Evaluating the Efficacy of an Extract for UV Defense and Mitigation of Oxidative Stress, Transitioning from Biomass to Bioprotection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Matter
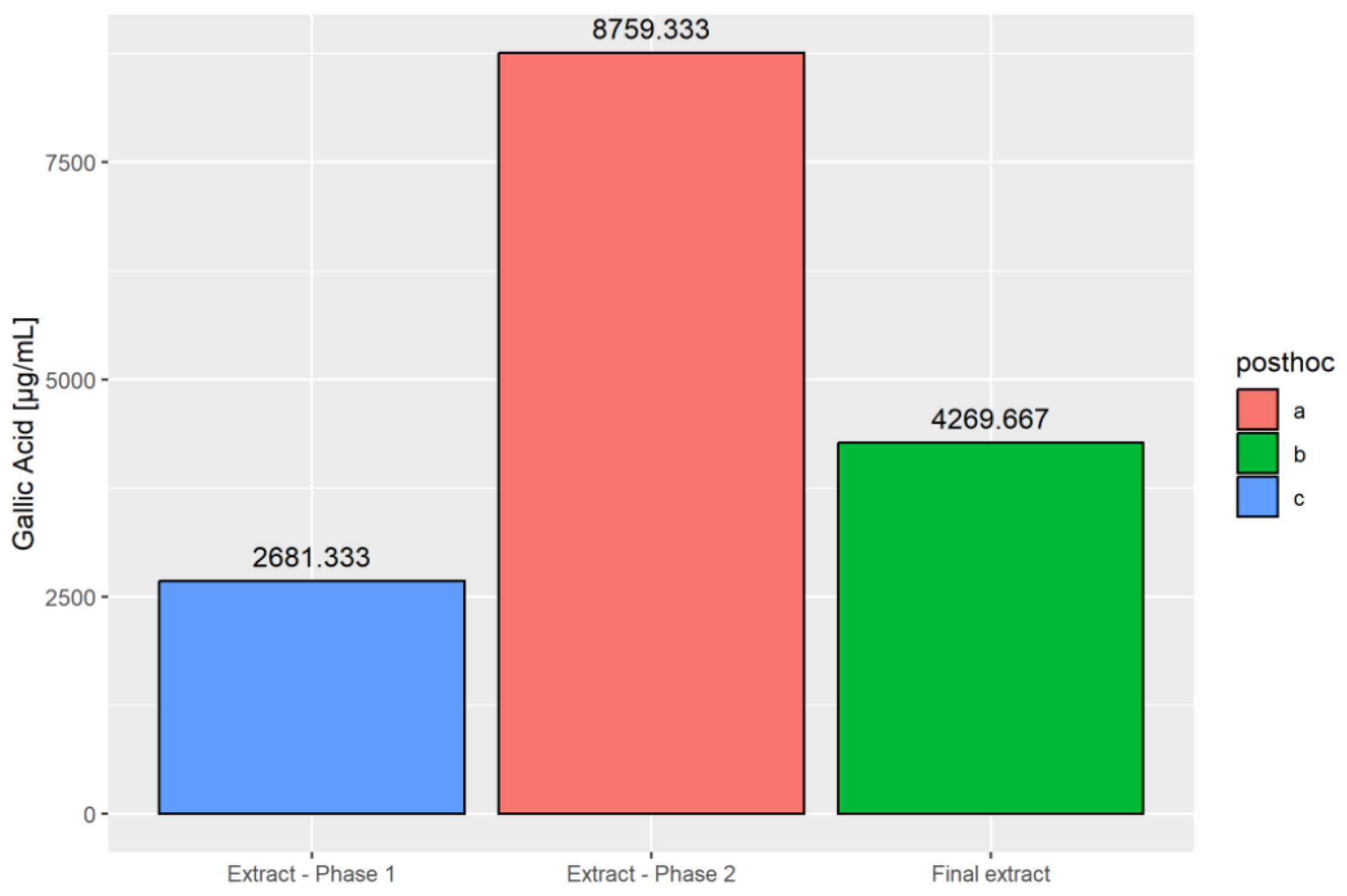
2.2. Extraction Process
2.3. Examination of the Bioactive Chemicals
2.4. Method for Analyzing Samples in a Controlled Laboratory Environment
2.5. HPLC-PDA Analysis of Major Phenolic Compounds
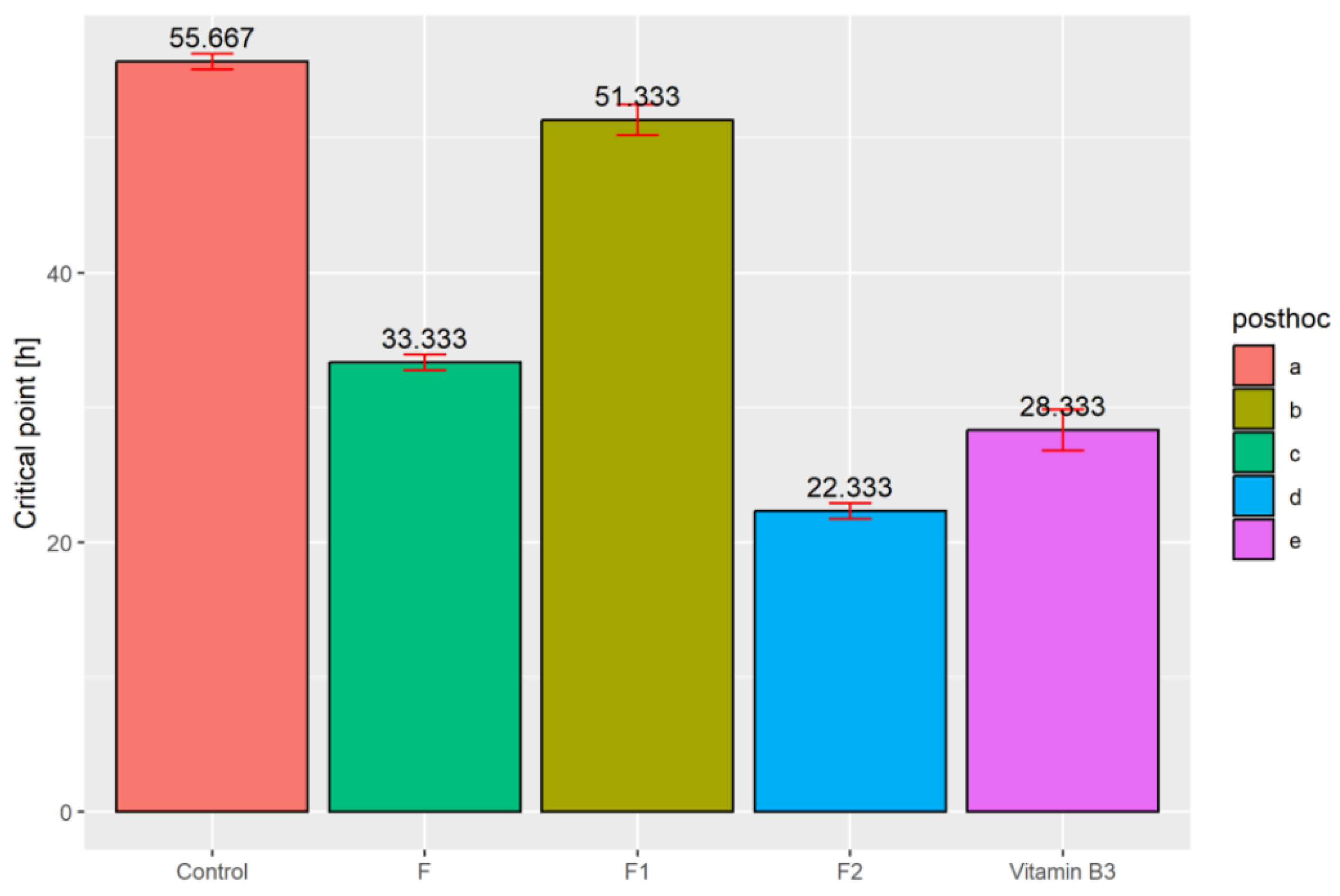
2.6. Determination of the In Vivo Antioxidant Potential under Blue Light
2.7. Statistical Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbanjan, N.P.; Eelager, M.P.; Korgaonkar, K.; Gonal, B.N.; Kadapure, A.J.; Arakera, S.B.; Kumar, S.P. Descriptive Review on Conversion of Waste Residues into Valuable Bionanocomposites for a Circular Bioeconomy. Nano-Struct. Nano-Objects 2024, 39, 101265. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224. [Google Scholar] [CrossRef]
- Krsmanović, N.; Rašeta, M.; Mišković, J.; Bekvalac, K.; Bogavac, M.; Karaman, M.; Isikhuemhen, O.S. Effects of UV Stress in Promoting Antioxidant Activities in Fungal Species Trametes versicolor (L.) Lloyd and Flammulina velutipes (Curtis) Singer. Antioxidants 2023, 12, 302. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(S) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Bachs-Herrera, A.; York, D.; Stephens-Jones, T.; Mabbett, I.; Yeo, J.; Martín-Martínez, F.J. Biomass Carbon Mining to Develop Nature-Inspired Materials for a Circular Economy. iScience 2023, 26, 106549. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Ketan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 6, 13. [Google Scholar] [CrossRef]
- Katiyar, S.K. Grape Seed Proanthocyanidines and Skin Cancer Prevention: Inhibition of Oxidative Stress and Protection of Immune System. Mol. Nutr. Food Res. 2008, 52, S71–S76. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Anim. Models Exp. Med. 2022, 6, 183–195. [Google Scholar] [CrossRef]
- Elroi, H.; Zbigniew, G.; Agnieszka, W.C.; Piotr, S. Enhancing Waste Resource Efficiency: Circular Economy for Sustainability and Energy Conversion. Front. Environ. Sci. 2023, 11, 1303792. [Google Scholar] [CrossRef]
- Marcelino, S.; Mandim, F.; Taofiq, O.; Pires, T.C.S.P.; Finimundy, T.C.; Prieto, M.A.; Barros, L. Valorization of Punica granatum L. Leaves Extracts as a Source of Bioactive Molecules. Pharmaceuticals 2023, 16, 342. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Avram, I.; Pelinescu, D.; Gatea, F.; Ionescu, R.; Barcan, A.; Rosca, R.; Zanfirescu, A.; Vamanu, E. Boletus edulis Extract—A New Modulator of Dysbiotic Microbiota. Life 2023, 13, 1481. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Sulaiman, C.; Balachandran, I. Total Phenolics and Total Flavonoids in Selected Indian Medicinal Plants. Indian J. Pharm. Sci. 2012, 74, 258. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS•+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Enescu, I.C.; Badea, G.; Vijan, L.E. The Influences of Genotype and Year on Some Biologically Active Compounds in Honeysuckle Berries. Horticulturae 2023, 9, 455. [Google Scholar] [CrossRef]
- Papa, C.M.; Suciu, A.; Dopcea, I.; Ene, N.; Singh, S.K.; Vamanu, E. Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments. Nutraceuticals 2023, 3, 306–314. [Google Scholar] [CrossRef]
- de Mendiburu, F. _agricolae: Statistical Procedures for Agricultural Research_. R Package Version 1.3-6. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 3 September 2024).
- Signorell, A. _DescTools: Tools for Descriptive Statistics_. R Package Version 0.99.54. 2024. Available online: https://CRAN.R-project.org/package=DescTools (accessed on 3 September 2024).
- Wickham, H. Ggplot2; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Lakicevic, M.; Povak, N.; Reynolds, K.M. Introduction to R for Terrestrial Ecology; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]

| Compound | Retention Time (Minute) | Concentration (µg/100 g Dry Extract) |
|---|---|---|
| Galic acid | 3.63 | 37.41 ± 2.74 |
| Catechin | 7.99 | 373.29 ± 27.65 |
| Epicatechin | 9.77 | 33.39 ± 0.08 |
| Hesperidin | 15.00 | 5.22 ± 1.12 |
| Resveratrol | 17.37 | 2.14 ± 0.36 |
| Rutin | 12.73 | 7.74 ± 3.10 |
| Isoquercetin | 13.50 | 3.74 ± 0.32 |
| Myricetin | 15.98 | 2.38 ± 0.47 |
| Quercetin | 18.49 | 1.32 ± 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamanu, E.; Lakićević, M.; Dedović, N.; Dumitru, G.; Badea, I.G.; Gatea, F.; Dinu, L.D. Evaluating the Efficacy of an Extract for UV Defense and Mitigation of Oxidative Stress, Transitioning from Biomass to Bioprotection. Agronomy 2024, 14, 2306. https://doi.org/10.3390/agronomy14102306
Vamanu E, Lakićević M, Dedović N, Dumitru G, Badea IG, Gatea F, Dinu LD. Evaluating the Efficacy of an Extract for UV Defense and Mitigation of Oxidative Stress, Transitioning from Biomass to Bioprotection. Agronomy. 2024; 14(10):2306. https://doi.org/10.3390/agronomy14102306
Chicago/Turabian StyleVamanu, Emanuel, Milena Lakićević, Nebojša Dedović, Georgiana Dumitru, Ileana Georgiana Badea, Florentina Gatea, and Laura Dorina Dinu. 2024. "Evaluating the Efficacy of an Extract for UV Defense and Mitigation of Oxidative Stress, Transitioning from Biomass to Bioprotection" Agronomy 14, no. 10: 2306. https://doi.org/10.3390/agronomy14102306









