Use of Beauveria bassiana and Bacillus amyloliquefaciens Strains as Gossypium hirsutum Seed Coatings: Evaluation of the Bioinsecticidal and Biostimulant Effects in Semi-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Protocol for Coating Seeds with B. bassiana and B. amyloliquefaciens
2.3. Experimental Set-Up
2.4. Recording of A. gossypii Population and Cotton Growth Characteristics
2.5. Total Chlorophyll Content
2.6. Proline Content
2.7. Statistical Analysis
3. Results
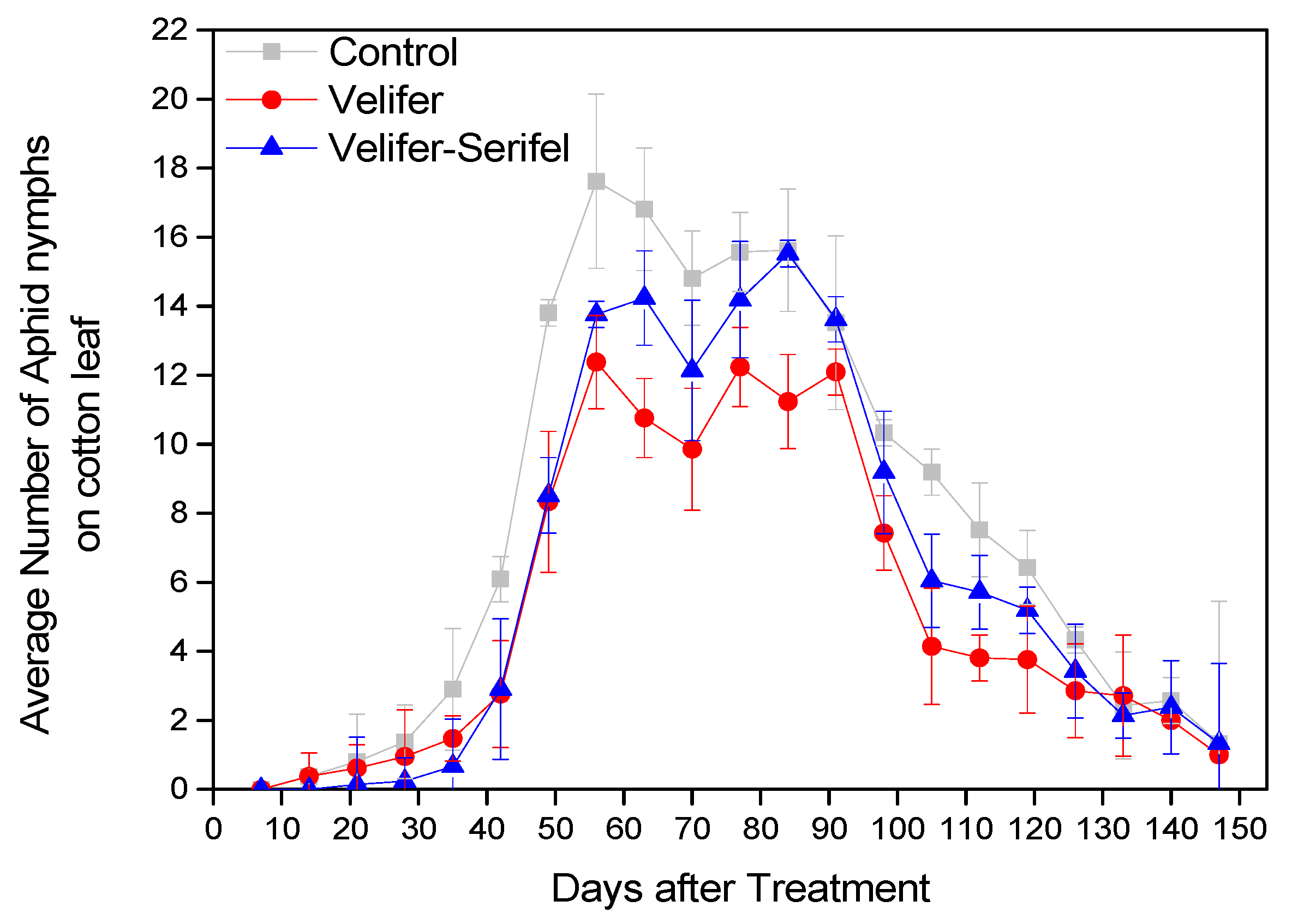
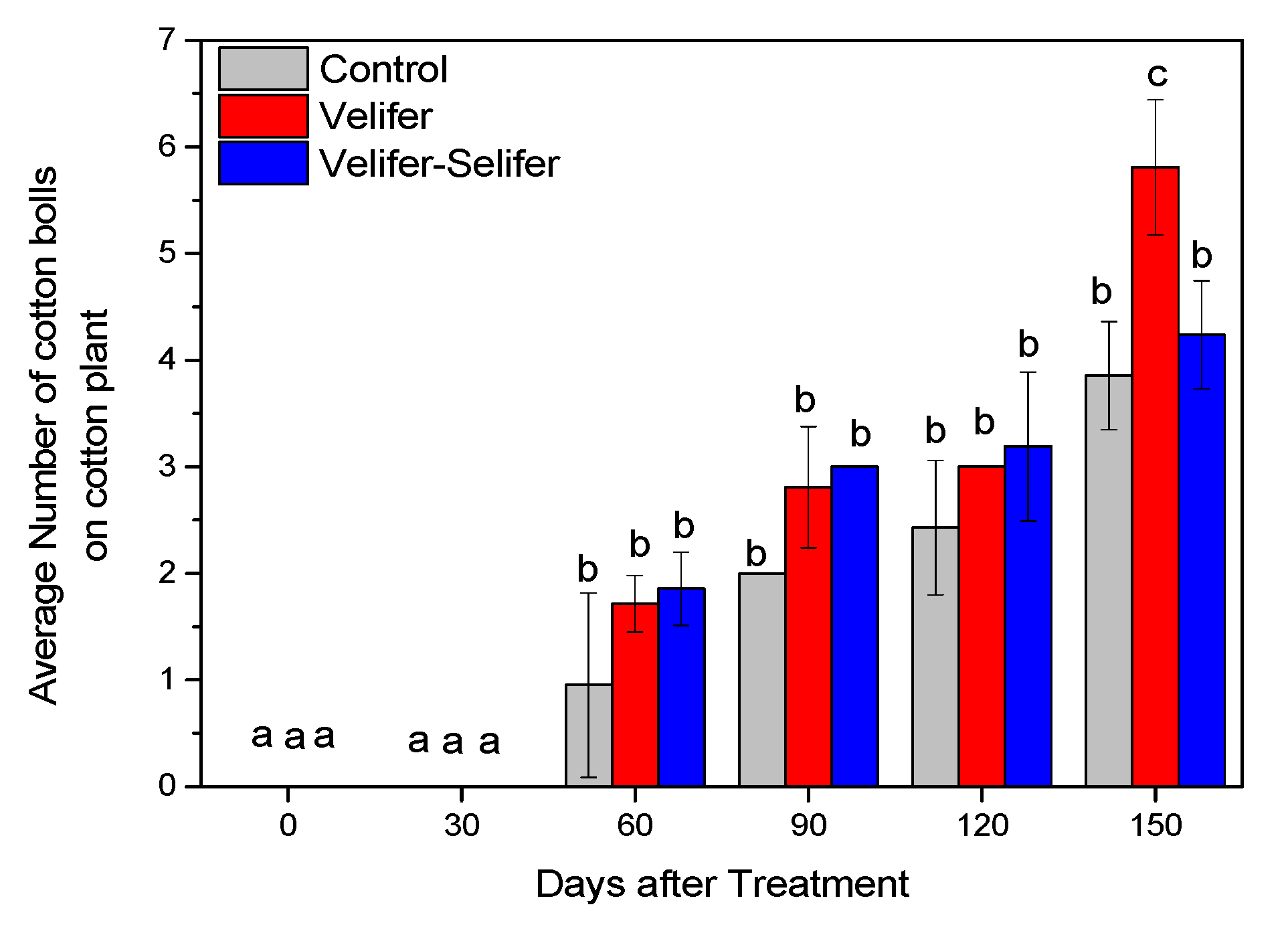
3.1. Insect Pest Population
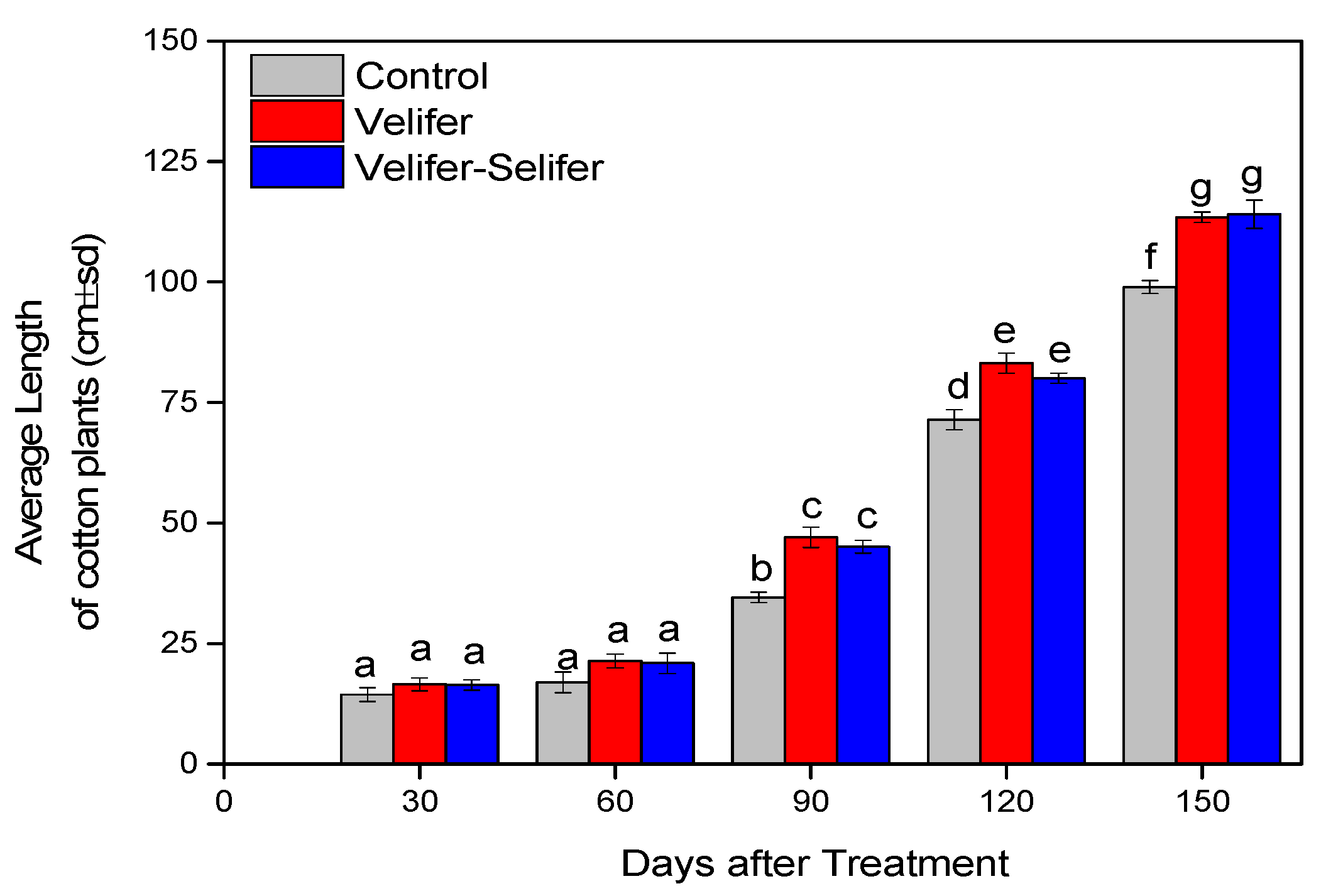
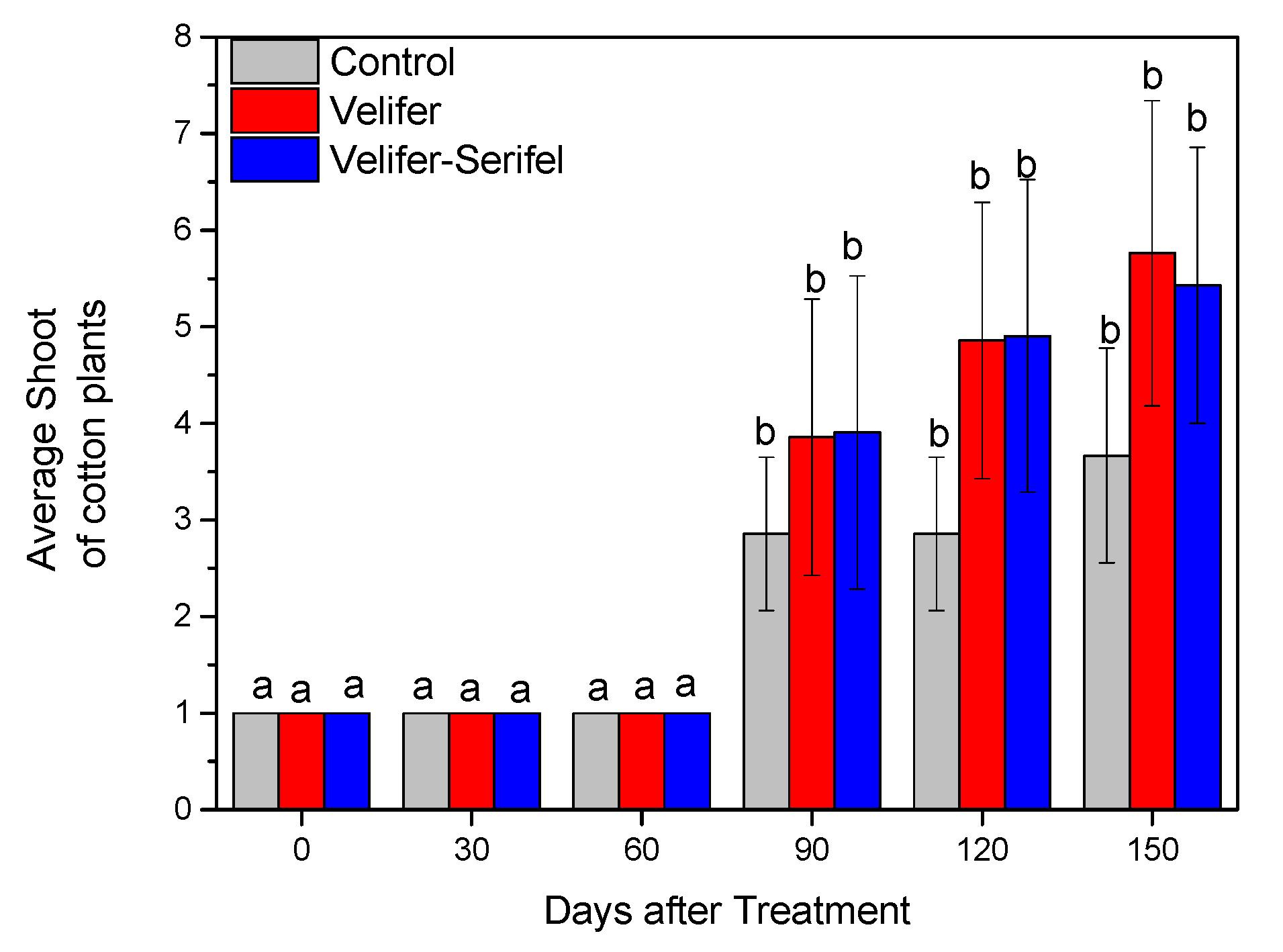
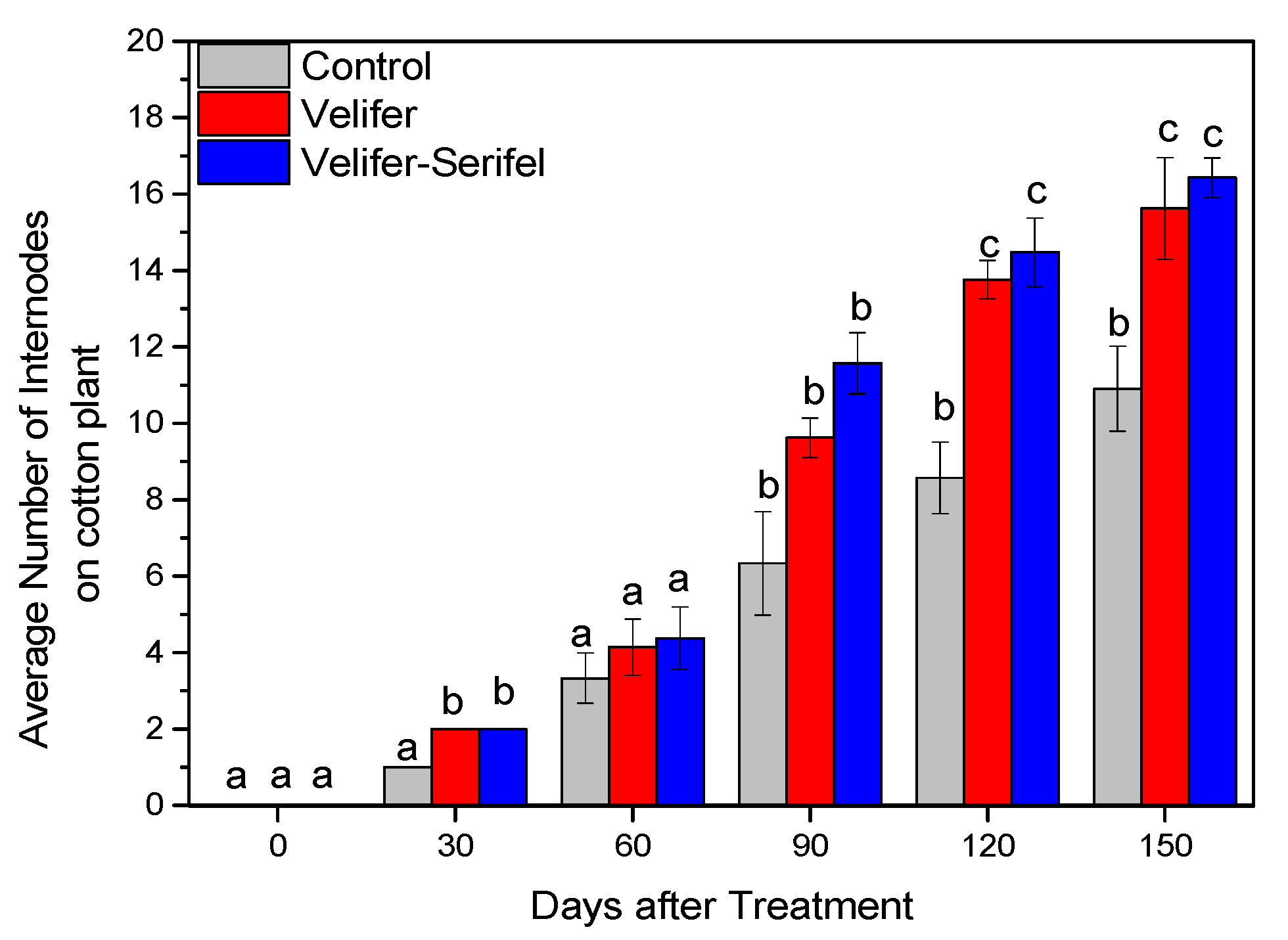
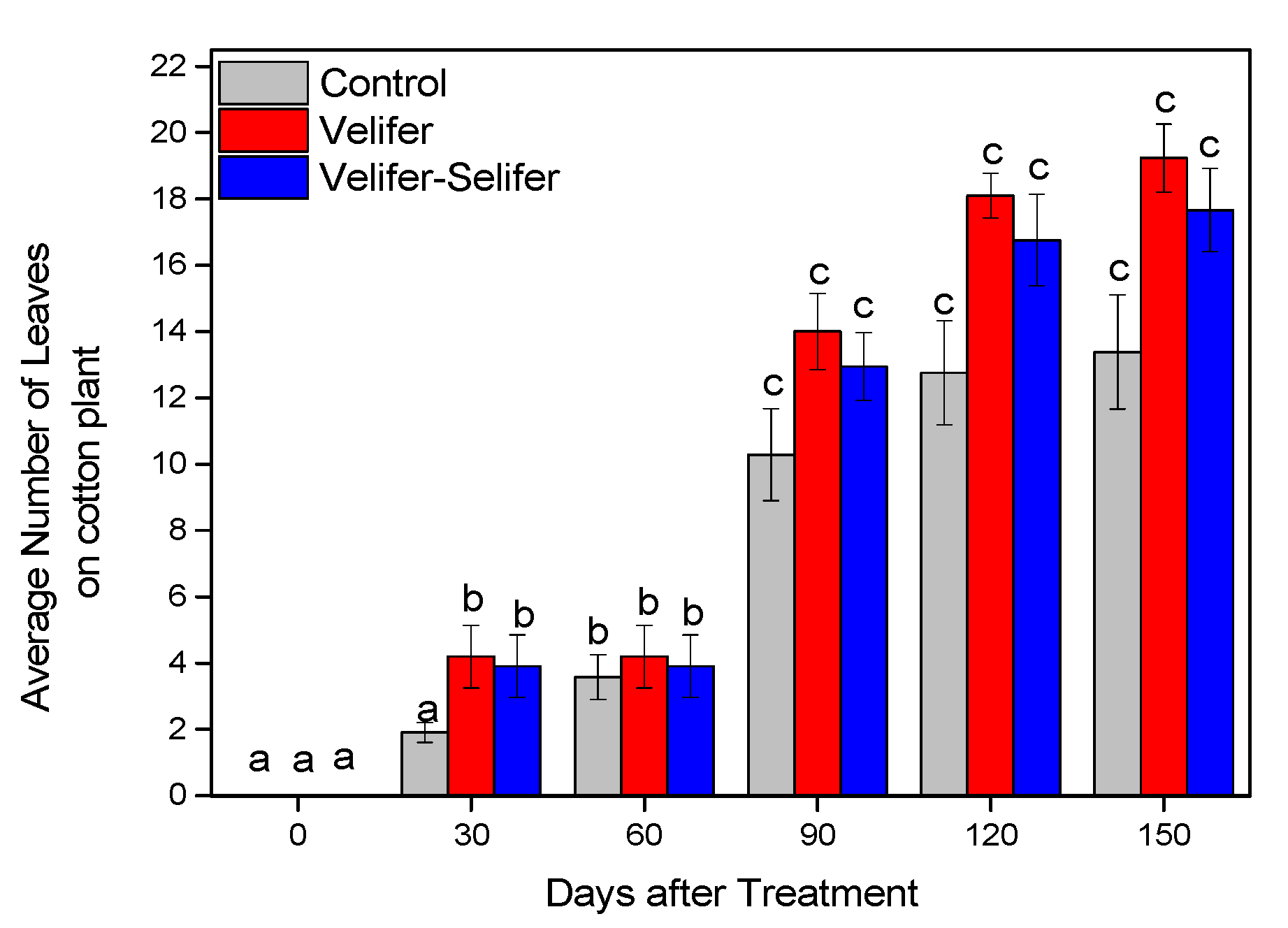
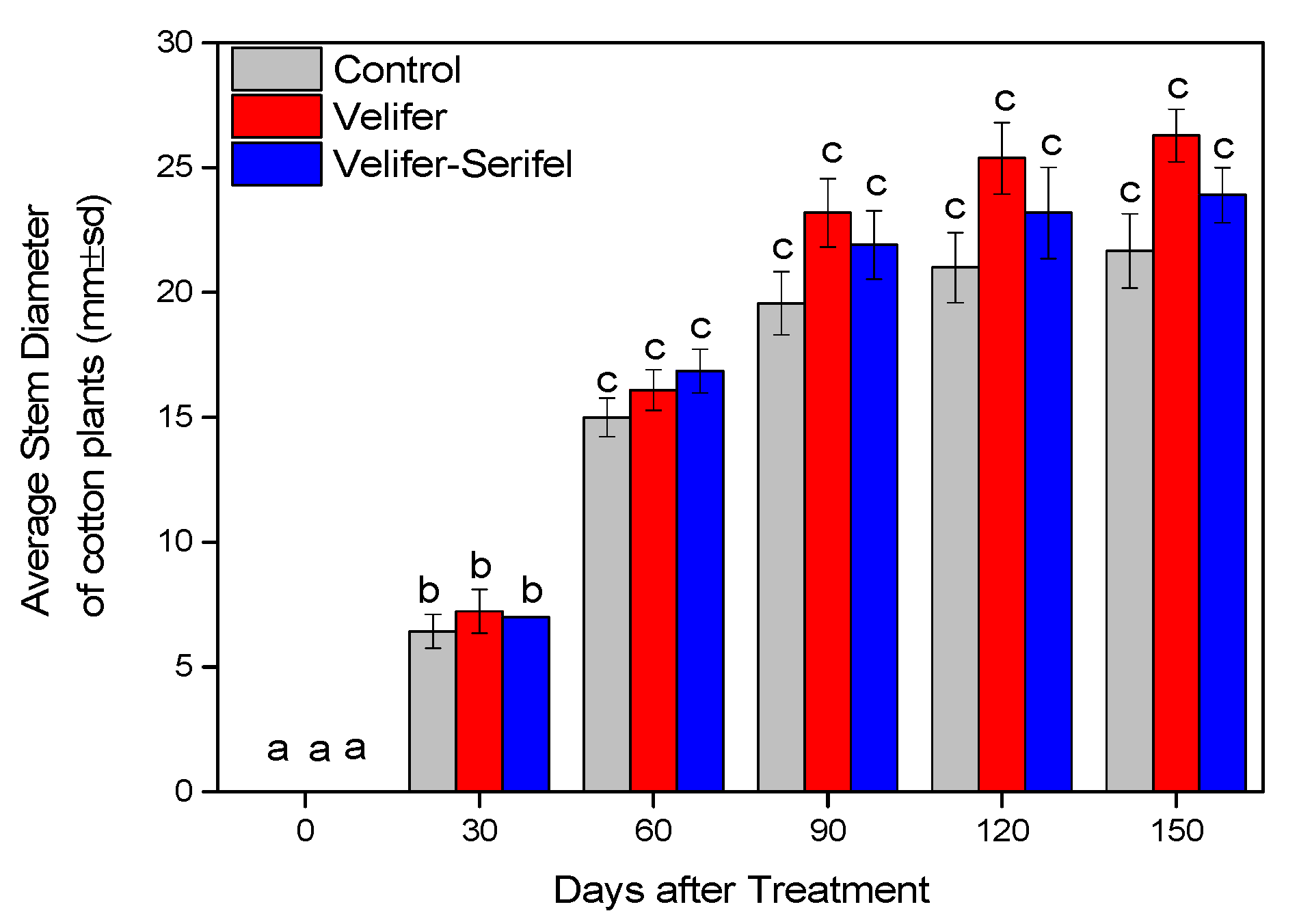
3.2. Effect on Plant Growth
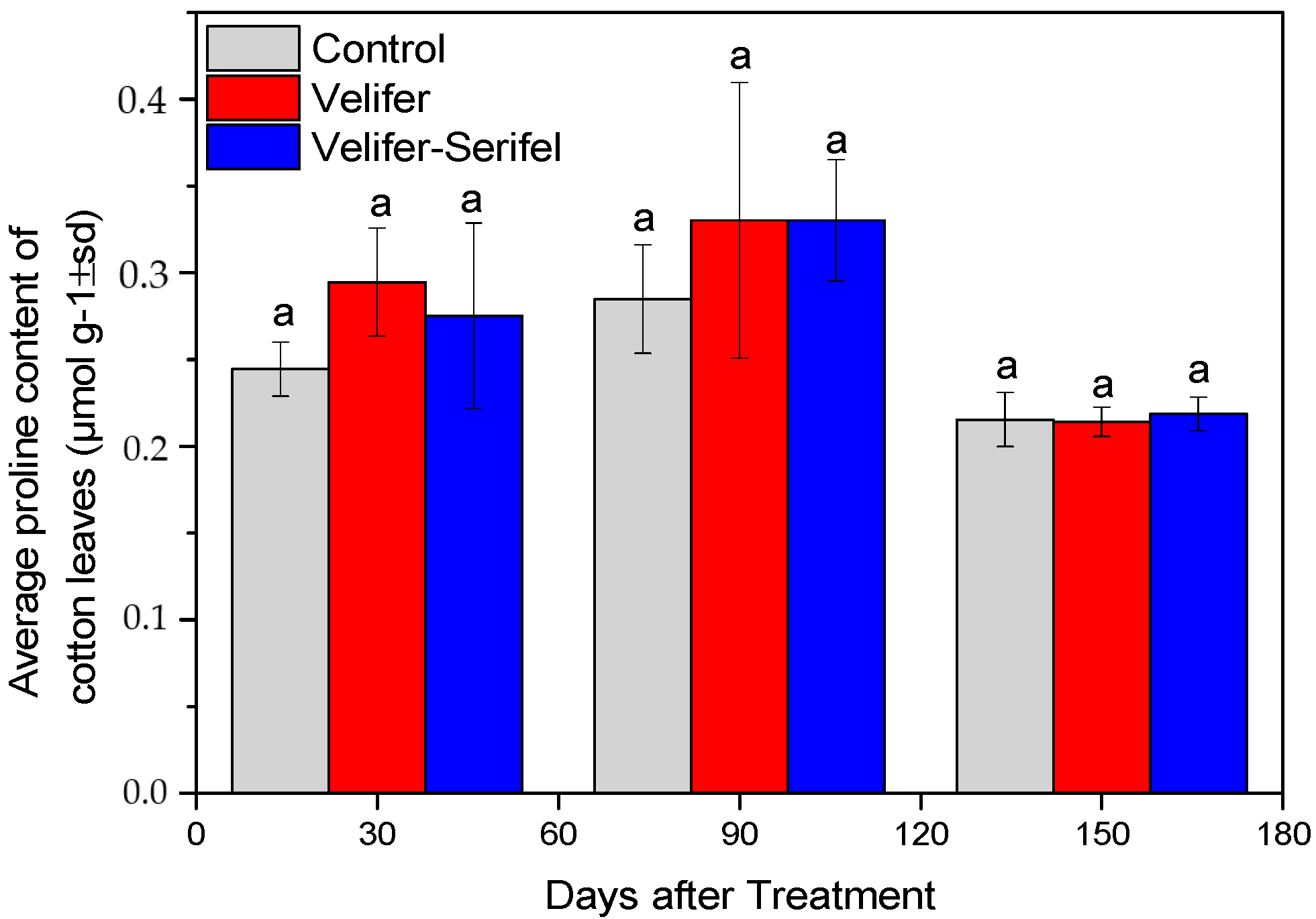
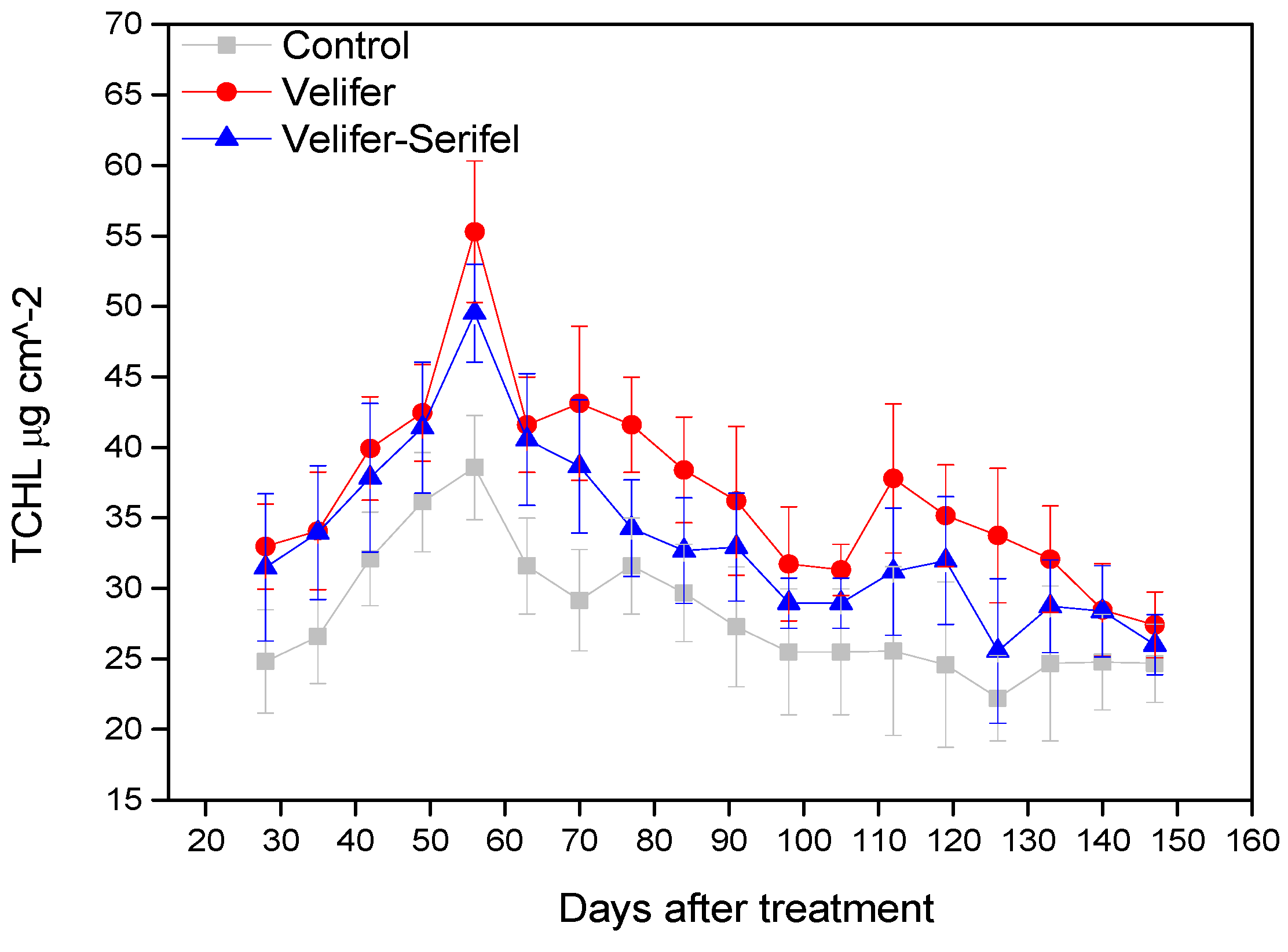
3.3. Effect on Proline and Total Chlorophyll Content (TCHL)
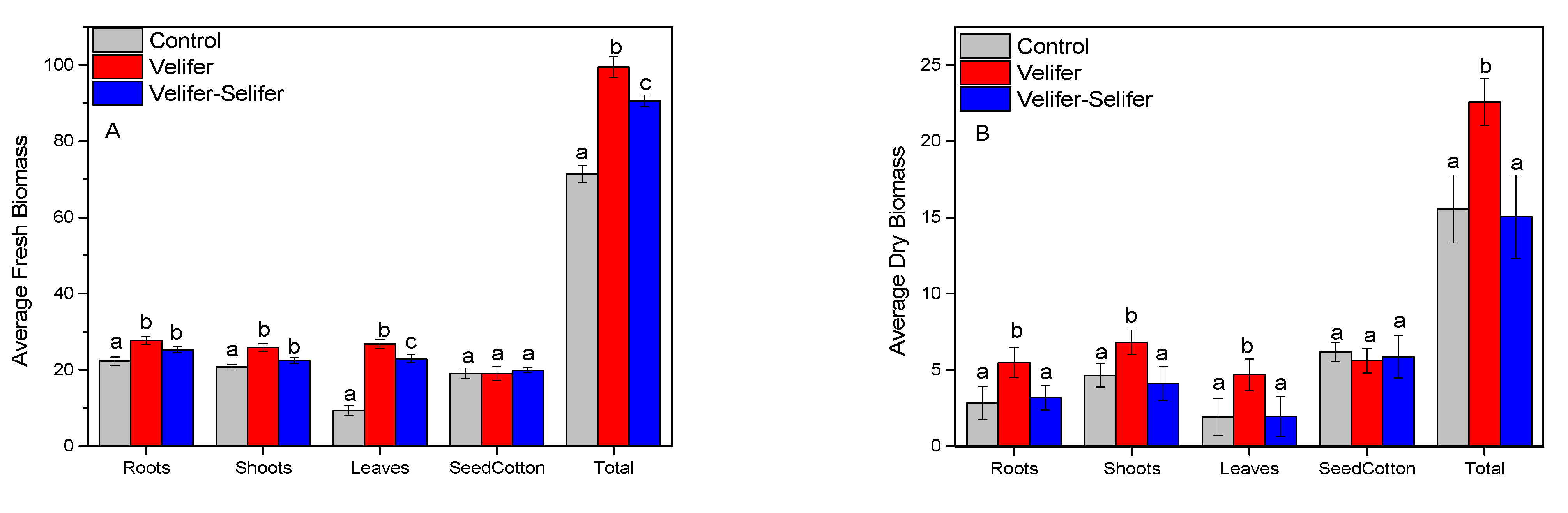
3.4. Cotton Fresh and Dry Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslan, R.; Aygun, Y.Z.; Mert, M. Yield and Fiber Quality Characteristics of Some Cotton (Gossypium hirsutum L.) Cultivars Grown in the Southeastern Anatolian Conditions. Turk. J. Field Crops 2022, 27, 285–292. [Google Scholar] [CrossRef]
- Namrata, P.B.; Hardik, L.S.; Ramani, H.; Rajkumar, B.K. Screening of Cotton (Gossypium hirsutum L.) Genotypes for Drought Tolerance. Pharma Innov. J. 2022, 11, 1634–1639. [Google Scholar]
- Ertekin, I.; Atis, I.; Aygun, Y.Z.; Yilmaz, S.; Kizilsimsek, M. Effects of Different Nitrogen Doses and Cultivars on Fermentation Quality and Nutritive Value of Italian Ryegrass (Lolium multiflorum Lam.) Silages. Anim. Biosci. 2022, 35, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, H.; Wang, T.; Li, Y.; Liu, Y.; Chen, X.; Hu, X. Impact of Climate Change on Cotton Growth and Yields in Xinjiang, China. Field Crops Res. 2020, 247, 107590. [Google Scholar] [CrossRef]
- Saleem, M.A.; Malik, W.; Qayyum, A.; Ul-Allah, S.; Ahmad, M.Q.; Afzal, H.; Amjid, M.W.; Ateeq, M.F.; Zia, Z.U. Impact of Heat Stress Responsive Factors on Growth and Physiology of Cotton (Gossypium hirsutum L.). Mol. Biol. Rep. 2021, 48, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Tabari, H. Climate Change Impact on Flood and Extreme Precipitation Increases with Water Availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef] [PubMed]
- Ul-Allah, S.; Rehman, A.; Hussain, M.; Farooq, M. Fiber Yield and Quality in Cotton under Drought: Effects and Management. Agric. Water Manag. 2021, 255, 106994. [Google Scholar] [CrossRef]
- Zafar, S.; Afzal, H.; Ijaz, A.; Mahmood, A.; Ayub, A.; Nayab, A.; Hussain, S.; UL-Hussan, M.; Sabir, M.A.; Zulfiqar, U.; et al. Cotton and Drought Stress: An Updated Overview for Improving Stress Tolerance. S. Afr. J. Bot. 2023, 161, 258–268. [Google Scholar] [CrossRef]
- Raza, M.M.; Bebber, D.P. Climate Change and Plant Pathogens. Curr. Opin. Microbiol. 2022, 70, 102233. [Google Scholar] [CrossRef]
- Nikam, T.A.; Latpate, C.B.; Bhosale, B.B. Estimation of Yield Losses Due to Sucking Pests of Bt Cotton under High Density Planting System. J. Cotton Res. Dev. 2019, 33, 273–280. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Desneux, N.; Lu, Y. Impact of Temperature on Survival Rate, Fecundity, and Feeding Behavior of Two Aphids, Aphis Gossypii and Acyrthosiphon Gossypii, When Reared on Cotton. Insects 2021, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Li, H.; Gao, Y.; Yang, Y.; Lu, Y. Bottom-Up Effects of Drought-Stressed Cotton Plants on Performance and Feeding Behavior of Aphis Gossypii. Plants 2023, 12, 2886. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, F.S.; Fernandes, F.S.; Nascimento, A.R.B.; Nascimento, J.L.; Malaquias, J.B.; Silva, C.A.D. Feeding Damage from Cotton Aphids, Aphis Gossypii Glover (Hemiptera: Heteroptera: Aphididae), in Cotton with Colored Fiber Intercropped with Fennel. Ann. Entomol. Soc. Am. 2012, 105, 20–27. [Google Scholar] [CrossRef]
- XiaoXia, D.; HaiLan, J.; Jun, P.; ZeMin, H.; TianWen, M.; JunGang, W. Physiological Responses of Cotton to Feeding by Aphis Gossypii during the Flower-Boiling Stage. Chin. J. Appl. Entomol. 2013, 50, 161–166. [Google Scholar]
- Shannag, H.K.; Thorvilson, H.; El-Shatnawi, M.K. Changes in Photosynthetic and Transpiration Rates of Cotton Leaves Infested with the Cotton Aphid, Aphis Gossypii: Unrestricted Infestation. Ann. Appl. Biol. 1998, 132, 13–18. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhao, Y.; Ren, Y.; Mu, W.; Liu, F. Efficacy of Granular Applications of Clothianidin and Nitenpyram against Aphis Gossypii (Glover) and Apolygus Lucorum (Meyer-Dür) in Cotton Fields in China. Crop Prot. 2015, 78, 27–34. [Google Scholar] [CrossRef]
- Mahas, J.W.; Hamilton, F.B.; Roberts, P.M.; Ray, C.H.; Miller, G.L.; Sharman, M.; Conner, K.; Bag, S.; Blythe, E.K.; Toews, M.D.; et al. Investigating the Effects of Planting Date and Aphis Gossypii Management on Reducing the Final Incidence of Cotton Leafroll Dwarf Virus. Crop Prot. 2022, 158, 106005. [Google Scholar] [CrossRef]
- Escriu, F.; Perry, K.L.; Garcia-Arenal, F. Transmissibility of Cucumber Mosaic Virus by Aphis Gossypii Correlates with Viral Accumulation and Is Affected by the Presence of Its Satellite RNA. Phytopathology 2000, 90, 1068–1072. [Google Scholar] [CrossRef]
- Tarazi, R.; Vaslin, M.F.S. The Viral Threat in Cotton: How New and Emerging Technologies Accelerate Virus Identification and Virus Resistance Breeding. Front. Plant Sci. 2022, 13, 851939. [Google Scholar] [CrossRef]
- Li, R.; Cheng, S.; Liang, P.; Chen, Z.; Zhang, Y.; Liang, P.; Zhang, L.; Gao, X. Status of the Resistance of Aphis Gossypii Glover, 1877 (Hemiptera: Aphididae) to Afidopyropen Originating from Microbial Secondary Metabolites in China. Toxins 2022, 14, 750. [Google Scholar] [CrossRef]
- Srivastava, R.; Shukla, A.C. Fusarium Pallidoroseum: A Potential Entomopathogenic Agent for the Biological Management of Aphis Gossypii. J. Appl. Nat. Sci. 2021, 13, 775–785. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Denholm, I. High Resistance of Field Populations of the Cotton Aphid Aphis Gossypii Glover (Homoptera: Aphididae) to Pyrethroid Insecticides in Pakistan. J. Econ. Entomol. 2003, 96, 875–878. [Google Scholar] [CrossRef]
- Wang, K.Y.; Guo, Q.L.; Xia, X.M.; Wang, H.Y.; Liu, T.X. Resistance of Aphis Gossypii (Homoptera: Aphididae) to Selected Insecticides on Cotton from Five Cotton Production Regions in Shandong, China. J. Pestic. Sci. 2007, 32, 372–378. [Google Scholar] [CrossRef]
- Gaber, A.S.; Abd-Ella, A.A.; Abou-Elhagag, G.H.; Abdel-Rahman, Y.A. Field Efficiency and Selectivity Effects of Selected Insecticides on Cotton Aphid, Aphis Gossypii Glover (Homoptera: Aphididea) and Its Predators. J. Phytopathol. Dis. Manag. 2015, 2, 22–35. [Google Scholar]
- Chamuene, A.; Araújo, T.A.D.; Lopes, M.C.; Ramos Pereira, R.; Berger, P.G.; Picanço, M.C. Investigating the Natural Mortality of Aphis Gossypii (Hemiptera: Aphididae) on Cotton Crops in Tropical Regions Using Ecological Life Tables. Environ. Entomol. 2020, 49, 66–72. [Google Scholar] [CrossRef]
- Eid, A.E.; El-Heneidy, A.H.; Hafez, A.A.; Shalaby, F.F.; Adly, D. On the Control of the Cotton Aphid, Aphis Gossypii Glov. (Hemiptera: Aphididae), on Cucumber in Greenhouses. Egypt. J. Biol. Pest. Control 2018, 28, 64. [Google Scholar] [CrossRef]
- Mbiza, N.I.T.; Hu, Z.; Zhang, H.; Zhang, Y.; Luo, X.; Wang, Y.; Wang, Y.; Liu, T.; Li, J.; Wang, X.; et al. GhCalS5 Is Involved in Cotton Response to Aphid Attack through Mediating Callose Formation. Front. Plant Sci. 2022, 13, 892630. [Google Scholar] [CrossRef]
- Kerns, D.L.; Yates, J.A.; Baugh, B.A. Economic Threshold for Cotton Aphid (Hemiptera: Aphididae) on Cotton in the Southwestern United States. J. Econ. Entomol. 2015, 108, 1795–1803. [Google Scholar] [CrossRef]
- Abang, A.F.; Srinivasan, R.; Hanna, R.; Fotso, A.K.; Kekeunou, S.; Tenkouano, A.; Bilong, C.F.B. Productivity and Resistance of Okra (Abelmoschus spp.) to the Cotton Aphid Aphis Gossypii Glover (Hemiptera: Aphididae) under Tropical Conditions. Int. J. Trop. Insect Sci. 2021, 41, 197–208. [Google Scholar] [CrossRef]
- Karamchedu, A. Dried up Bt Cotton Narratives: Climate, Debt and Distressed Livelihoods in Semi-Arid Smallholder India. Clim. Dev. 2023, 16, 289–300. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Del Campillo, M.C.; Quesada-Moraga, E. Beauveria Bassiana: An Entomopathogenic Fungus Alleviates Fe Chlorosis Symptoms in Plants Grown on Calcareous Substrates. Sci. Hortic. 2015, 197, 193–202. [Google Scholar] [CrossRef]
- Dannon, H.F.; Dannon, A.E.; Douro-Kpindou, O.K.; Zinsou, A.V.; Houndete, A.T.; Toffa-Mehinto, J.; Elegbede, I.A.T.M.; Olou, B.D.; Tamò, M. Toward the Efficient Use of Beauveria Bassiana in Integrated Cotton Insect Pest Management. J. Cotton Res. 2020, 3, 24. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The Endophytic Fungal Entomopathogens Beauveria Bassiana and Purpureocillium Lilacinum Enhance the Growth of Cultivated Cotton (Gossypium hirsutum) and Negatively Affect Survival of the Cotton Bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Kanasagra, J.R.; Valu, M.; Raval, L.J.; Rupapara, S. Heterosis, Combining Ability and Gene Action for Seed Cotton Yield and Its Contributing Characters in Cotton (Gossypium hirsutum L.). Pharma Innov. J. 2022, 11, 2050–2056. [Google Scholar]
- Khashaba, E.H.K. Inoculation and Colonization of Isolated Entomopathogenic Fungi Beauveria Bassiana in Rice Plants, Oryza sativa L. through Seed Immersion Method. Egypt. J. Biol. Pest. Control 2021, 31, 92. [Google Scholar] [CrossRef]
- Nawaz, A.; Razzaq, F.; Razzaq, A.; Gogi, M.D.; Fernández-Grandon, G.M.; Tayib, M.; Ayub, M.A.; Sufyan, M.; Shahid, M.R.; Qayyum, M.A.; et al. Compatibility and Synergistic Interactions of Fungi, Metarhizium Anisopliae, and Insecticide Combinations against the Cotton Aphid, Aphis Gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2022, 12, 4843. [Google Scholar] [CrossRef]
- Dara, S.K. Non-Entomopathogenic Roles of Entomopathogenic Fungi in Promoting Plant Health and Growth. Insects 2019, 10, 277. [Google Scholar] [CrossRef]
- Kasim Ongun, A.; Karademir, C. The Effect of Application of Different Doses of Acid Mixture Seed Coating Method on Cotton (Gossypium hirsutum L.) Yield and Fiber Technological Characteristics. Technology and Engineering Management. J. Agron. Technol. Eng. Manag. 2022, 2022, 841. [Google Scholar] [CrossRef]
- Canassa, F.; Tall, S.; Moral, R.A.; de Lara, I.A.R.; Delalibera, I.; Meyling, N.V. Effects of Bean Seed Treatment by the Entomopathogenic Fungi Metarhizium Robertsii and Beauveria Bassiana on Plant Growth, Spider Mite Populations and Behavior of Predatory Mites. Biol. Control 2019, 132, 199–208. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; López-Díaz, C.; Landa, B.B. The Hidden Habit of the Entomopathogenic Fungus Beauveria Bassiana: First Demonstration of Vertical Plant Transmission. PLoS ONE 2014, 9, e89278. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Effect of Seed Treatment Duration on Growth and Colonization of Vicia Faba by Endophytic Beauveria Bassiana and Metarhizium Brunneum. Biol. Control 2016, 103, 187–195. [Google Scholar] [CrossRef]
- Brownbridge, M.; Reay, S.D.; Nelson, T.L.; Glare, T.R. Persistence of Beauveria Bassiana (Ascomycota: Hypocreales) as an Endophyte Following Inoculation of Radiata Pine Seed and Seedlings. Biol. Control 2012, 61, 194–200. [Google Scholar] [CrossRef]
- Rasool, S.; Vidkjær, N.H.; Hooshmand, K.; Jensen, B.; Fomsgaard, I.S.; Meyling, N.V. Seed Inoculations with Entomopathogenic Fungi Affect Aphid Populations Coinciding with Modulation of Plant Secondary Metabolite Profiles across Plant Families. New Phytol. 2021, 229, 1715–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, B. Entomopathogenic Fungi Beauveria Bassiana and Metarhizium Anisopliae Play Roles of Maize (Zea mays) Growth Promoter. Sci. Rep. 2022, 12, 15706. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Daskalaki, E.; Kitsiou, F.; Papantzikos, V.; Servis, D.; Bitivanos, S.; Patakioutas, G.; Eliopoulos, P.A. Dual Action of Beauveria Bassiana (Hypocreales; Cordycipitaceae) Endophytic Stains as Biocontrol Agents against Sucking Pests and Plant Growth Biostimulants on Melon and Strawberry Field Plants. Microorganisms 2022, 10, 2306. [Google Scholar] [CrossRef]
- Afandhi, A.; Widjayanti, T.; Emi, A.A.L.; Tarno, H.; Afiyanti, M.; Handoko, R.N.S. Endophytic Fungi Beauveria Bassiana Balsamo Accelerates Growth of Common Bean (Phaeseolus vulgaris L.). Chem. Biol. Technol. Agric. 2019, 6, 11. [Google Scholar] [CrossRef]
- El Husseini, M.M.; Bochow, H.; Junge, H. The Biofertilising Effect of Seed Dressing with PGPR Bacillus Amyloliquefaciens FZB 42 Combined with Two Levels of Mineral Fertilising in African Cotton Production. Arch. Phytopathol. Plant Prot. 2012, 45, 2261–2271. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Bacillus Amyloliquefaciens Alters Gene Expression, ROS Production and Lignin Synthesis in Cotton Seedling Roots. J. Appl. Microbiol. 2018, 124, 1589–1603. [Google Scholar] [CrossRef]
- Bakr, E.M. A New Software for Measuring Leaf Area, and Area Damaged by Tetranychus Urticae Koch. J. Appl. Entomol. 2005, 129, 173–175. [Google Scholar] [CrossRef]
- Priya, S.; Ghosh, R. Monitoring Effects of Heavy Metal Stress on Biochemical and Spectral Parameters of Cotton Using Hyperspectral Reflectance. Environ. Monit. Assess. 2023, 195, 112. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. PROTOCOL: Extraction and Determination of Proline. Prometh. Wiki 2011, 2011, 1–5. [Google Scholar]
- Mnasri, N.; Chennaoui, C.; Gargouri, S.; Mhamdi, R.; Hessini, K.; Elkahoui, S.; Djébali, N. Efficacy of Some Rhizospheric and Endophytic Bacteria in Vitro and as Seed Coating for the Control of Fusarium Culmorum Infecting Durum Wheat in Tunisia. Eur. J. Plant Pathol. 2017, 147, 501–515. [Google Scholar] [CrossRef]
- García-Espinoza, F.; García, M.J.; Quesada-Moraga, E.; Yousef-Yousefa, M. Entomopathogenic Fungus-Related Priming Defense Mechanisms in Cucurbits Impact Spodoptera Littoralis (Boisduval) Fitness. Appl. Environ. Microbiol. 2023, 89, e00940-23. [Google Scholar] [CrossRef]
- Faiz, A.; Faiz, A.; Wang, W.; Bennett, C. Sustainable Rural Roads for Livelihoods and Livability. Procedia Soc. Behav. Sci. 2012, 53, 1–8. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Papantzikos, V.; Katsogiannou, S.; Papanikou, A.; Koukidis, C.; Servis, D.; Eliopoulos, P.; Patakioutas, G. Biostimulant and Bioinsecticidal Effect of Coating Cotton Seeds with Endophytic Beauveria Bassiana in Semi-Field Conditions. Microorganisms 2023, 11, 2050. [Google Scholar] [CrossRef]
- Kuzhuppillymyal-Prabhakarankutty, L.; Ferrara-Rivero, F.H.; Tamez-Guerra, P.; Gomez-Flores, R.; Rodríguez-Padilla, M.C.; Ek-Ramos, M.J. Effect of Beauveria Bassiana-Seed Treatment on Zea mays L. Response against Spodoptera Frugiperda. Appl. Sci. 2021, 11, 2887. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Malik, A. Preparation, Characterization, and Insecticidal Activity Evaluation of Three Different Formulations of Beauveria Bassiana against Musca Domestica. Parasitol. Res. 2013, 112, 3485–3495. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Zhang, T.; Bai, S.; Zhi, Y.; Chen, J.; Wang, Z. Synergistic Effect of Beauveria Bassiana and Trichoderma Asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia Furnacalis (Lepidoptera, Crambidae) and Larval Immune Response. Int. J. Mol. Sci. 2020, 21, 8215. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus Amyloliquefaciens as an Excellent Agent for Biofertilizer and Biocontrol in Agriculture: An Overview for Its Mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus Amyloliquefaciens Inoculation Alters Physiology of Rice (Oryza sativa L. Var. IR-36) through Modulating Carbohydrate Metabolism to Mitigate Stress Induced by Nutrient Starvation. Int. J. Biol. Macromol. 2020, 143, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Kramski, D.J.; Nowinski, D.; Kowalczuk, K.; Kruszyński, P.; Radzimska, J.; Greb-Markiewicz, B. Beauveria Bassiana Water Extracts’ Effect on the Growth of Wheat. Plants 2023, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An Endophytic Beauveria Bassiana Strain Increases Spike Production in Bread and Durum Wheat Plants and Effectively Controls Cotton Leafworm (Spodoptera littoralis) Larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Rivas-Franco, F.; Hampton, J.G.; Morán-Diez, M.E.; Narciso, J.; Rostás, M.; Wessman, P.; Jackson, T.A.; Glare, T.R. Effect of Coating Maize Seed with Entomopathogenic Fungi on Plant Growth and Resistance against Fusarium Graminearum and Costelytra Giveni. Biocontrol Sci. Technol. 2019, 29, 877–900. [Google Scholar] [CrossRef]
- Shaalan, R.S.; Gerges, E.; Habib, W.; Ibrahim, L. Endophytic Colonization by Beauveria Bassiana and Metarhizium Anisopliae Induces Growth Promotion Effect and Increases the Resistance of Cucumber Plants against Aphis Gossypii. J. Plant Prot. Res. 2021, 61, 358. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest Management via Endophytic Colonization of Tobacco Seedlings by the Insect Fungal Pathogen Beauveria Bassiana. Pest. Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Akter, T.; Mimma, A.A.; Haque, M.A.; Hossain, M.M.; Ghosh, T.K.; Zinan, N.; Chowdhury, M.Z.H.; Islam, S.M.N. Seed Priming with Beauveria Bassiana Improves Growth and Salt Stress Response in Rice. Environ. Exp. Bot. 2023, 213, 105427. [Google Scholar] [CrossRef]
- Veloz-Badillo, G.M.; Riveros-Ramírez, J.; Angel-Cuapio, A.; Arce-Cervantes, O.; Flores-Chávez, B.; Espitia-López, J.; Loera, O.; Garza-López, P.M. The Endophytic Capacity of the Entomopathogenic Fungus Beauveria Bassiana Caused Inherent Physiological Response in Two Barley (Hordeum vulgare) Varieties. 3 Biotech. 2019, 9, 12. [Google Scholar] [CrossRef]
- Saragih, M.; Trizelia; Nurbailis; Yusniwati. Endophytic Colonization and Plant Growth Promoting Effect by Entomopathogenic Fungus, Beauveria Bassiana to Red Chili (Capsicum annuum L.) with Different Inoculation Methods. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 12070. [Google Scholar] [CrossRef]
- Homayoonzadeh, M.; Esmaeily, M.; Talebi, K.; Allahyari, H.; Reitz, S.; Michaud, J.P. Inoculation of Cucumber Plants with Beauveria Bassiana Enhances Resistance to Aphis Gossypii (Hemiptera: Aphididae) and Increases Aphid Susceptibility to Pirimicarb. Eur. J. Entomol. 2022, 119, 1–11. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Card, S.D.; Bastías, D.A.; Caradus, J.R. Antagonism to Plant Pathogens by Epichloë Fungal Endophytes—A Review. Plants 2021, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Römmert, A.K.; Dammann, U.; Aust, H.J.; Strack, D. The Endophyte-Host Interaction: A Balanced Antagonism? Mycol. Res. 1999, 103, 1275–1283. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, R.; Saravanakumar, D.; Raguchander, T. Combination of Endophytic Bacillus and Beauveria for the Management of Fusarium Wilt and Fruit Borer in Tomato. Pest. Manag. Sci. 2014, 70, 1742–1750. [Google Scholar] [CrossRef]
- Komagata, Y.; Sekine, T.; Oe, T.; Kakui, S.; Yamanaka, S. Simultaneous Use of Beauveria Bassiana and Bacillus Subtilis-Based Biopesticides Contributed to Dual Control of Trialeurodes Vaporariorum (Hemiptera: Aleyrodidae) and Tomato Powdery Mildew without Antagonistic Interactions. Egypt. J. Biol. Pest. Control 2024, 34, 18. [Google Scholar] [CrossRef]
- Malik, M.A.; Ahmad, S.J.N.; Ahmad, J.N.; Abbasi, A.; Sufyan, M.; Arif, M.J. Efficacy of Bacillus Thuringiensis and Beauveria Bassiana against Red Palm Weevil Rhynchophorus Ferrugineus Olivier (Coleoptera: Curculionidae). Afr. Entomol. 2019, 27, 386–394. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, N.; Hashem, A.; Dawoud, T.M.; Abd_Allah, E.F. Insecticidal Potential of Bacillus Thuringiensis, Beauveria Bassiana and Metarhizium Anisopliae Individually and Their Synergistic Effect with Barazide against Spodoptera Litura. Heliyon 2024, 10, e37175. [Google Scholar] [CrossRef]
- Wang, X.M.; Yang, B.; Wang, H.W.; Yang, T.; Ren, C.G.; Zheng, H.L.; Dai, C.C. Consequences of Antagonistic Interactions between Endophytic Fungus and Bacterium on Plant Growth and Defense Responses in Atractylodes Lancea. J. Basic. Microbiol. 2015, 55, 659–670. [Google Scholar] [CrossRef]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The Bacterial Superoxide Dismutase and Glutathione Reductase Are Crucial for Endophytic Colonization of Rice Roots by Gluconacetobacter Diazotrophicus PAL5. Mol. Plant-Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef]
- Mack, K.M.L.; Rudgers, J.A.; Mack, K.M.L.; Rudgers, J.A. Balancing Multiple Mutualists: Asymmetric Interactions among Plants, Arbuscular Mycorrhizal Fungi, and Fungal Endophytes. Oikos 2008, 117, 310–320. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High Nitrogen Supply and Carbohydrate Content Reduce Fungal Endophyte and Alkaloid Concentration in Lolium Perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Parsons, A.J.; Xue, H.; Fraser, K.; Ryan, G.D.; Newman, J.A.; Rasmussen, S. Competition between Foliar Neotyphodium Lolii Endophytes and Mycorrhizal Glomus spp. Fungi in Lolium perenne Depends on Resource Supply and Host Carbohydrate Content. Funct. Ecol. 2011, 25, 910–920. [Google Scholar] [CrossRef]
- Omacini, M.; Eggers, T.; Bonkowski, M.; Gange, A.C.; Jones, T.H. Leaf Endophytes Affect Mycorrhizal Status and Growth of Co-Infected and Neighbouring Plants. Funct. Ecol. 2006, 20, 226–232. [Google Scholar] [CrossRef]
- Russo, M.L.; Scorsetti, A.C.; Vianna, M.F.; Allegrucci, N.; Ferreri, N.A.; Cabello, M.N.; Pelizza, S.A. Effects of Endophytic Beauveria Bassiana (Ascomycota: Hypocreales) on Biological, Reproductive Parameters and Food Preference of the Soybean Pest Helicoverpa Gelotopoeon. J. King Saud. Univ. Sci. 2019, 31, 1077–1082. [Google Scholar] [CrossRef]
- Zimmermann, G. The ‘Galleria Bait Method’ for Detection of Entomopathogenic Fungi in Soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Kannadan, S.; Rudgers, J.A. Endophyte Symbiosis Benefits a Rare Grass under Low Water Availability. Funct. Ecol. 2008, 22, 706–713. [Google Scholar] [CrossRef]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria Bassiana (Balsamo) Vuillemin as an Endophyte in Tissue Culture Banana (Musa spp.). J. Invertebr. Pathol. 2007, 96, 34–42. [Google Scholar] [CrossRef]
- Jaber, L.R.; Vidal, S. Fungal Endophyte Negative Effects on Herbivory Are Enhanced on Intact Plants and Maintained in a Subsequent Generation. Ecol. Entomol. 2010, 35, 25–36. [Google Scholar] [CrossRef]
- Wagner, B.L.; Lewis, L.C. Colonization of Corn, Zea mays, by the Entomopathogenic Fungus Beauveria Bassiana. Appl. Environ. Microbiol. 2000, 66, 3468. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria Bassiana as an Endophyte: A Critical Review on Associated Methodology and Biocontrol Potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantzikos, V.; Mantzoukas, S.; Koutsompina, A.; Karali, E.M.; Eliopoulos, P.A.; Servis, D.; Bitivanos, S.; Patakioutas, G. Use of Beauveria bassiana and Bacillus amyloliquefaciens Strains as Gossypium hirsutum Seed Coatings: Evaluation of the Bioinsecticidal and Biostimulant Effects in Semi-Field Conditions. Agronomy 2024, 14, 2335. https://doi.org/10.3390/agronomy14102335
Papantzikos V, Mantzoukas S, Koutsompina A, Karali EM, Eliopoulos PA, Servis D, Bitivanos S, Patakioutas G. Use of Beauveria bassiana and Bacillus amyloliquefaciens Strains as Gossypium hirsutum Seed Coatings: Evaluation of the Bioinsecticidal and Biostimulant Effects in Semi-Field Conditions. Agronomy. 2024; 14(10):2335. https://doi.org/10.3390/agronomy14102335
Chicago/Turabian StylePapantzikos, Vasileios, Spiridon Mantzoukas, Alexandra Koutsompina, Evangelia M. Karali, Panagiotis A. Eliopoulos, Dimitrios Servis, Stergios Bitivanos, and George Patakioutas. 2024. "Use of Beauveria bassiana and Bacillus amyloliquefaciens Strains as Gossypium hirsutum Seed Coatings: Evaluation of the Bioinsecticidal and Biostimulant Effects in Semi-Field Conditions" Agronomy 14, no. 10: 2335. https://doi.org/10.3390/agronomy14102335
APA StylePapantzikos, V., Mantzoukas, S., Koutsompina, A., Karali, E. M., Eliopoulos, P. A., Servis, D., Bitivanos, S., & Patakioutas, G. (2024). Use of Beauveria bassiana and Bacillus amyloliquefaciens Strains as Gossypium hirsutum Seed Coatings: Evaluation of the Bioinsecticidal and Biostimulant Effects in Semi-Field Conditions. Agronomy, 14(10), 2335. https://doi.org/10.3390/agronomy14102335








