Abstract
The corn stunt disease complex, caused by the mollicutes Spiroplasma kunkelii (Css) and the maize bushy stunt (MBS) phytoplasma, is a major phytosanitary issue for corn production in the neotropical region of Brazil. In this study, we investigated the presence of alternative hosts for S. kunkelii (Css) in the Brazilian Cerrado biome and explored the potential for asymptomatic Css infections in maize plants. To identify possible alternative hosts, we collected samples from ornamental and native plants located in Cerrado regions with a high incidence of corn stunt disease. We also monitored the disease’s progression over time and calculated the area under the disease progression curve (AUDPC). Additionally, we sampled healthy, asymptomatic maize plants growing near symptomatic ones and analyzed all the samples using qPCR to detect the pathogen. Our results showed no Css infection in the sampled alternative host species and no asymptomatic infections in the nearby maize plants. The incidence of maize stunting varied significantly among the sample years and counties. In Aparecida do Rio Negro, the infection rate was six times higher in 2020 compared to 2019, while Porto Nacional showed an 11-fold increase. During surveillance from March to July, the infection rate at the Sierra site went from less than 3% in March 2019 to 6% in July (F1,4 = 415.84; p = 0.0003). MBS infection increased significantly, while S. kunkelii remained stable below 3%. In 2020, MBS increased from 3% to more than 12%. A similar trend was observed at the Sede site, where MBS increased from 9% in 2019 to 11% in 2020. An increase in the AUDPC was observed both within individual years and between 2019 and 2020, indicating a worsening trend in disease severity. Overall, our findings reinforce the need for preventive measures in managing maize crop fields.
1. Introduction
According to the Food and Agriculture Organization (FAO) of the United Nations, global corn production is estimated to grow to 3.1 b tons by 2032 [1]. This is because, compared with wheat and rice, maize is a more versatile, multi-purpose crop. In addition, maize has been responsible for supplying around 75% of the world’s starch demand in the last few years [2]. Brazil is among the top three corn-exporting countries, with an estimated production of 125 million metric tons of grain, grown on 22.1 million hectares of land, with an average yield of 5.56 Kg per hectare [3]. However, its position as a major corn producer is threatened by pests and diseases that affect corn plants during their vegetative phase.
Among the diseases affecting maize in Brazil, the corn stunt complex is notable. This complex is caused by the mollicute S. kunkelii (corn stunt spiroplasm—Css), which leads to pale stunt, and phytoplasma Candidatus Phytoplasma asteris (maize bushy stunt—MBS), which causes red stunt. Galvão, et al. [4] reported that pale stunt and red stunt infections can occur either individually or in combination. Transmission occurs primarily through the leafhopper Dalbulus maidis (Hemiptera: Cicadellidae), which acts as the main vector by sustaining a complete systematic circulation of the pathogen throughout its life [5,6]. In addition to this, D. maidis frequently engages in numerous test bites, which can further contribute to the spread of the pathogen [7]. Due to the insect vector’s high dispersion and migration capacity, coupled with its ability to survive between harvests (facilitated by the presence of maize plants remaining or growing after the harvest, which serve as green bridges between different planting areas or times [8]), the pathogen can be transmitted over long distances and periods.
Corn stunt was regarded as a secondary disease for many years and occurred sporadically in maize-producing areas until the 1990s. However, since the early 2000s, its capacity to induce crop losses has become well established [9]. Over the last decade, Brazil has expanded its corn cultivation area, primarily in the off-season, leading to an increased in disease incidence [10]. Due to the high prevalence of the corn stunt vector in maize fields, the incidence of the disease can range from 6% to 100% in plants showing corn stunt symptoms [11]. Infected corn plants typically exhibit symptoms 30 days after infection, particularly during the plant’s reproductive phase, and these symptoms vary depending on the pathogen that infects and colonizes the plant’s phloem [12]. Pale stunt primarily causes lesions in the form of chlorotic streaks that appear parallel to the leaf veins [9]. Additionally, it can lead to alterations in plant development, such as shortening internodes, reduced plant height, and causing defective ears with either incomplete grain formation or flat grains [13]. Red stunt symptoms are usually characterized by the reddening of the leaves, which progresses from the edges toward the center, and similar plant development problems as those seen in pale stunt [14].
Various studies on S. kunkelli (Css) are being conducted to control corn stunt, with a focus on identifying maize varieties that are resistant to both the pathogens and the vector insects [9,15]. The application of insecticides is a common practice for controlling insect pests, particularly D. maidis, in neotropical corn fields. However, previous research has shown that D. maidis can adapt and use certain grass species as an alternative host for several months [10,16,17,18]. This adaptation raises the possibility of the pathogen persisting in alternative hosts during periods when maize plants are absent. Additionally, it has been reported that these pathogens can cause asymptomatic infections in plants [19,20].
Thus, it is reasonable to suggest that several studies are needed to understand the biology of Spiroplasma kunkelli (Css) in alternative hosts, the progression of the disease over time, the potential asymptomatic nature of Css, and the identification of alternative host plants that could serve as sources of inoculum for the pathogens and the vector. Here, we evaluated the possibility of naturally infected alternative hosts for pale stunt caused by Css, the presence of asymptomatic and latently infected corn plants with Css, and assessed the temporal progression of the disease complex (Css, MBS, and mixed infection) in two corn-producing areas in Tocantins State, Brazil.
2. Materials and Methods
2.1. Assessing the Natural Infection of Spiroplasma kunkelii (CSS) in Alternative Hosts near High-Incidence Corn Stunt Disease Areas
Here we adapted the methodology purpose by Esquivel-Fariña, et al. [21]. Briefly, during 2019 and 2020, a total of 212 plants were collected from areas around a corn production site with a high incidence of vector presence and corn stunt disease symptoms. Similarly, maize plants with pale stunt symptoms were also collected from the same location as a positive control. The sampling was conducted on a corn-producing farm (10°29′32.1″ S, 48°18′51.7″ W) in the municipality of Porto Nacional, Tocantins, Brazil. This location was selected due to its central position in the Brazilian Cerrado region. Sample collections were carried out opportunistically during field monitoring, with an emphasis on gathering the maximum number of plants and different varieties possible during this period. The fragments of leaves from the sampled plants were stored in plastic tubes and sent to the laboratory for DNA extraction, where the potential transmission was analyzed. The extracted DNA was subjected to a qPCR analysis to verify the presence of S. kunkelii (Css).
2.2. Evaluation of Possible Spiroplasma kunkelii (Css) Infection in Asymptomatic Maize Plants Adjacent to Symptomatic Plants
Initially, we identified a commercial maize field, with 30F53 hybrids, where approximately 20% of the plants exhibited external symptoms of pale stunt [9,22]. Samples were collected from visibly healthy, asymptomatic plants (n = 140) and from symptomatic plants (n = 35). The healthy plants were collected in close proximity to or from the same rows as the symptomatic plants, in a ratio of 1:4 (Figure 1). In the laboratory, the leaves with and without symptoms were separated, identified, and analyzed via qPCR to confirm the presence of S. kunkelii (Css) in the symptomatic plants and to check for possible latent infection in the asymptomatic plants.
Figure 1.
Diagram of the sample collection for corn-infected plants and nearby asymptomatic plants (A) and the municipalities where the temporal progression of corn stunt disease was evaluated (B).
2.3. DNA Extraction and qPCR Reaction
The DNA extraction and PCR analysis protocol were conducted by a partner laboratory. The optimized DNA extraction protocol followed the method described by Truett et al. [23], while the PCR reaction protocol was adapted from the method described by Barros et al. [24] using the primer set CSSF2/R6 for the detection of S. kunkelii. For the detection of maize bushy stunt (MBS), an adaptation of the protocol described by Harrison et al. [25] with the primer set MBSF1/MBSR1 was used. Both of the protocols were optimized for verification using a qPCR machine Rotor-Gene, (Qigen, Düsseldorf, Germany) with SYBR Green dye. The results indicated the presence or absence of the pathogen in the samples.
2.4. Measuring the Occurrence of Maize Plants Infected with Spiroplasma kunkelii, Maize Bushy Stunt Phytoplasma, and Mixed Infections in Two Commercial, Maize-Producing Areas
This study was conducted from March to July in both 2019 and 2020 in two commercial, maize-producing areas. One area was located in the municipality of Porto Nacional (Sede; 10°29′30.4″ S 48°19′55.9″ E, at an altitude of 212 m above the mean sea level) and the other in Aparecida do Rio Negro (Sierra; 10°06′05.0″ S 48°09′48.9″ E, at an altitude of 265 m above the mean sea level), both in Tocantins State, Brazil. Five visual evaluations were conducted monthly, starting with the phenological stage in March (V5–V7), followed by April (V7–V10), May (V10–VT), June (R1–R4), and July (R4–R6), to identify maize plants of the commercial hybrid, 30F53, that were exhibiting the symptoms of pale stunt (Css), red stunt (MBS), or mixed infections. The sampled plants were then placed in kraft paper packages and sent to the laboratory for a qPCR analysis to confirm pathogen presence and infection status.
2.5. Temporal Progression of Corn Stunt Disease and the Assessment of the Area under the Disease Progression Curve (AUDPC)
The data collected from the evaluation of corn stunt incidence in the two areas over two years were used to implement a linear regression model to demonstrate the disease’s progression over time. SigmaPlot® Software (version 12.0; Systat Software, San Jose, CA, USA) was used for curve fitting. The area under the disease progression curve (AUDPC) was calculated, as described by [26]:
where yi represents the incidence of infection at the initial time point, yi+1 represents the incidence at the subsequent time point, and (ti+1 − ti) represents the time interval between consecutive evaluations.
AUDPC = ∑[(yi + yi+1)/2] × (ti+1 − ti)]
3. Results
3.1. Potential Alternative Hosts for Natural Infection by Spiroplasma kunkelii (Css)
Of the 212 ornamental plants, spanning 22 species across 12 families, sampled from communal areas of corn-producing farms in the Cerrado region, known for its high rate of corn stunt infection, none tested positive for S. kunkelii (Css) in qPCR analyses (see Table 1).

Table 1.
The qPCR analysis for Spiroplasma kunkelii in plants from corn crops of the Cerrado region in Tocantins State.
3.2. Presence of Spiroplasma kunkelii (Css) Infection in Asymptomatic Maize Plants Adjacent to Symptomatic Plants
Spiroplasma kunkelii was undetectable in the asymptomatic plants neighboring Css-positive, symptomatic plants. Our qPCR analysis confirmed that all 35 of the sampled plants with corn stunt symptoms were infected with Css. In contrast, the 140 asymptomatic plants tested negative for Css. Additionally, we observed normal development in the asymptomatic plants that were located near to the infected ones.
3.3. Incidence of Maize Plants Infected with Spiroplasma kunkelii, Maize Bushy Stunt Phytoplasma, or Mixed Infections in Two Commercial, Maize-Producing Areas
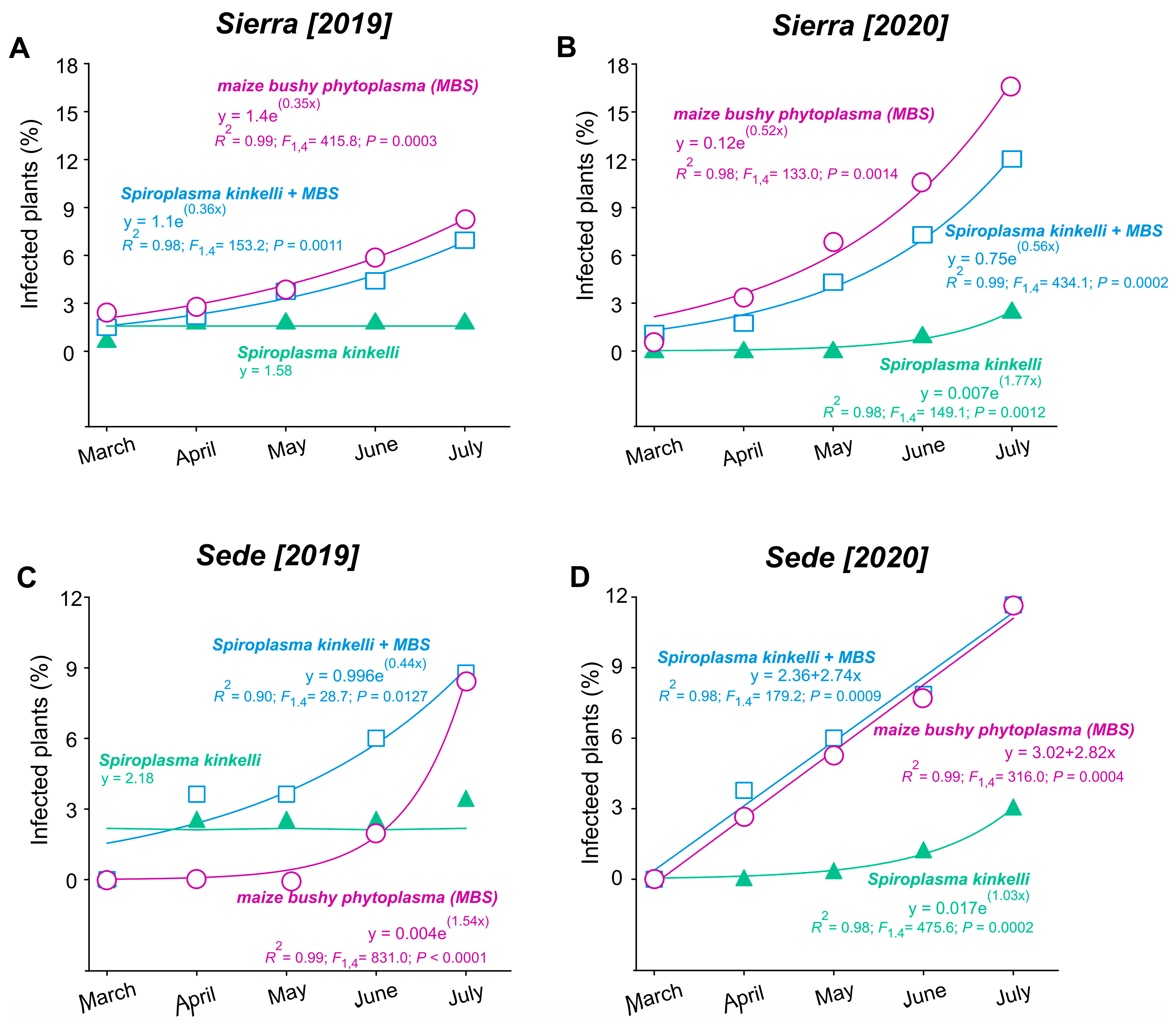
During monitoring from March to July in both 2019 and 2020, the incidence of corn stunt varied significantly. In the Sierra location, the infection rate was less than 3% in March of 2019, rising to 6% by July of the same year. The increase in infected plants was observed for maize bushy stunt (MBS) (F1,4 = 415.84; p = 0.0003) and the combined infection of S. kunkelii + MBS (F1,4 = 153.20; p = 0.0011), while the infection rate of S. kunkelii remained less than 3% and stable throughout 2019 (Figure 2A). In the same region, the infestation of MBS was greater in the second year (starting with 3% in March and surpassing 12% by July for MBS (F1,4 = 132.96; p = 0.0014)) and was also greater for S. kunkelii + MBS (F1,4 = 434.11; p = 0.0002); a slight increase was observed for S. kunkelii (F1,4 = 149.11; p = 0.0012) (Figure 2B). Similar trends were observed at the Sede location in 2019, with a 9% increase in infected plants from March to July for MBS (F1,4 = 831.03; p = 0.0001) and for S. kunkelii + MBS (F1,4 = 28.72; p = 0.012), while the percentage for S. kunkelii remained around 2.18% throughout all the periods (Figure 2C). In 2020, the increase was 11% for MBS (F1,4 = 179.17; p = 0.0009) and for S. kunkelii + MBS (F1,4 = 316.01; p = 0.0004), with less than 3% for S. kunkelii (F1,4 = 475.63; p = 0.0002) (Figure 2D).
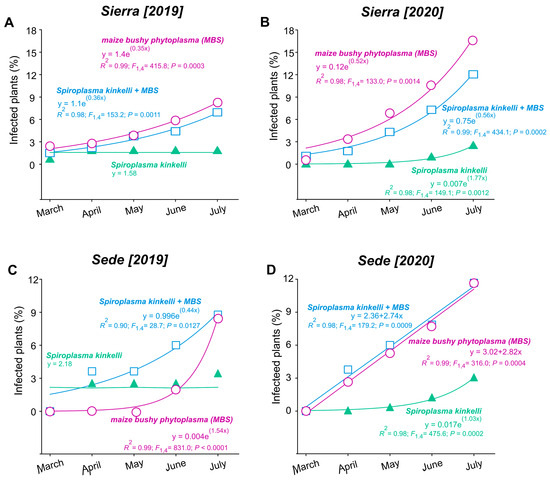
Figure 2.
Temporal progression of corn stunt disease over two periods of time, from March to July, in two cultivation areas, located in the municipalities of Aparecida of Rio Negro (Sierra: A,B) and Porto Nacional (Sede: C,D), Tocantins State, Brazil.
3.4. Temporal Disease Progression and the Area under the Disease Progression Curve (AUDPC) in Two Commercial, Maize-Producing Areas
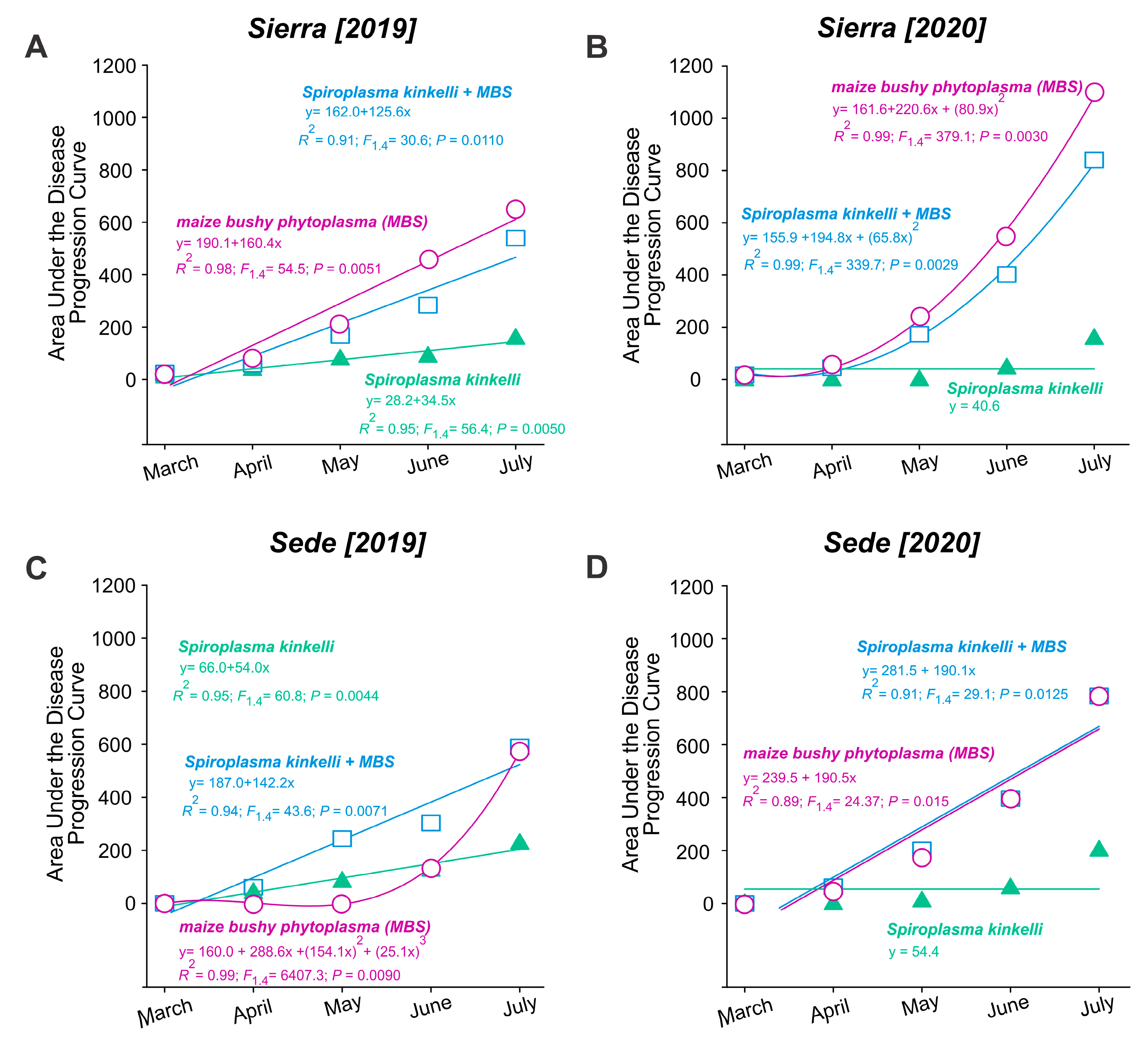
An increase in the AUDPC was observed both within the same year and between the first year (2019) and the second year (2020). At the Sierra location, the AUDPC for maize bushy stunt phytoplasma (MBS) (F1,4 = 54.48; p = 0.006) and S. kunkelii + MBS (F1,4 = 30.62; p = 0.011) increased from 50 in March to over 500 in July 2019 (Figure 3A). Similarly, the AUDPC increased from 50 to over 800 for MBS (F2,4 = 379.10; p = 0.003) and S. kunkelii + MBS (F2,4 = 339.65; p = 0.003) in 2020 (Figure 3B). The increase for S. kunkelii was less pronounced in 2019 (F1,4 = 95.41; p = 0.0019) and remained stable in 2020. At the Sede location, the AUDPC increased from zero to approximately 500 for MBS (F3,4 = 6407.31; p = 0.009) and S. kunkelii + MBS (F1,4 = 43.59; p = 0.007). For S. kunkelii, the increase was less than 200 (F1,4 = 60.75; p = 0.004) (Figure 3C). A similar increase was observed in 2020 for MBS (F1,4 = 24.38; p = 0.016) and S. kunkelii + MBS (F1,4 = 29.11; p = 0.013); however, S. kunkelii remained stable (Figure 3D).
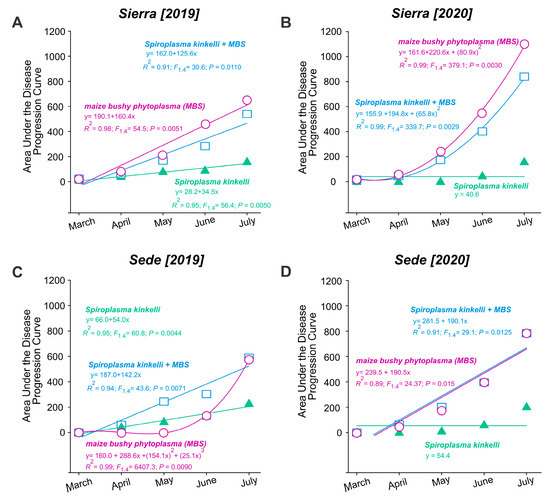
Figure 3.
Area under the disease progression curve (AUDPC) of corn stunt disease over two periods of time, from March to July, in two cultivation areas, located in the municipalities of Aparecida of Rio Negro (Sierra: A,B) and Porto Nacional (Sede: C,D), Tocantins State, Brazil.
4. Discussion
In this study, we analyzed plant tissue from the Cerrado region, including ornamental plants from the same family as maize. We found that all 212 of the samples from the areas near corn fields with a high incidence of corn stunt tested negative for Spiroplasma kunkeli (Css). This indicates that there are no alternative hosts for S. kunkelii in the Cerrado region that are infected through natural routes. These results indicate that S. kunkelii, unlike maize bushy stunt phytoplasma (MBS), which can infect some other plants [17], does not have hosts other than maize, since all the qPCR evaluations of the maize were positive for Css.
The absence of an alternative host for S. kunkelii detected through this investigation is consistent with previous studies on other spiroplasms. Sagouti et al. [27] noted that spiroplasms have a highly specific relationship with their hosts, and alternative hosts are rarely found unless the infection is forced, such as through graft transmission. Additionally, our study confirmed that S. kunkelii was not detected in asymptomatic maize plants exhibiting pale stunt. All 140 of the healthy plants in the reproductive phase (R1) that were situated near diseased plants tested negative for the pathogen. In contrast, the maize plants that were positive for Css that were analyzed in this study displayed the characteristic symptoms of pale stunt. These findings align with observations from studies on carrot crops [28] and ornamental plants [29], in which asymptomatic plants infected with spiroplasms were not found.
S. kunkelii induces numerous alterations in the primary metabolism of plants, particularly in leaf tissues [30,31]. One of the key elements diverted from the plants to support the metabolism of S. kunkelii is magnesium (Mg), which contributes to the observed leaf yellowing [32]. Oliveira et al. [33] found that plants with corn stunt symptoms had a 46% reduction in the available Mg. Our analysis of corn stunt incidence in 2019 and 2020 confirmed the presence of the disease in both of the maize-producing areas. Additionally, the qPCR analysis identified three types of infections in the maize plants: S. kunkelii (Css) alone, maize bushy stunt (MBS) alone, and mixed infection. The highest infection rates were observed for MBS alone in the Sierra region in both 2019 and 2020. Compared to MBS, Css showed a lower incidence of infection. These results are consistent with the incidence surveys conducted in other Brazilian states, which demonstrate a high rate of the disease in maize [4,34]. However, our analysis observed a lower incidence of Css in both the study area and the two years (2021 and 2022).
By analyzing disease progression through the regression of the incidence data, we identified a relationship between the season and the appearance of new plants infected with maize bushy stunt (MBS), mixed infections, or Spiroplasma kunkelii (Css). The highest emergence of infected plants in both years occurred between the V10 and VT stages, with a continuous increase in incidence in subsequent stages until grain maturation. These results align with a previous study [34], where it was reported that corn stunt symptoms typically appear 30 to 40 days after seedling infection. Another relevant aspect is the appearance of spikelets and their development in subsequent periods, which can act as a drain for nutrients and compete with pathogens for nutrients. This scenario can lead to severe nutritional deficiency in the maize plants, resulting in an easier expression of symptoms, such as chlorosis [33].
By monitoring the evolution of the disease in the study area over the years, we were able to analyze pathogen progression, even in areas of higher altitude. Some insects are not adapted to high altitudes [35]; cultivating maize in mountainous areas might be considered a potential strategy for disease control [36]. However, when analyzing the AUDPCs, we revealed an increase in disease epidemiology in both of the areas. This suggests that the relatively small differences in altitude between the two areas do not significantly impact disease distribution and should not be relied upon as a preventive measure.
Unlike S. kunkelii, phytoplasma possess biological characteristics that enhance its competition with other pathogens. For example, infected plants can become more attractive to vector insects due to color changes, such as turning red when infected with phytoplasma, either alone or in a mixed infection [14]. Additionally, another known factor of phytoplasma’s biology is their ability to directly manipulate the behavior of vector insects through changes in the composition of the volatiles emitted by infected plants, inducing again the insect feeding preference [37]. In contrast to the results described here for S. kunkelii, previous investigations indicate that maize bushy stunt (MBS) can occasionally infect and be transmitted by asymptomatic plants [19].
These results are consistent with findings from other studies on vector-transmitted plant diseases [38]. Introducing a pathogen into an area without effective control measures initiates a series of secondary epidemiological cycles, leading to an increase in infected plants. This trend persists over the years, causing a cumulative impact as the pathogen remains in the field and grows with each new cycle [39]. Additionally, the Sierra region (Aparecida do Rio Negro) experienced a higher growth rate of maize planting compared to the Sede area (Porto Nacional) [3]. This contradicts the principle of avoiding disease by limiting the amount of the pathogen and vectors in the area [40], resulting in higher infection rates.
5. Conclusions
Our findings indicate that, despite the increase in corn stunt cases between 2019 and 2020, as confirmed through qPCR, S. kunkelii shows high host specificity, as none of the tested alternative hosts were infected. We also confirmed that maize plants infected with S. kunkelii do not exhibit latent infections, as symptoms appear once the plant is infected. These results are crucial for agronomy, as they highlight the need for more targeted management practices and disease-resistant breeding programs. However, it is important to note that while our findings are pioneering for the region studied and may serve as an alert to other areas, their implications may not be directly applicable to regions with different conditions (e.g., the Amazon rainforest). Such extrapolations are only appropriate when sufficient information is available regarding the population dynamics of the insect vector, D. maidis, and the pathogen, S. kunkelii. Factors such as climate, altitude, temperature, humidity, and cultivar resistance can significantly influence this interaction, necessitating further studies in various regions and under different conditions. Therefore, the results presented here can be integrated into epidemiological research on the spatial and temporal progression of maize stunting, the population dynamics of D. maidis, and contribute valuable data for advancing disease control efforts in areas with climatic conditions similar to those of the Brazilian Cerrado. Future research should explore the pathogen’s host selectivity mechanisms and identify genetic resistance markers to improve maize protection strategies.
Author Contributions
Conceptualization, G.R.S. and R.S.T.; methodology, G.R.S., R.S.T., J.M.R. and N.P.N.; software, G.R.S., R.W.S.A., M.A.O. and R.S.T.; validation, G.R.S., R.W.S.A., M.V.G. and E.E.O.; formal analysis, G.R.S., R.S.T., L.O.V.J., F.S.C., M.A.O. and E.E.O.; investigation, G.R.S., R.S.T., J.M.R., M.V.G., A.S.R.C. and F.S.C.; resources, G.R.S., R.W.S.A., and E.E.O.; data curation, G.R.S., R.S.T., L.O.V.J., E.E.O., M.A.O. and R.W.S.A.; writing—original draft preparation, G.R.S., R.S.T., L.O.V.J. and E.E.O.; writing—review and editing, G.R.S., R.S.T., L.O.V.J., E.E.O., M.V.G. and N.P.N.; visualization, L.O.V.J., E.E.O. and J.M.R.; supervision, G.R.S., R.W.S.A. and E.E.O.; project administration, G.R.S., R.W.S.A. and E.E.O.; funding acquisition, G.R.S., R.W.S.A. and E.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tocantins Federal University (PROPESQ–UFT), the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES; Finance Code 001), the National Council of Scientific and Technological Development (CNPq; 309890/2022-5, 152366/2022-9 and 307290/2023-9), the Tocantins State Foundation for Research Aid (FAPTO), and the Minas Gerais State Foundation for Research Aid (APQ 03771-18).
Data Availability Statement
All the data are contained in the article and can be made available in the event of requests.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- OECD-FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Vilpoux, O.F.; Santos Silveira Junior, J.F. Chapter 3—Global production and use of starch. In Starchy Crops Morphology, Extraction, Properties and Applications; Pascoli Cereda, M., François Vilpoux, O., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 43–66. [Google Scholar]
- CONAB. Companhia Nacional de Abastecimento. Available online: https://www.conab.gov.br/info-agro/safras (accessed on 29 August 2023).
- Galvão, S.R.; Sabato, E.O.; Bedendo, I.P. Occurrence and distribution of single or mixed infection of phytoplasma and spiroplasma causing corn stunting in Brazil. Trop. Plant Pathol. 2021, 46, 152–155. [Google Scholar] [CrossRef]
- Jones, T.-k.L.; Medina, R.F. Corn Stunt Disease: An ideal insect–microbial–plant pathosystem for comprehensive studies of vector-Borne plant diseases of corn. Plants 2020, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Özbek, E.; Miller, S.A.; Meulia, T.; Hogenhout, S.A. Infection and replication sites of Spiroplasma kunkelii (Class: Mollicutes) in midgut and Malpighian tubules of the leafhopper Dalbulus maidis. J. Invertebr. Pathol. 2003, 82, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Carpane, P.; Catalano, M.I. Probing behavior of the corn leafhopper Dalbulus maidis on susceptible and resistant maize hybrids. PLoS ONE 2022, 17, e0259481. [Google Scholar] [CrossRef]
- da Silva, D.D.; Aguiar, F.M.; Cota, L.V.; da Costa, R.V.; Mendes, S.M. Molicutes em milho: A diversificação de sistemas de produção pode ser a solução? Coordenação Nefit/Gestão 2017, 2017, 32–52. [Google Scholar]
- Oleszczuk, J.D.; Catalano, M.I.; Dalaisón, L.; Di Rienzo, J.A.; Giménez Pecci, M.d.l.P.; Carpane, P. Characterization of components of resistance to corn stunt disease. PLoS ONE 2020, 15, e0234454. [Google Scholar] [CrossRef]
- Oliveira, C.M.d.; Frizzas, M.R. Eight decades of Dalbulus maidis (DeLong & Wolcott) (Hemiptera, Cicadellidae) in Brazil: What we know and what we need to know. Neotrop. Entomol. 2022, 51, 1–17. [Google Scholar] [CrossRef]
- Santos, J.M.S.M.; Talamini, V.; SABATO, E.d.O.; de OLIVIERA, F.; Diniz, L.E.C.; dos Santos, C.C. Monitoramento de enfezamentos causados por Molicutes e de ciigarrinhas na cultura do milho nos estados de Sergipe e Bahia na safra 2013. In Proceedings of the IV Seminário de Iniciação Científica e Pós-Graduação, Aracajú, Sergipe, Brazil, 9 January 2014. [Google Scholar]
- Sabato, E.O.; Landau, E.C.; Barros, B.A.; Oliveira, C.M. Differential transmission of phytoplasma and spiroplasma to maize caused by variation in the environmental temperature in Brazil. Eur. J. Plant Pathol. 2020, 157, 163–171. [Google Scholar] [CrossRef]
- Ávila, C.J.; Oliveira, C.; Moreira, S.; Bianco, R.; Tamai, M.A. A cigarrinha Dalbulus maidis e os enfezamentos do milho no Brasil. Rev. Plantio Direto 2021, 18–25. [Google Scholar]
- Ramos, A.; Esteves, M.B.; Cortés, M.T.B.; Lopes, J.R.S. Maize bushy stunt phytoplasma favors its spread by changing host preference of the insect vector. Insects 2020, 11, 600. [Google Scholar] [CrossRef]
- Freitas, L.M.; Souza, B.H.S.; Eghrari, K.; Nascimento, A.M.; Brito, A.H. Effect of Bt zygosity in transgenic maize hybrids to the non-target pest Dalbulus maidis. J. Pest Sci. 2023, 96, 281–298. [Google Scholar] [CrossRef]
- Istchuk, A.N.; da Silva, P.R.; Borges, A.R.P.; Neves, T.C.N.d.; Ramos Pereira, R.; Schwertner, M.H.; Ishizuka, T.K.; Pietrowski, V. Does the corn leafhopper Dalbulus maidis (DeLong & Wolcott) (Hemiptera: Cicadellidae) reproduce in hosts other than maize? EntomoBrasilis 2023, 16, e1044. [Google Scholar] [CrossRef]
- Bedendo, I.P.; Lopes, J.R.S. Impact and Management of Major Phytoplasma Diseases in Brazil. In Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt: Biology and Detection; Olivier, C.Y., Dumonceaux, T.J., Pérez-López, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 251–268. [Google Scholar]
- Hemmati, C.; Al-Subhi, A.M.; Al-Sadi, A.M. Chapter 14—Updates on phytoplasma diseases associated with fodder crops in Asia. In Phytoplasma Diseases of Major Crops, Trees, and Weeds; Tiwari, A.K., Caglayan, K., Hoat, T.X., Subhi, A.A., Nejat, N., Reddy, G., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 2, pp. 337–345. [Google Scholar]
- García Gonzalez, J.; Giraldo Jaramillo, M.; Roberto Spotti Lopes, J. Undetected infection by maize bushy stunt phytoplasma enhances host-plant preference to Dalbulus maidis (Hemiptera: Cicadellidae). Environ. Entomol. 2018, 47, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, E.; Olivier, C.Y.; Luna-Rodríguez, M.; Rodríguez, Y.; Iglesias, L.G.; Castro-Luna, A.; Adame-García, J.; Dumonceaux, T.J. Maize bushy stunt phytoplasma affects native corn at high elevations in Southeast Mexico. Eur. J. Plant Pathol. 2016, 145, 963–971. [Google Scholar] [CrossRef]
- Esquivel-Fariña, A.; Rezende, J.A.M.; Wintermantel, W.M.; Hladky, L.J.; Bampi, D. Natural infection rate of known tomato chlorosis virus-susceptible hosts and the influence of the host plant on the virus relationship with Bemisia tabaci MEAM1. Plant Dis. 2021, 105, 1390–1397. [Google Scholar] [CrossRef]
- Carpane, P.; Laguna, I.G.; Virla, E.G.; Paradell, S.; Murúa, L.; Giménez-Pecci, M.d.l.P. Experimental transmission of corn stunt spiroplasma present in different regions of Argentina. Maydica 2006, 51, 461–468. [Google Scholar]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 2000, 29, 52–54. [Google Scholar] [CrossRef]
- Barros, T.S.L.; Davis, R.E.; Resende, R.O.; Dally, E.L. Design of a Polymerase Chain Reaction for specific detection of corn stunt spiroplasma. Plant Dis. 2001, 85, 475–480. [Google Scholar] [CrossRef]
- Harrison, N.; Richardson, P.; Tsai, J.; Ebbert, M.; Kramer, J. PCR assay for detection of the phytoplasma associated with maize bushy stunt disease. Phytopathology 1996, 68, 677–680. [Google Scholar] [CrossRef]
- Simko, I. IdeTo: Spreadsheets for calculation and analysis of area under the disease progress over time data. PhytoFrontiers™ 2021, 1, 244–247. [Google Scholar] [CrossRef]
- Sagouti, T.; Belabess, Z.; Rhallabi, N.; Barka, E.A.; Tahiri, A.; Lahlali, R. Citrus stubborn disease: Current insights on an enigmatic problem prevailing in citrus orchards. Microorganisms 2022, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Munyaneza, J.E.; Lemmetty, A.; Nissinen, A.I.; Sengoda, V.G.; Fisher, T.W. Molecular detection of aster yellows phytoplasma and “Candidatus Liberibacter Solacacearum” in carrots affected by the Psyllid Trioza apicalis (Hemiptera: Triozidae) in Finland. Plant Pathol. J. 2011, 93, 697–700. [Google Scholar]
- Tahat, M.M.; Nejat, N.; Sijam, K. Glomus mosseae bioprotection against aster yellows phytoplasma (16srI-B) and Spiroplasma citri infection in Madagascar periwinkle. Physiol. Mol. Plant Pathol. 2014, 88, 1–9. [Google Scholar] [CrossRef]
- Gross, J.; Gallinger, J.; Görg, L.M. Interactions between phloem-restricted bacterial plant pathogens, their vector insects, host plants, and natural enemies, mediated by primary and secondary plant metabolites. Entomol. Gen. 2022, 42, 185–215. [Google Scholar] [CrossRef]
- Nome, C.; Magalhães, P.C.; Oliveira, E.; Nome, S.; Laguna, I.G. Differences in intracellular localization of corn stunt spiroplasmas in magnesium treated maize. Biocell 2009, 33, 133–136. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil. 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Oliveira, E.; Magalhães, P.C.; Gomide, R.L.; Vasconcelos, C.A.; Souza, I.R.P.; Oliveira, C.M.; Cruz, I.; Schaffert, R.E. Growth and nutrition of mollicute-infected maize. Plant Dis. 2002, 86, 945–949. [Google Scholar] [CrossRef]
- Oliveira, E.; Resende, R.O.; Pecci, G.M.; Laguna, I.G.; Herrera, P.; Cruz, I. Incidência de viroses e enfezamentos e estimativa de perdas causadas por molicutes em milho no Paraná. Pesqui. Agropecu. Bras. 2003, 38, 19–25. [Google Scholar] [CrossRef]
- Herrera Vásquez, J.A.; Jaén Sanjur, J.N.; Zachrisson Salamina, B.A.; Rubio Miguélez, L.; Alvarado, B.; Amet, A.; Aguilera Cogley, V.A.; Valdespino, R.A.; Mejía Franco, L.C. Occurrence and dístríbutíon of Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodídae) on tomato crops in Panama. Acta Agron. 2022, 71, 96–105. [Google Scholar] [CrossRef]
- Magenya, O.; Mueke, J.; Omwega, C. Association of maize streak virus disease and its vectors (Homoptera: Cicadelidae) with soil macronutrients and altitudes in Kenya. Afr. J. Agric. Res. 2009, 4, 1284–1290. [Google Scholar]
- Weintraub, P.G.; Trivellone, V.; Krüger, K. The Biology and Ecology of Leafhopper Transmission of Phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria-II: Transmission and Management of Phytoplasma-Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 27–51. [Google Scholar]
- Macedo, M.A.; Inoue-Nagata, A.K.; Silva, T.N.Z.; Freitas, D.M.S.; Rezende, J.A.M.; Barbosa, J.C.; Michereff-Filho, M.; Nascimento, A.R.; Lourenção, A.L.; Bergamin Filho, A. Temporal and spatial progress of the diseases caused by the crinivirus tomato chlorosis virus and the begomovirus tomato severe rugose virus in tomatoes in Brazil. Plant Pathol. 2019, 68, 72–84. [Google Scholar] [CrossRef]
- Filho, A.B.; Inoue-Nagata, A.K.; Bassanezi, R.B.; Belasque, J.; Amorim, L.; Macedo, M.A.; Barbosa, J.C.; Willocquet, L.; Savary, S. The importance of primary inoculum and area-wide disease management to crop health and food security. Food Secur. 2016, 8, 221–238. [Google Scholar] [CrossRef]
- Arsia, S.K.; Bharti, O.P.; Sharma, S. Integrated Approach in Plants Disease Management. In Modern Approaches in Plant Pathology; Sharma, S.K.A.S., Kaur, A., Poorvasandhya, R., Dhaka, S., Eds.; Elite publishing House: New Delhi, India, 2023; pp. 12–21. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).