Effects of Long-Term Sustainable Inorganic Fertilization on Rice Productivity and Fertility of Quaternary Red Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Experimental Design
2.3. Determination of Soil Properties and Rice Yield
2.4. Sampling and DNA Extraction for 16s RNA and ITS2 Sequence
2.5. Sequence Data Analysis
2.6. Statistical Analyses
3. Results
3.1. Soil Physical and Chemical Properties
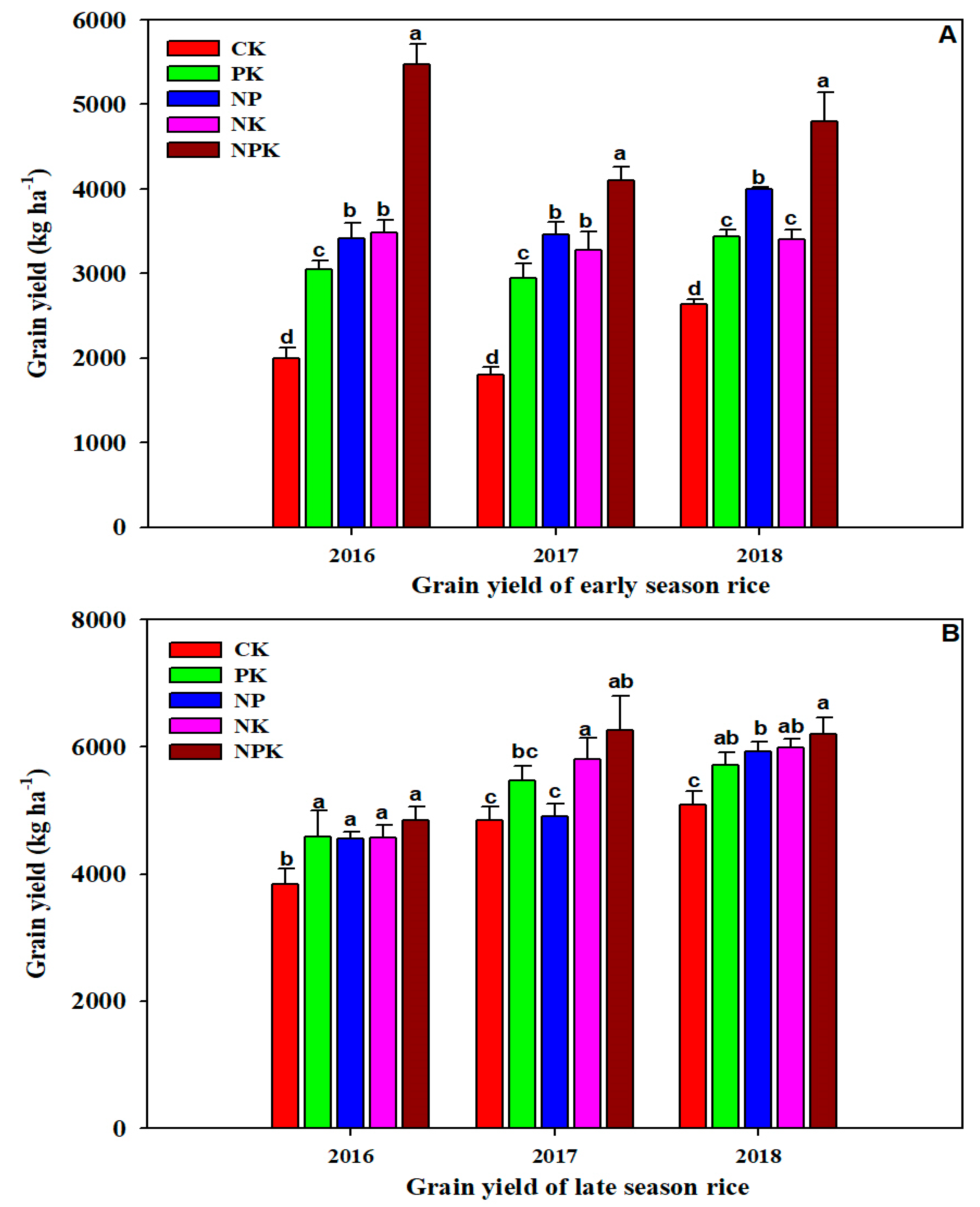
3.2. Rice Yield in Recent Three Years
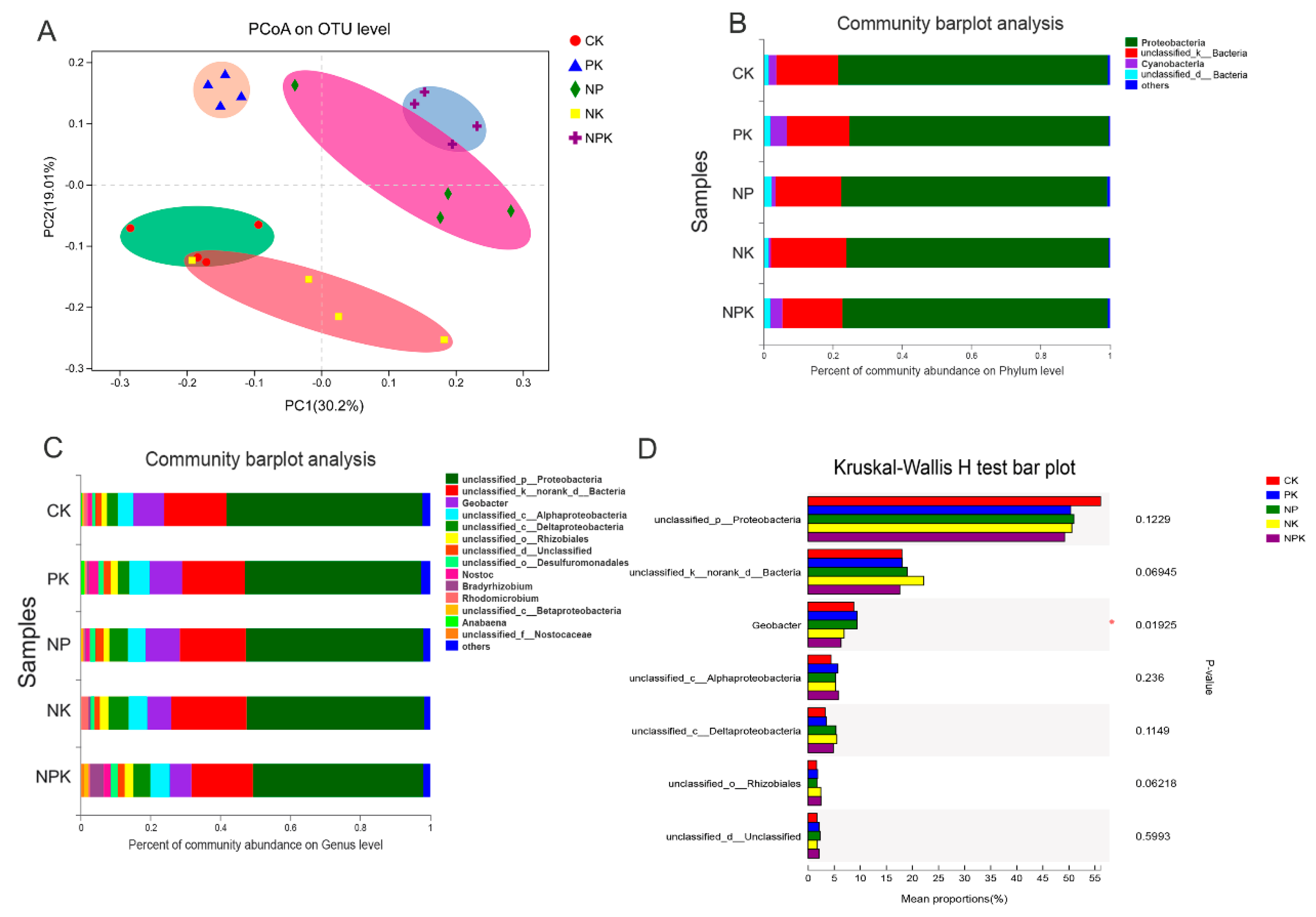
3.3. Effects of Different Fertilization Strategies on Bacterial Diversity
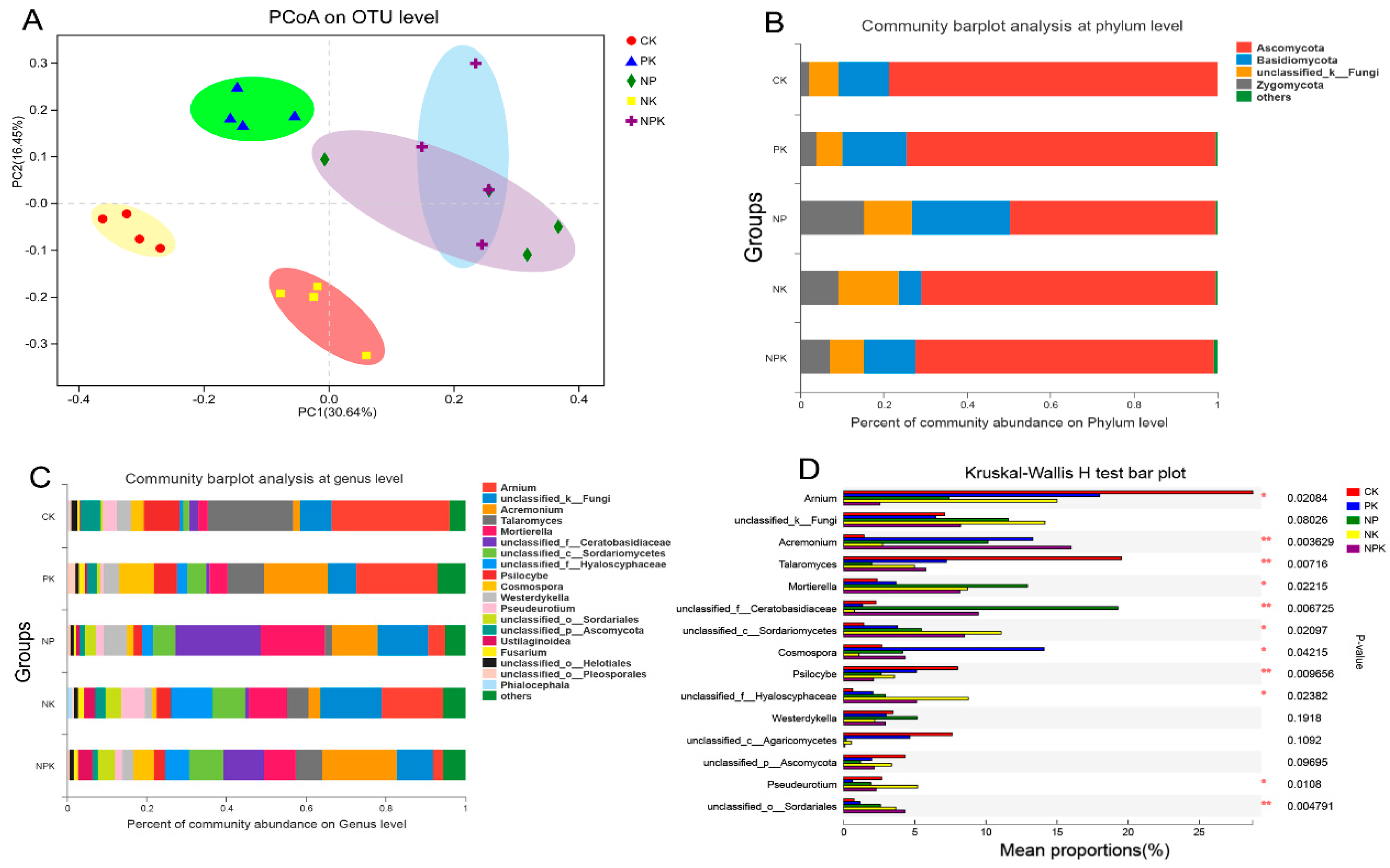
3.4. Effects of Different Fertilization Strategies on Fungal Diversity
3.5. Effects of Different Fertilization Strategies on Nitrogen-Fixing Bacteria
3.6. Effects of Different Fertilization Strategies on Denitrifying Bacteria
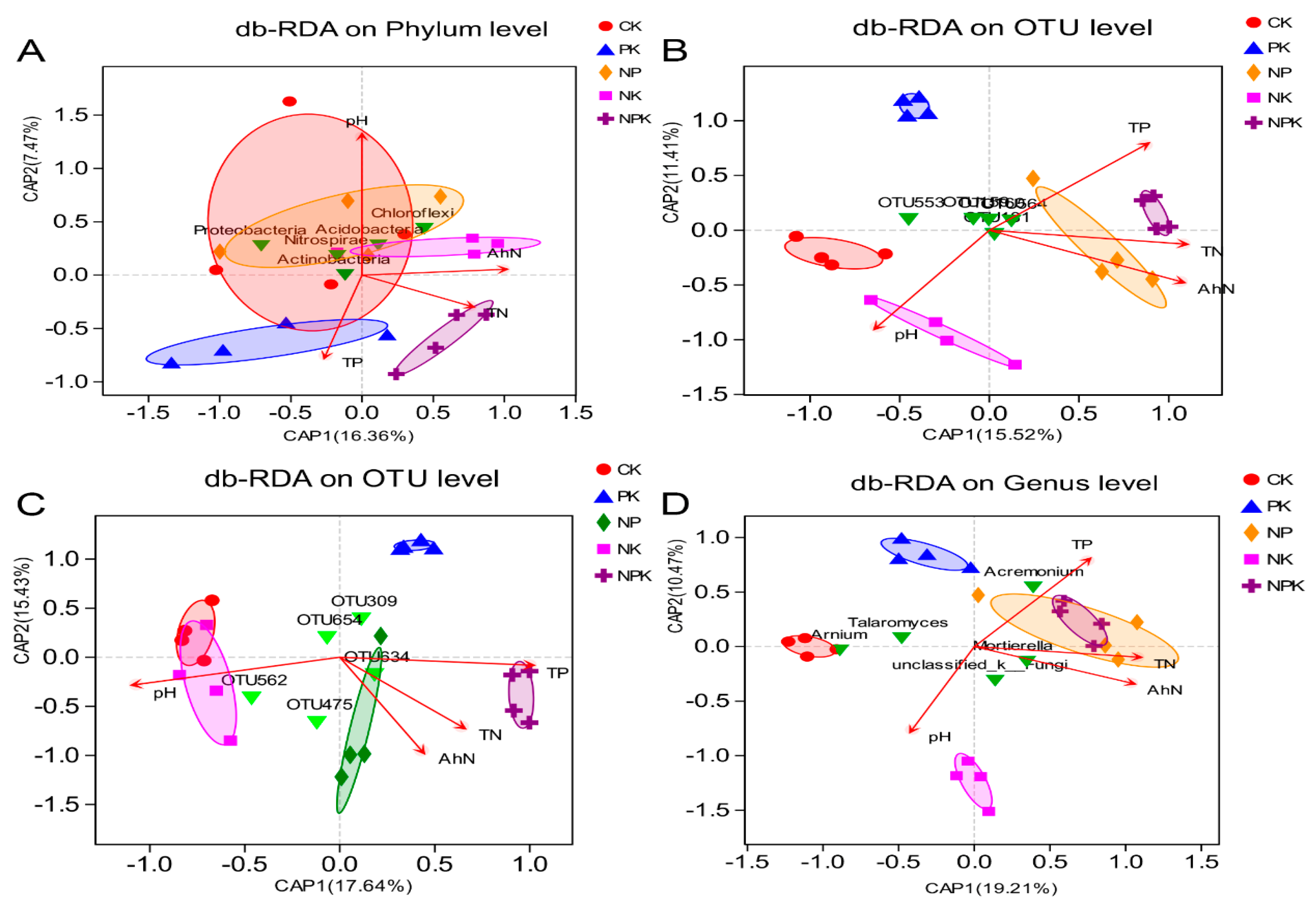
3.7. Potential Drivers of Bacterial Community Compositions
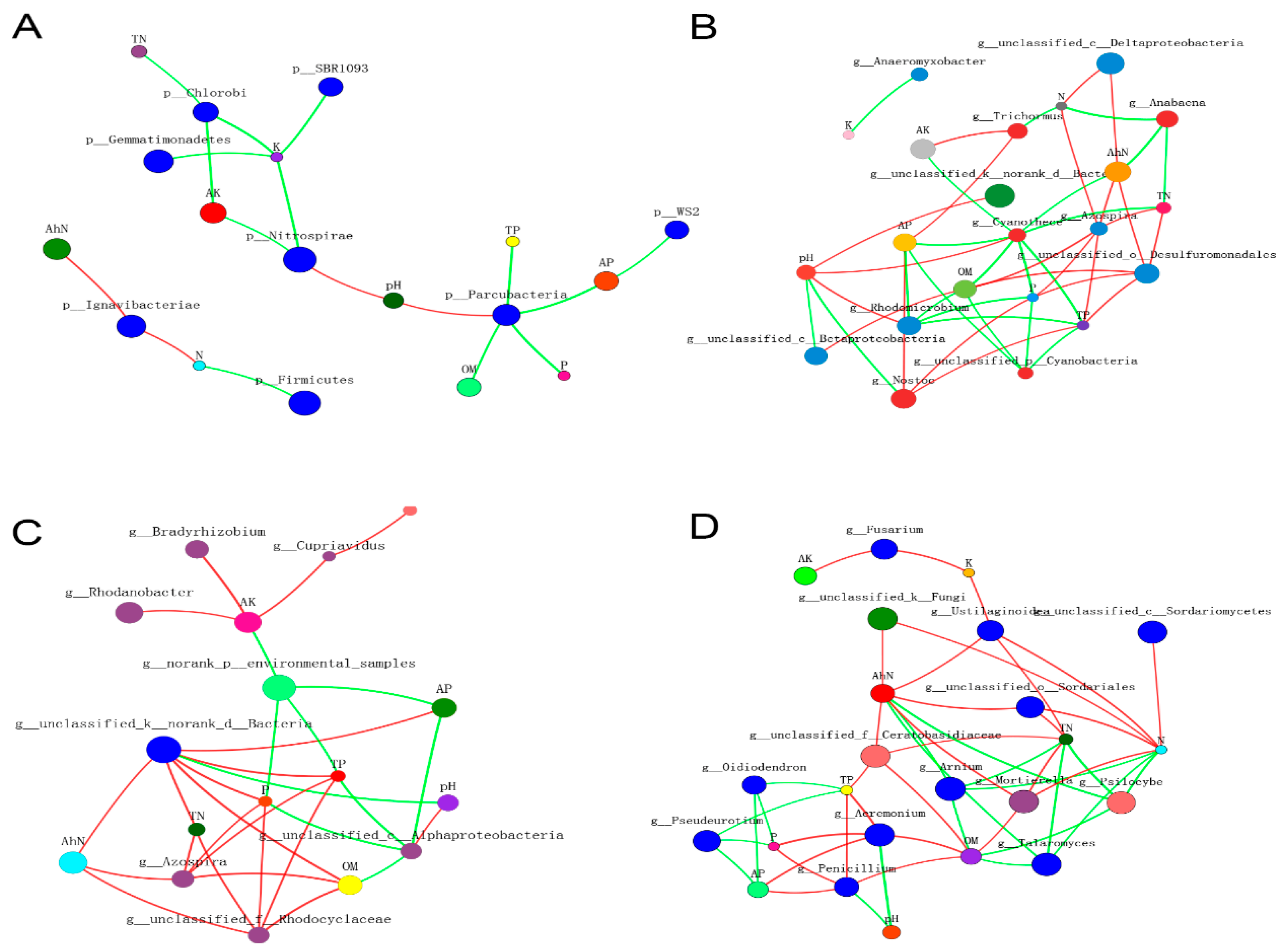
3.8. Two-Way Correlation Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khmelevtsova, L.; Konstantinova, E.; Karchava, S.; Klimova, M.; Azhogina, T.; Polienko, E.; Khammami, M.; Sazykin, I.; Sazykina, M. Influence of pesticides and mineral fertilizers on the bacterial community of arable soils under pea and chickpea crops. Agronomy 2023, 13, 750. [Google Scholar] [CrossRef]
- Bedano, J.C.; Lavelle, P.; Zou, X. Soil biodiversity for the sustainability of agroecosystems. Acta Oecol. 2021, 110, 103705. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Y.; Wang, J. Spatiotemporal patterns of gross ecosystem product across China’s cropland ecosystems over the past two decades. Front. Ecol. Evol. 2022, 10, 959329. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Sun, Z.; Li, S.; Liang, Y. Research progress on influencing factors of soil microbial biomass and diversity. North Hortic. 2022, 503, 116–121. [Google Scholar]
- Jin, C.; Zheng, M.-J.; Pang, D.-W.; Yin, Y.-P.; Han, M.-M.; Li, Y.-X.; Luo, Y.-L.; Xu, X.; Yong, L.; Wang, Z.-L. Straw return and appropriate tillage method improve grain yield and nitrogen efficiency of winter wheat. J. Integr. Agric. 2017, 16, 1708–1719. [Google Scholar]
- Li, L.; Feng, J.; Liu, M.; Mei, H.; Kang, Z.; Cai, Q. Effect of crop planting patterns on soil microorganisms and crop pests in farmland. Chin. Agri. Sci. Bull. 2021, 37, 99–106. [Google Scholar]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Gao, X.F.; Yan, B.S.; Wu, C.X.; Wang, G. Effects of long term fertilization on soil quality and millet yield on slope farmland in loess hilly areas. Agric. Res. Arid Areas 2021, 39, 76–83. [Google Scholar]
- Li, X.; Zhao, B.Q.; Li, X.H.; Li, Y.T.; Sun, R.L.; Zhu, L.S.; Xu, J.; Wang, L.X.; Li, X.P.; Zhang, Z.F. Effects of different fertilization systems on soil microbe and its relation to soil fertility. Zhongguo Nongye Kexue 2005, 38, 1591–1599. [Google Scholar]
- Wu, M.; Qin, H.; Chen, Z.; Wu, J.; Wei, W. Effect of long-term fertilization on bacterial composition in rice paddy soil. Biol. Fertil. Soils 2011, 47, 397–405. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Mo, R.; Deng, W.; Zhu, Z.; Liu, D.; Hu, X. Effects of long-term straw retention on soil microorganisms under a rice–wheat cropping system. Arch. Microbiol. 2020, 202, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Kang, G.; Beri, V.; Sidhu, B.; Rupela, O. A new index to assess soil quality and sustainability of wheat-based cropping systems. Biol. Fertil. Soils 2005, 41, 389–398. [Google Scholar] [CrossRef]
- Nakhro, N.; Dkhar, M. Populations and biomass carbon in paddy field soil. Agron. J. 2010, 9, 102–110. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Strauss, S.L.; González-López, J.; Bedmar, E.J. Changes in the diversity and predicted functional composition of the bulk and rhizosphere soil bacterial microbiomes of tomato and common bean after inorganic N-fertilization. Rhizosphere 2021, 18, 100362. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Mei, N.; Zhang, X.; Wang, X.; Peng, C.; Gao, H.; Zhu, P.; Gu, Y. Effects of 40 years applications of inorganic and organic fertilization on soil bacterial community in a maize agroecosystem in northeast China. Eur. J. Agron. 2021, 130, 126332. [Google Scholar] [CrossRef]
- Su, P.; Lou, J.; Brookes, P.C.; Luo, Y.; He, Y.; Xu, J. Taxon-specific responses of soil microbial communities to different soil priming effects induced by addition of plant residues and their biochars. J. Soils Sediments 2017, 17, 674–684. [Google Scholar] [CrossRef]
- Lu, Z.; Deng, Y.; Van Nostrand, J.D.; He, Z.; Voordeckers, J.; Zhou, A.; Lee, Y.-J.; Mason, O.U.; Dubinsky, E.A.; Chavarria, K.L. Microbial gene functions enriched in the deep-water horizon deep-sea oil plume. ISME J. 2012, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dwevedi, A.; Kumar, P.; Kumar, P.; Kumar, Y.; Sharma, Y.K.; Kayastha, A.M. Soil sensors: Detailed insight into research updates, significance, and future prospects. In New Pesticides and Soil Sensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 561–594. [Google Scholar]
- Feng, Z.F.Z.; Wang, P.W.P.; Liu, X.L.X.; Chen, X.C.X.; Ji, J.J.J.; Hou, H.H.H.; Liu, Y.L.Y.; Xia, W.X.W.; Lv, Z.L.Z.; Liu, G.L.G. Research progress and perspective of red soil improvement and utilization technologies in China. Acta Agric. Jiangxi 2017, 29, 57–61. [Google Scholar]
- Cao, C.; Jiang, S.; Ying, Z.; Zhang, F.; Han, X. Spatial variability of soil nutrients and microbiological properties after the establishment of leguminous shrub Caragana microphylla Lam. plantation on sand dune in the Horqin Sandy Land of Northeast China. Ecol. Eng. 2011, 37, 1467–1475. [Google Scholar] [CrossRef]
- Campbell, B.J.; Polson, S.W.; Hanson, T.E.; Mack, M.C.; Schuur, E.A. The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ. Microbiol. 2010, 12, 1842–1854. [Google Scholar] [CrossRef]
- Wessén, E.; Hallin, S.; Philippot, L. Differential responses of bacterial and archaeal groups at high taxonomical ranks to soil management. Soil Biol. Biochem. 2010, 42, 1759–1765. [Google Scholar] [CrossRef]
- Gorfer, M.; Blumhoff, M.; Klaubauf, S.; Urban, A.; Inselsbacher, E.; Bandian, D.; Mitter, B.; Sessitsch, A.; Wanek, W.; Strauss, J. Community profiling and gene expression of fungal assimilatory nitrate reductases in agricultural soil. ISME J. 2011, 5, 1771–1783. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Teotia, P.; Kumar, V.; Kumar, M.; Shrivastava, N.; Varma, A. Rhizosphere microbes: Potassium solubilization and crop productivity–present and future aspects. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 315–325. [Google Scholar]
- Youssef, M.; Eissa, M. Biofertilizers and their role in management of plant parasitic nematodes. A review. J. Biotechnol. Pharma Res. 2014, 5, 1–6. [Google Scholar]
- Homer, D.C.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; University of California: Davis, CA, USA, 1961; pp. 1–99. [Google Scholar]
- Celi, L.; Said-Pullicino, D.; Bol, R.; Lang, F.; Luster, J. Interconnecting soil organic matter with nitrogen and phosphorus cycling. Multi-Scale Biogeochemical Processes in Soil Ecosystems: Critical Reactions and Resilience to Climate Changes; Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 51–77. [Google Scholar]
- Wang, J.L.; Liu, K.L.; Zhao, X.Q.; Zhang, H.Q.; Li, D.; Li, J.J.; Shen, R.F. Balanced fertilization over four decades has sustained soil microbial communities and improved soil fertility and rice productivity in red paddy soil. Sci. Total Environ. 2021, 793, 148664. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, T.; Lei, X.-Y.; Cui, X.-W.; Lu, Y.-X.; Fan, P.-F.; Long, S.-P.; Huang, J.; Gao, J.-S.; Zhang, Z.-H. Improvement of soil fertility and rice yield after long-term application of cow manure combined with inorganic fertilizers. J. Integr. Agric. 2023, 22, 2221–2232. [Google Scholar]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Bai, Y.-C.; Chang, Y.-Y.; Hussain, M.; Lu, B.; Zhang, J.-P.; Song, X.-B.; Lei, X.-S.; Pei, D. Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Ding, L.J.; Xue, K.; Yao, H.Y.; Quensen, J.; Bai, S.J.; Wei, W.X.; Wu, J.S.; Zhou, J.; Tiedje, J.M. Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol. Ecol. 2015, 24, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.; Park, K.-C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Coolon, J.D.; Jones, K.L.; Todd, T.C.; Blair, J.M.; Herman, M.A. Long-term nitrogen amendment alters the diversity and assemblage of soil bacterial communities in tallgrass prairie. PLoS ONE 2013, 8, e67884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Cao, F.; Shen, D.; Guan, D.; Li, J. Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci. Rep. 2017, 7, 3267. [Google Scholar] [CrossRef]
- de Quadros, P.D.; Zhalnina, K.; Davis-Richardson, A.G.; Drew, J.C.; Menezes, F.B.; Camargo, F.A.d.O.; Triplett, E.W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, Y.; He, T.; Liao, R.; Liu, R.; Yi, M.; Huang, L.; Yang, Z.; Fu, T.; Li, X. Soil nitrogen leaching decreases as biogas slurry DOC/N ratio increases. Appl. Soil Ecol. 2017, 111, 105–113. [Google Scholar] [CrossRef]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Qasim, W.; Zhao, Y.; Wan, L.; Lv, H.; Lin, S.; Gettel, G.M.; Butterbach-Bahl, K. The potential importance of soil denitrification as a major N loss pathway in intensive greenhouse vegetable production systems. Plant Soil 2022, 471, 157–174. [Google Scholar] [CrossRef]
- Wang, J.W.J.; Song-Yang, S.Y.; Ma, M.M.T.; Raza, W.; Li Jing, L.J.; Howland, J.; Huang, Q.H.Q.; Shen, Q.S.Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173, 330–338. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Detheridge, A.P.; Brand, G.; Fychan, R.; Crotty, F.V.; Sanderson, R.; Griffith, G.W.; Marley, C.L. The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agric. Ecosyst. Environ. 2016, 228, 49–61. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Kumla, D.; Dethoup, T.; Buttachon, S.; Singburaudom, N.; Silva, A.M.; Kijjoa, A. Spiculisporic acid E, a new spiculisporic acid derivative and ergosterol derivatives from the marine-sponge associated fungus Talaromyces trachyspermus (KUFA 0021). Nat. Prod. Commun. 2014, 9, 1147–1150. [Google Scholar] [CrossRef]
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef]
- Dethoup, T.; Kaewsalong, N.; Songkumorn, P.; Jantasorn, A. Potential application of a marine-derived fungus, Talaromyces tratensis KUFA 0091 against rice diseases. Biol. Control 2018, 119, 1–6. [Google Scholar] [CrossRef]
- Park, S.W.; Nguyen, T.T.; Lee, H.B. Characterization of two species of Acremonium (unrecorded in Korea) from soil samples: A. variecolor and A. persicinum. Mycobiology 2017, 45, 353–361. [Google Scholar] [CrossRef]
- Nicoletti, R.; Vinale, F. Bioactive compounds from marine-derived Aspergillus, Penicillium, Talaromyces and Trichoderma species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef]
- Veldre, V.; Abarenkov, K.; Bahram, M.; Martos, F.; Selosse, M.-A.; Tamm, H.; Kõljalg, U.; Tedersoo, L. Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol. 2013, 6, 256–268. [Google Scholar] [CrossRef]
- Jenkins, B.D.; Spinette, R.; Jones, A.; Puggioni, G.; Ehrlich, A.; Brown, S.M. Nitrogen fixation by anaerobes is stimulated by low oxygen and insensitive to combined nitrogen in coastal sediments. In Proceedings of the American Geophysical Union, Ocean Sciences Meeting, New Orleans, LA, USA, 21–26 February 2016; p. B34B-0366. [Google Scholar]
- Zhu, L.; Zeng, C.; Gao, F.; Shi, A.; Mao, H.; Wei, W. Characteristic analysis of microbial diversity in crud fertilizer from compost of rice straw. Trans. Chin. Soc. Agric. Mach. 2018, 49, 228–234. [Google Scholar]
- Turner, M. The evolutionary tree of fungi grows a new branch. Nat. News 2011, 11. [Google Scholar] [CrossRef]
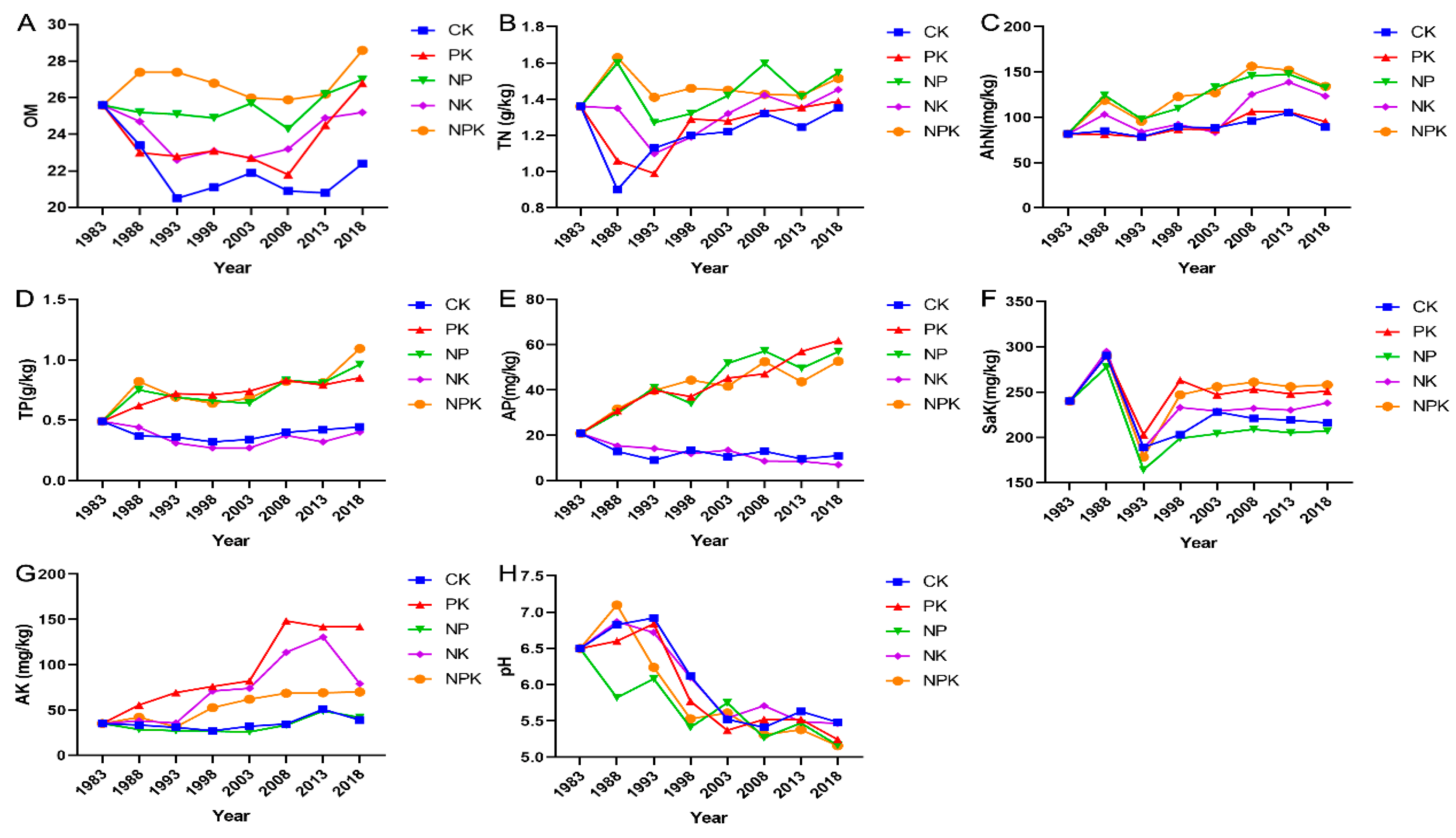



| OM g kg−1 | TN g kg−1 | TP g kg−1 | Sap mg kg−1 | AhN mg kg−1 | AP mg kg−1 | AK mg kg−1 | Cec Cmol kg−1 | pH |
|---|---|---|---|---|---|---|---|---|
| 25.6 | 1.36 | 0.49 | 240 | 81.6 | 20.8 | 35.0 | 7.54 | 6.50 |
| Groups | Plant | Basic Fertilizer | Tillering Stage | Panicle Differentiation Stage | |||
|---|---|---|---|---|---|---|---|
| P2O5 | N | N | K2O | N | K2O | ||
| CK | Early | 0 | 0 | 0 | 0 | 0 | 0 |
| Late | 0 | 0 | 0 | 0 | 0 | 0 | |
| PK | Early | 60.0 | 0 | 0 | 75.0 | 0 | 75.0 |
| Late | 60.0 | 0 | 0 | 75.0 | 0 | 75.0 | |
| NP | Early | 60.0 | 74.9 | 37.4 | 0 | 37.4 | 0 |
| Late | 60.0 | 89.8 | 44.9 | 0 | 44.9 | 0 | |
| NK | Early | 0 | 74.9 | 37.4 | 75.0 | 37.4 | 75 |
| Late | 0 | 89.8 | 44.9 | 75.0 | 44.9 | 75 | |
| NPK | Early | 60.0 | 74.9 | 37.4 | 75.0 | 37.4 | 75 |
| Late | 60.0 | 89.8 | 44.9 | 75.0 | 44.9 | 75 | |
| Shannon | Simpson | Ace | Chao | ||
|---|---|---|---|---|---|
| Bacteria | CK | 6.97 a | 0.0027 b | 3967.3 | 3987.6 a |
| PK | 6.93 a | 0.0029 b | 3907.1 | 3924.4 b | |
| NP | 6.96 a | 0.0026 b | 3842.7 | 3829.1 c | |
| NK | 6.89 a | 0.0025 b | 3707.2 | 3720.0 d | |
| NPK | 6.79 a | 0.0033 a | 3689.3 | 3697.9 e | |
| Fungi | CK | 2.95 c | 0.1271 a | 602.7 c | 609.8 c |
| PK | 3.36 b | 0.0939 b | 745.8 a | 736.5 a | |
| NP | 3.88 a | 0.0492 c | 607.4 cade | 609.8 c | |
| NK | 3.47 a | 0.0673 b | 678.3 b | 657.9 b | |
| NPK | 3.33 b | 0.0884 b | 563.7 dc | 554.1 d | |
| Nitrogen-fixing bacteria | CK | 5.69 a | 0.0070 b | 963.7 a | 955.7 a |
| PK | 5.68 a | 0.0071 b | 918.0 b | 920.6 c | |
| NP | 5.76 a | 0.0058 b | 905.2 c | 899.8 d | |
| NK | 5.64 a | 0.0093 a | 922.5 b | 939.9 b | |
| NPK | 5.67 a | 0.0067 b | 868.9 d | 851.8 e | |
| Denitrifying bacteria | CK | 4.40 a | 0.0264 a | 555.9 a | 524.7 a |
| PK | 4.31 ac | 0.0272 ac | 483.6 bc | 481.6 b | |
| NP | 4.41 b | 0.0315 b | 505.1 b | 494.2 b | |
| NK | 4.08 ab | 0.0346 b | 422.0 c | 414.8 d | |
| NPK | 4.01 b | 0.0452 b | 438.8 bc | 441.7 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hou, H.; Lan, X.; Ji, J.; Liu, X.; Lv, Z.; Chen, L. Effects of Long-Term Sustainable Inorganic Fertilization on Rice Productivity and Fertility of Quaternary Red Soil. Agronomy 2024, 14, 2311. https://doi.org/10.3390/agronomy14102311
Liu Y, Hou H, Lan X, Ji J, Liu X, Lv Z, Chen L. Effects of Long-Term Sustainable Inorganic Fertilization on Rice Productivity and Fertility of Quaternary Red Soil. Agronomy. 2024; 14(10):2311. https://doi.org/10.3390/agronomy14102311
Chicago/Turabian StyleLiu, Yiren, Hongqian Hou, Xianjin Lan, Jianhua Ji, Xiumei Liu, Zhenzhen Lv, and Liumeng Chen. 2024. "Effects of Long-Term Sustainable Inorganic Fertilization on Rice Productivity and Fertility of Quaternary Red Soil" Agronomy 14, no. 10: 2311. https://doi.org/10.3390/agronomy14102311




