Improving Inoculum Production of Arbuscular Mycorrhizal Fungi in Zea mays L. Using Light-Emitting Diode (LED) Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Condition
2.2. Optimizing the Conditions of Light-Emitting Diodes (LEDs) for Promoting Maize Growth and Fungal Abundance
2.3. Assessment of AM Fungal Colonization
- 1.
- Frequency of mycorrhiza in the root system (F%)
- 2.
- Intensity of mycorrhizal colonization in the root system (M%)
- 3.
- Intensity of mycorrhizal colonization in mycorrhizal root fragments (m%)
- 4.
- Arbuscule abundance in mycorrhizal root fragments (a%)
- 5.
- Arbuscule Abundance in the Entire Root System (A%)
2.4. Isolation of R. irregularis Spores
2.5. Gene References and Primer Design
2.6. RNA Extraction and qRT-PCR
2.7. Statistical Analyses
3. Results
3.1. Optimum Blue-and-Red LED Light Ratio Expedited Maize Seedling Growth and Increased Biomass
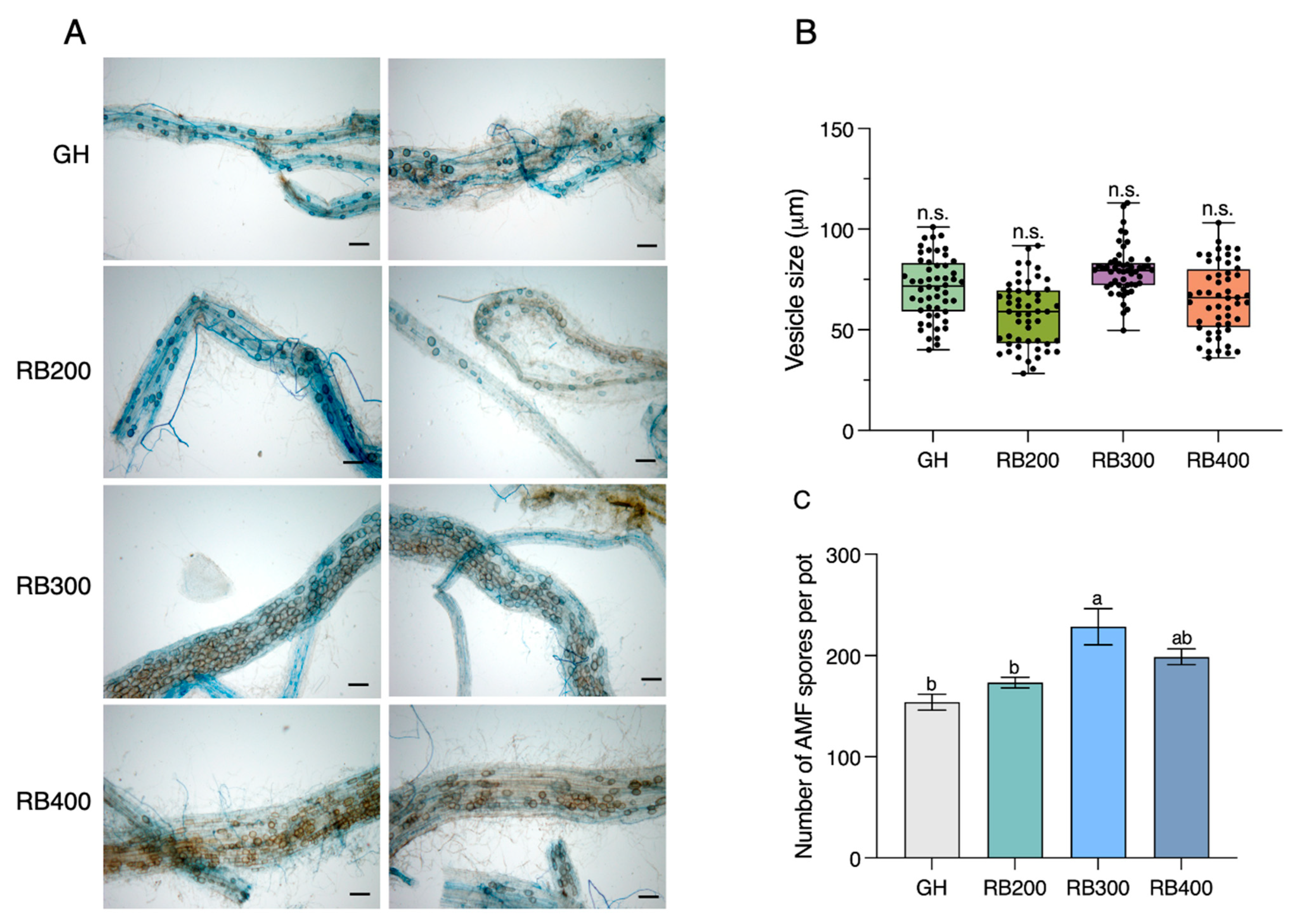
3.2. LED Light Intensity Affected Fungal Abundance in Maize Root
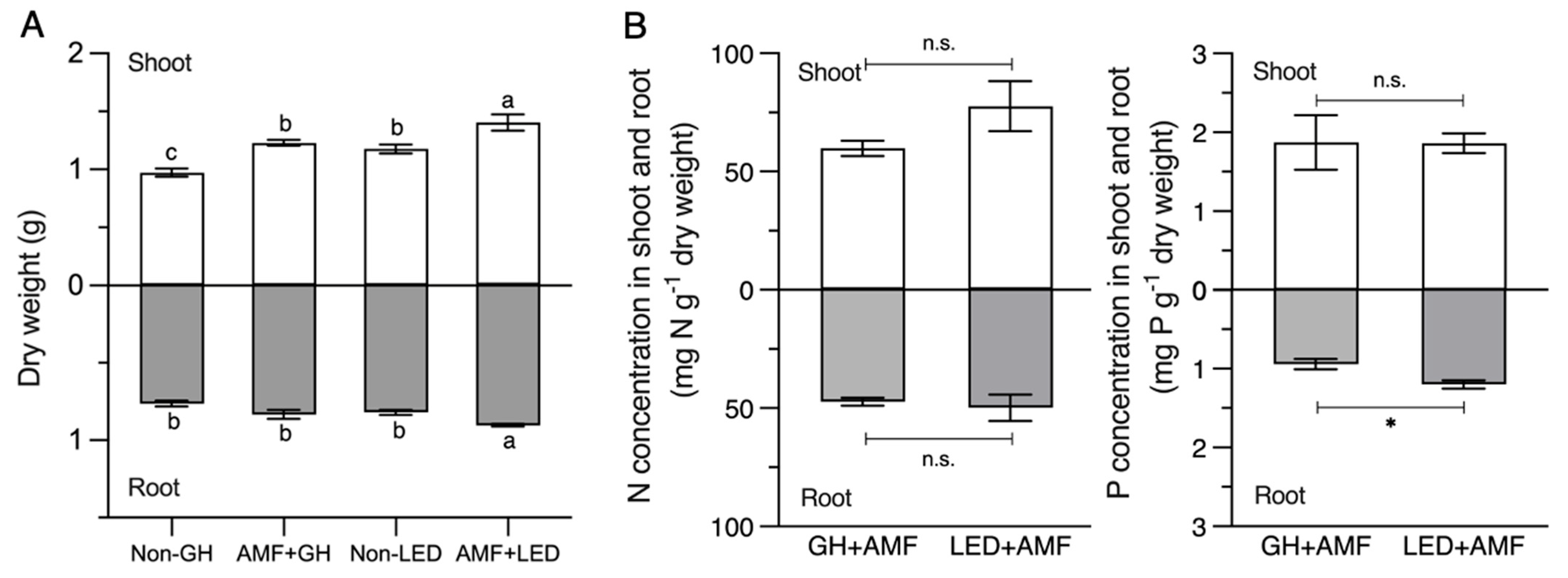
3.3. The Optimized LED Condition Enhanced Nutrient Accumulation, AM Fungal Colonization, and Spore Production in Maize Better than the GH Condition
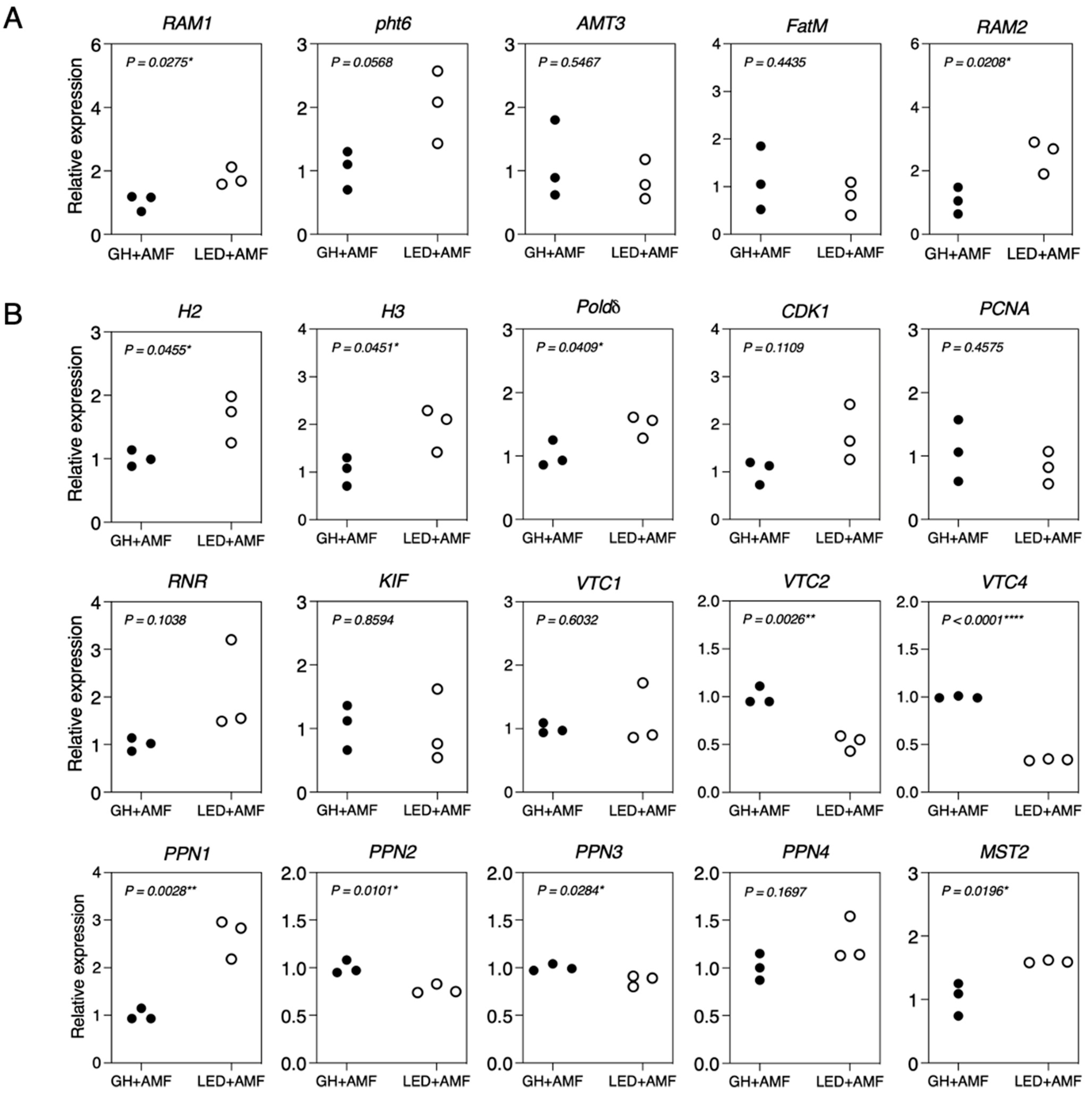
3.4. Gene Expression in Maize and R. irregularis during Symbiosis in Response to Growth Treatment under LED Light and GH Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kivlin, S.N.; Hawkes, C.V.; Treseder, K.K. Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2011, 43, 2294–2303. [Google Scholar] [CrossRef]
- Lee, E.H.; Eo, J.K.; Ka, K.H.; Eom, A.H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Mei, L.; Yang, X.; Cao, H.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alter plant and soil C:N:P stoichiometries under warming and nitrogen input in a semiarid meadow of China. Int. J. Environ. Res. Public Health 2019, 16, 397. [Google Scholar] [CrossRef]
- Prasad, K.; Aggarwal, A.; Yadav, K.; Tanwar, A. Impact of different levels of superphosphate using arbuscular mycorrhizal fungi and Pseudomonas fluorescens on Chrysanthemum indicum L. J. Soil Sci. Plant Nutr. 2012, 12, 451–462. [Google Scholar]
- Sudheer, S.; Johny, L.; Srivastava, S.; Adholeya, A. The trade-in-trade: Multifunctionalities, current market and challenges for arbuscular mycorrhizal fungal inoculants. Symbiosis 2023, 89, 259–272. [Google Scholar] [CrossRef]
- IJdo, M.; Cranenbrouck, S.; Declerck, S. Methods for large-scale production of AM fungi: Past, present, and future. Mycorrhiza 2011, 21, 1–16. [Google Scholar] [CrossRef]
- Sayeed Akhtar, M.; Nor, S.; Abdullah, A. Mass production techniques of arbuscular mycorrhizal fungi: Major advantages and disadvantages: A review. Biosci. Biotechnol. Res. Asia 2014, 11, 1199–1204. [Google Scholar] [CrossRef]
- Chourasiya, D.; Gajghate, R.; Prakash, A.; Sharma, M.P. Low-cost technologies for AMF inoculum production using various agro-wastes and other by-products. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Inoculum Production and Application; Springer Nature: Singapore, 2024; pp. 145–165. [Google Scholar]
- Bencherif, K.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounés-Hadj Sahraoui, A. Engineering approach for production of arbuscular mycorrhizal inoculum adapted to saline soil management. Stresses 2023, 3, 404–423. [Google Scholar] [CrossRef]
- Genre, A.; Bonfante, P. Building a mycorrhizal cell: How to reach compatibility between plants and arbuscular mycorrhizal fungi. J. Plant Interact. 2005, 1, 3–13. [Google Scholar] [CrossRef]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018, 8, 10235. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, A.; Lahrmann, U.; Langen, G. Broad compatibility in fungal root symbioses. Curr. Opin. Plant Biol. 2014, 20, 135–145. [Google Scholar] [CrossRef] [PubMed]
- INVAM International Culture Colletion of (Vesicular) Arbuscular Micorrhizal Fungi. Available online: https://invam.ku.edu/ (accessed on 16 September 2024).
- Kiddee, S.; Yuttavanichakul, W.; Boonkerd, N.; Teaumroong, N.; Saito, K.; Tittabutr, P. Secretion compounds from Brevibacillus Sp. SUT47 promote spore Propagation of Acaulospora Tuberculata colonizing maize roots (Zea mays L. cultivar Suwan 5). ScienceAsia 2020, 46, 634–638. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloem loading in zmsweet13a, b, c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Xu, Y.; Chang, Y.; Chen, G.; Lin, H. The Research on LED supplementary lighting system for plants. Optik 2016, 127, 7193–7201. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirb. Agric. 2010, 97, 99–104. [Google Scholar]
- He, D.; Kozai, T.; Niu, G.; Zhang, X. Light-emitting diodes for horticulture. In Light-Emitting Diodes Materials, Processes, Devices and Applications; Springer: Cham, Switzerland, 2019; pp. 513–547. [Google Scholar]
- Viršile, A.; Olle, M.; Duchovskis, P. LED lighting in horticulture. In Light Emitting Diodes for Agriculture: Smart Lighting; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–147. [Google Scholar]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.P.; Wang, J.Z.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Cruz, A.F. Effect of light-emitting diodes on arbuscular mycorrhizal fungi associated with bahiagrass (Paspalum notatum Flügge) and millet [Pennisetum glaucum (L.) R. Br]. Bioagro 2016, 28, 163–170. [Google Scholar]
- Tenzin, U.W.; Noirungsee, N.; Runsaeng, P.; Noppradit, P.; Klinnawee, L. Dry-season soil and co-cultivated host plants enhanced propagation of arbuscular mycorrhizal fungal spores from sand dune vegetation in trap culture. J. Fungi 2022, 8, 1061. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Saito, K. Role of cell wall polyphosphates in phosphorus transfer at the arbuscular interface in mycorrhizas. Front. Plant Sci. 2021, 12, 725939. [Google Scholar] [CrossRef]
- Songsaeng, A.; Tittabutr, P.; Umnajkitikorn, K.; Boonkerd, N.; Wongdee, J.; Songwattana, P.; Piromyou, P.; Greetatorn, T.; Girdthai, T.; Teaumroong, N. Application of light-emitting diodes with plant growth-promoting rhizobacteria and arbuscular mycorrhiza fungi for tomato seedling production. Agronomy 2022, 12, 2458. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Daniels, B.A.; Skipper, H. Methods for the Recovery and quantitative estimation of propagules from soil. In Methods & Principles of Mycorrhizal Research, American Phytopathological Society; American Phytopathological Society: Saint Paul, MN, USA, 1982; pp. 29–35. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Kiddee, S.; Wongdee, J.; Piromyou, P.; Songwattana, P.; Greetatorn, T.; Boonkerd, N.; Teaumroong, N.; Saito, K.; Tittabutr, P. Unveiling the tripartite synergistic interaction of plant-arbuscular mycorrhizal fungus symbiosis by endophytic Bacillus velezensis S141 in Lotus japonicus. Symbiosis 2024, 92, 355–367. [Google Scholar] [CrossRef]
- Sugimura, Y.; Kawahara, A.; Maruyama, H.; Ezawa, T. Plant foraging strategies driven by distinct genetic modules: Cross-ecosystem transcriptomics approach. Front. Plant Sci. 2022, 13, 903539. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Zhang, J.; Xiao, Y.; Yue, Y.; Duan, L.; Zhang, M.; Li, Z. Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.). PLoS ONE 2013, 8, e52126. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Ezawa, T.; Saito, K. Polyphosphate polymerizing and depolymerizing activity of VTC4 protein in an arbuscular mycorrhizal fungus. Soil Sci. Plant Nutr. 2022, 68, 256–267. [Google Scholar] [CrossRef]
- Kitazaki, K.; Fukushima, A.; Nakabayashi, R.; Okazaki, Y.; Kobayashi, M.; Mori, T.; Nishizawa, T.; Reyes-Chin-Wo, S.; Michelmore, R.W.; Saito, K.; et al. Metabolic reprogramming in leaf lettuce grown under different light quality and intensity conditions using narrow-band LEDs. Sci. Rep. 2018, 8, 7914. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.O.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’and ‘Purple Star’. Physiol. Plant. 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, A.; Cheng, Z.M. (Max) Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Hao, X.; Papadopoulos, A.P. Effects of supplemental lighting and cover materials on growth, photosynthesis, biomass partitioning, early yield and quality of greenhouse cucumber. Sci. Hortic. 1999, 80, 1–18. [Google Scholar] [CrossRef]
- Jones, M.A. Using light to improve commercial value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Jackson, L.E.; Six, J.; Ferris, H.; Goyal, S.; Asami, D.; Scow, K.M. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 2006, 282, 209–225. [Google Scholar] [CrossRef]
- Lupo, M.; Bashir, M.A.; Silvestri, C.; Brunori, E.; Pica, A.L.; Cristofori, V. LED lighting effects on plant growth and quality of Pyrus communis L. propagated in vitro. Agronomy 2022, 12, 2531. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED Lighting: A Grower’s Guide to Light Spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Campos-Soriano, L.; García-Martínez, J.; Segundo, B.S. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/Blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Matsumura, A.; Horii, S.; Ishii, T. Effects of arbuscular mycorrhizal fungi and intercropping with bahiagrass on growth and anti-oxidative enzyme activity of radish. J. Jpn. Soc. Hortic. Sci. 2007, 76, 224–229. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2018, 17, 25–36. [Google Scholar] [CrossRef]
- Petrillo, E.; Herz, M.A.G.; Barta, A.; Kalyna, M.; Kornblihtt, A.R. Let there be light: Regulation of gene expression in plants. RNA Biol. 2014, 11, 1215–1220. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. N. Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Thompson, J.P. Soilless culture of vesicular–arbuscular mycorrhizae of cereals: Effects of nutrient concentration and nitrogen source. Can. J. Bot. 1986, 64, 2282–2294. [Google Scholar] [CrossRef]
- Harris, S.D. Branching of Fungal Hyphae: Branching of fungal hyphae: Regulation, mechanisms and comparison with other branching systems. Mycologia 2008, 100, 823–832. [Google Scholar] [CrossRef]
- Sugimura, Y.; Saito, K. Transcriptional profiling of arbuscular mycorrhizal roots exposed to high levels of phosphate reveals the repression of cell cycle-related genes and secreted protein genes in Rhizophagus irregularis. Mycorrhiza 2017, 27, 139–146. [Google Scholar] [CrossRef]
- Ezawa, T.; Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121. [Google Scholar] [CrossRef]
- Kobae, Y.; Kawachi, M.; Saito, K.; Kikuchi, Y.; Ezawa, T.; Maeshima, M.; Hata, S.; Fujiwara, T. Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi. Mycorrhiza 2015, 25, 411–417. [Google Scholar] [CrossRef] [PubMed]
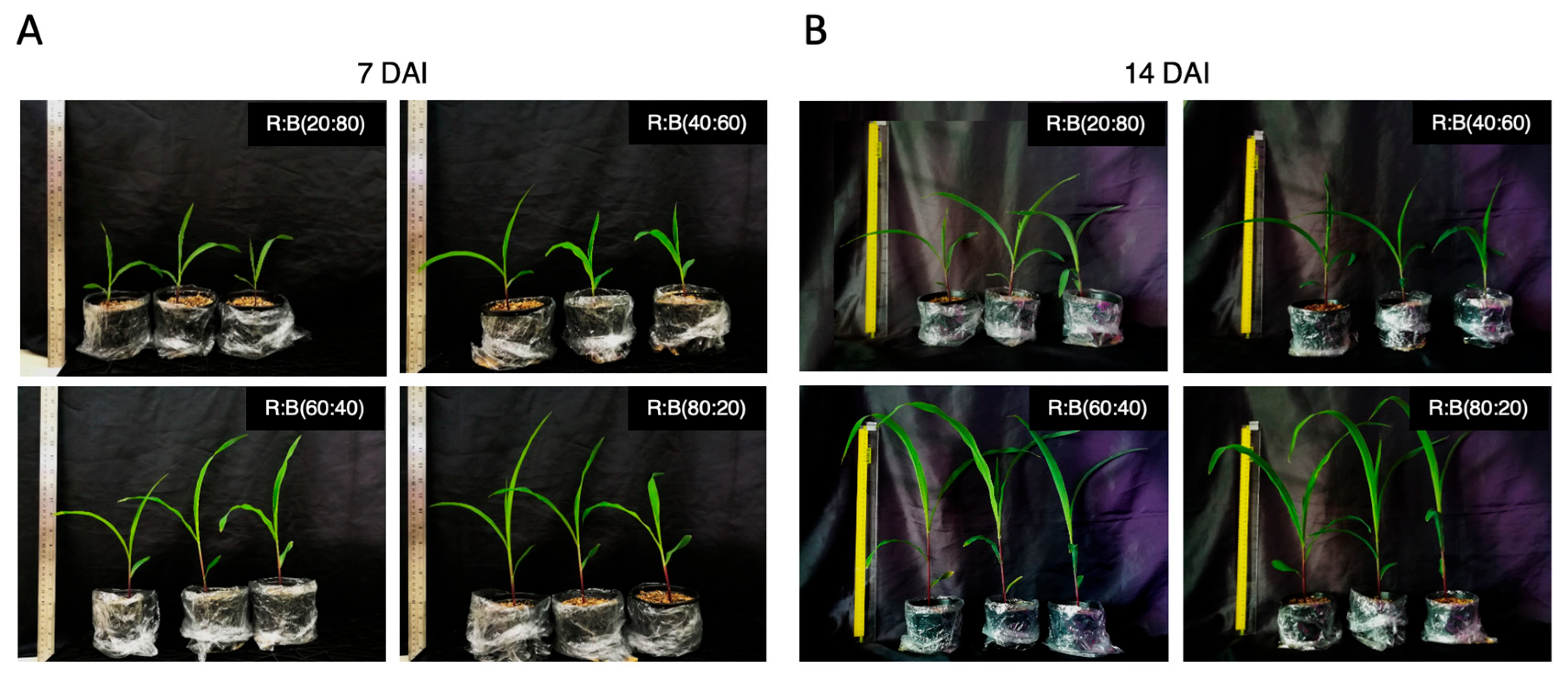



| Light Ratios | Plant Height (cm) | Chlorophyll Content | Plant Biomass (g/plant) | ||
|---|---|---|---|---|---|
| (SPAD Unit) | Shoot DW | Root DW | |||
| 7 DAE | R:B (20:80) | 13.83 ± 0.6 b | 25.86 ± 0.1 a | - | - |
| R:B (40:60) | 11.63 ± 0.8 b | 25.37 ± 0.0 a | - | - | |
| R:B (60:40) | 19.67 ± 2.1 a | 23.63 ± 0.3 b | - | - | |
| R:B (80:20) | 21.17 ± 1.0 a | 23.44 ± 0.3 b | - | - | |
| 14 DAE | R:B (20:80) | 16.00 ± 1.0 c | 32.74 ± 0.8 a | 0.22 ± 0.02 b | 0.33 ± 0.03 ab |
| R:B (40:60) | 16.67 ± 0.6 c | 29.04 ± 0.1 b | 0.14 ± 0.02 b | 0.22 ± 0.01 b | |
| R:B (60:40) | 31.13 ± 1.1 b | 26.49 ± 0.3 bc | 0.36 ± 0.02 a | 0.44 ± 0.03 a | |
| R:B (80:20) | 36.93 ± 0.8 a | 27.38 ± 0.1 c | 0.41 ± 0.02 a | 0.38 ± 0.04 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiddee, S.; Lakkasorn, N.; Wongdee, J.; Piromyou, P.; Songwattana, P.; Greetatorn, T.; Teamtisong, K.; Boonkerd, N.; Saito, K.; Teaumroong, N.; et al. Improving Inoculum Production of Arbuscular Mycorrhizal Fungi in Zea mays L. Using Light-Emitting Diode (LED) Technology. Agronomy 2024, 14, 2342. https://doi.org/10.3390/agronomy14102342
Kiddee S, Lakkasorn N, Wongdee J, Piromyou P, Songwattana P, Greetatorn T, Teamtisong K, Boonkerd N, Saito K, Teaumroong N, et al. Improving Inoculum Production of Arbuscular Mycorrhizal Fungi in Zea mays L. Using Light-Emitting Diode (LED) Technology. Agronomy. 2024; 14(10):2342. https://doi.org/10.3390/agronomy14102342
Chicago/Turabian StyleKiddee, Sutee, Niramon Lakkasorn, Jenjira Wongdee, Pongdet Piromyou, Pongpan Songwattana, Teerana Greetatorn, Kamonluck Teamtisong, Nantakorn Boonkerd, Katsuharu Saito, Neung Teaumroong, and et al. 2024. "Improving Inoculum Production of Arbuscular Mycorrhizal Fungi in Zea mays L. Using Light-Emitting Diode (LED) Technology" Agronomy 14, no. 10: 2342. https://doi.org/10.3390/agronomy14102342






