled winery wastewater used for irrigation pose risks to soil structural stability? Agric. Water Manag. 2021, 243, 106422. [Google Scholar] [CrossRef]
- Cuin, T.A.; Shabala, S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol. 2005, 46, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qi, H.; Qiao, W.; Shi, J.; Xu, Q.; Zhou, H.; Yan, G.; Huang, Q. Cotton (Gossypium hirsutum L.) genotypes with contrasting K+/Na+ ion homeostasis: Implications for salinity tolerance. Acta Physiol. Plant 2017, 39, 77. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Xiong, C.; Gu, J.; Zheng, Y.; Ju, F.; Wang, S.; Hu, W.; Zhao, W.; Zhou, Z.; et al. Improving the soil K+/Na+ ratio under moderate salt stress synergistically increases the yield and quality of cotton fiber and cottonseed. Ind. Crops Prod. 2024, 213, 118441. [Google Scholar] [CrossRef]

| Indicator | Value |
|---|---|
| Sand (% by weight) | 8.10 |
| Silt (% by weight) | 60.62 |
| Clay (% by weight) | 31.28 |
| Soil texture (USDA classification) | Silty clay loam |
| Bulk density (g cm−3) | 1.35 |
| pH | 7.66 |
| Electrical conductivity of saturated soil extract (ECe, dS m−1) | 0.72 |
| Water-soluble ion concentration (mmol L−1) | |
| Sodium (Na+) | 0.1 |
| Potassium (K+) | <0.01 |
| Calcium (Ca2+) | 0.54 |
| Magnesium (Mg2+) | 0.32 |
| Chloride (Cl−) | 0.23 |
| Sulfate (SO42−) | 2.18 |
| Carbonate + bicarbonate (CO32− + HCO3−) | 0.60 |
| Organic matter content (g kg−1) | 10.25 |
| Total nitrogen (g kg−1) | 0.93 |
| Total potassium (g kg−1) | 10.27 |
| Available phosphorus (mg kg−1) | 15.67 |
| Available potassium (mg kg−1) | 103.35 |
| Treatment | Adding Salt (mmolc L−1) | K/Na | EC (dS m−1) | Cation Ratio (mmolc L−1)0.5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | Targeted | Measured | SAR | PAR | ||
| CK | 0 | 0 | 0 | 0 | 0 | - | 0 | 0.15 | - | - |
| P0S1 | 35.00 | 0.00 | 2.25 | 2.25 | 40 | 0:1 | 4 | 4.20 | 14.77 | 0.01 |
| P1S1 | 17.50 | 17.50 | 2.25 | 2.25 | 40 | 1:1 | 4 | 4.31 | 7.56 | 7.22 |
| P1S0 | 0.00 | 35.00 | 2.25 | 2.25 | 40 | 1:0 | 4 | 4.44 | 0.36 | 14.42 |
| Tap water | 0.87 | 0.03 | 0.6 | 0.8 | 10 | 0.034 | - | 0.15 | 0.74 | 0.03 |
| SA or ST | First Batch Experiment | Second Batch Experiment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | P0S1 | P1S1 | P1S0 | CK | P0S1 | P1S1 | P1S0 | ||
| Soil → Root | SAK/Na | 2.96 d | 39.71 a | 15.64 b | 4.73 c | 6.23 c | 50.23 a | 37.27 a | 10.26 b |
| SACa/Na | 86.12 b | 228.16 a | 247.78 a | 45.29 c | 31.36 c | 43.60 b | 84.94 a | 13.08 d | |
| SAMg/Na | 5.17 b | 7.39 a | 8.21 a | 1.27 c | 2.66 b | 2.60 b | 5.35 a | 0.88 c | |
| Root → Stem | STK/Na | 111.94 a | 51.19 b | 55.56 b | 65.11 b | 159.74 a | 59.11 b | 63.72 b | 65.30 b |
| STCa/Na | 0.95 a | 0.87 a | 0.90 a | 1.08 a | 0.96 a | 0.82 a | 0.86 a | 1.04 a | |
| STMg/Na | 5.30 a | 1.64 b | 1.61 b | 3.94 b | 5.74 a | 2.85 b | 2.99 b | 3.47 b | |
| Root → Leaf | STK/Na | 168.79 b | 237.48 a | 114.28 c | 76.92 d | 187.37 b | 322.29 a | 86.52 c | 26.38 d |
| STCa/Na | 2.35 b | 3.30 a | 1.87 b | 2.05 b | 10.65 b | 29.72 a | 8.59 b | 8.93 b | |
| STMg/Na | 6.89 b | 8.66 a | 5.52 c | 4.81 d | 24.47 b | 45.61 a | 21.91 b | 14.26 c | |
| Treatment | Chlorophyll Concentration (μmol m−2) | |
|---|---|---|
| First Batch Pot Trial | Second Batch Pot Trial | |
| CK | 262.55 ± 2.14 a | 293.30 ± 3.62 a |
| P0S1 | 255.32 ± 3.67 b | 279.00 ± 5.21 b |
| P1S1 | 258.59 ± 3.13 b | 284.67 ± 5.85 b |
| P1S0 | 241.73 ± 5.15 c | 264.02 ± 4.31 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhang, T.; Gao, W.; Kuang, Y.; Liang, Q.; Feng, H.; Galymzhan, S. An Excessive K/Na Ratio in Soil Solutions Impairs the Seedling Establishment of Sunflower (Helianthus annuus L.) through Reducing the Leaf Mg Concentration and Photosynthesis. Agronomy 2024, 14, 2301. https://doi.org/10.3390/agronomy14102301
Cheng Y, Zhang T, Gao W, Kuang Y, Liang Q, Feng H, Galymzhan S. An Excessive K/Na Ratio in Soil Solutions Impairs the Seedling Establishment of Sunflower (Helianthus annuus L.) through Reducing the Leaf Mg Concentration and Photosynthesis. Agronomy. 2024; 14(10):2301. https://doi.org/10.3390/agronomy14102301
Chicago/Turabian StyleCheng, Yu, Tibin Zhang, Weiqiang Gao, Yuxin Kuang, Qing Liang, Hao Feng, and Saparov Galymzhan. 2024. "An Excessive K/Na Ratio in Soil Solutions Impairs the Seedling Establishment of Sunflower (Helianthus annuus L.) through Reducing the Leaf Mg Concentration and Photosynthesis" Agronomy 14, no. 10: 2301. https://doi.org/10.3390/agronomy14102301





