Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Obtaining of Ginger Extracts
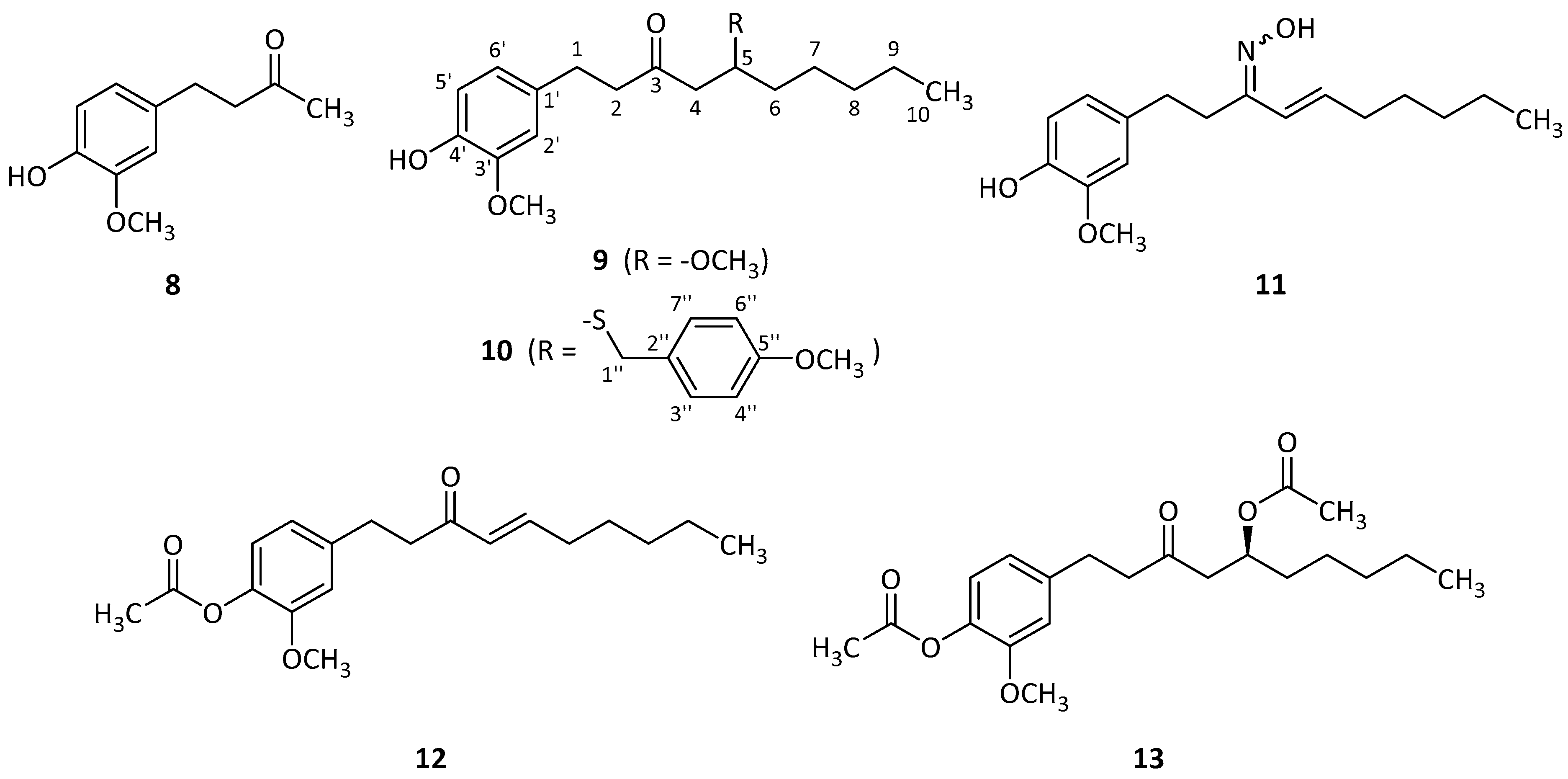
2.3. Purification and Characterization of Compounds from the Most Phytotoxic Extract
2.4. Synthesis of Derivatives
2.5. Bioassays
3. Results and Discussion
3.1. Obtaining and Study of the Phytotoxicity of Ginger Extracts
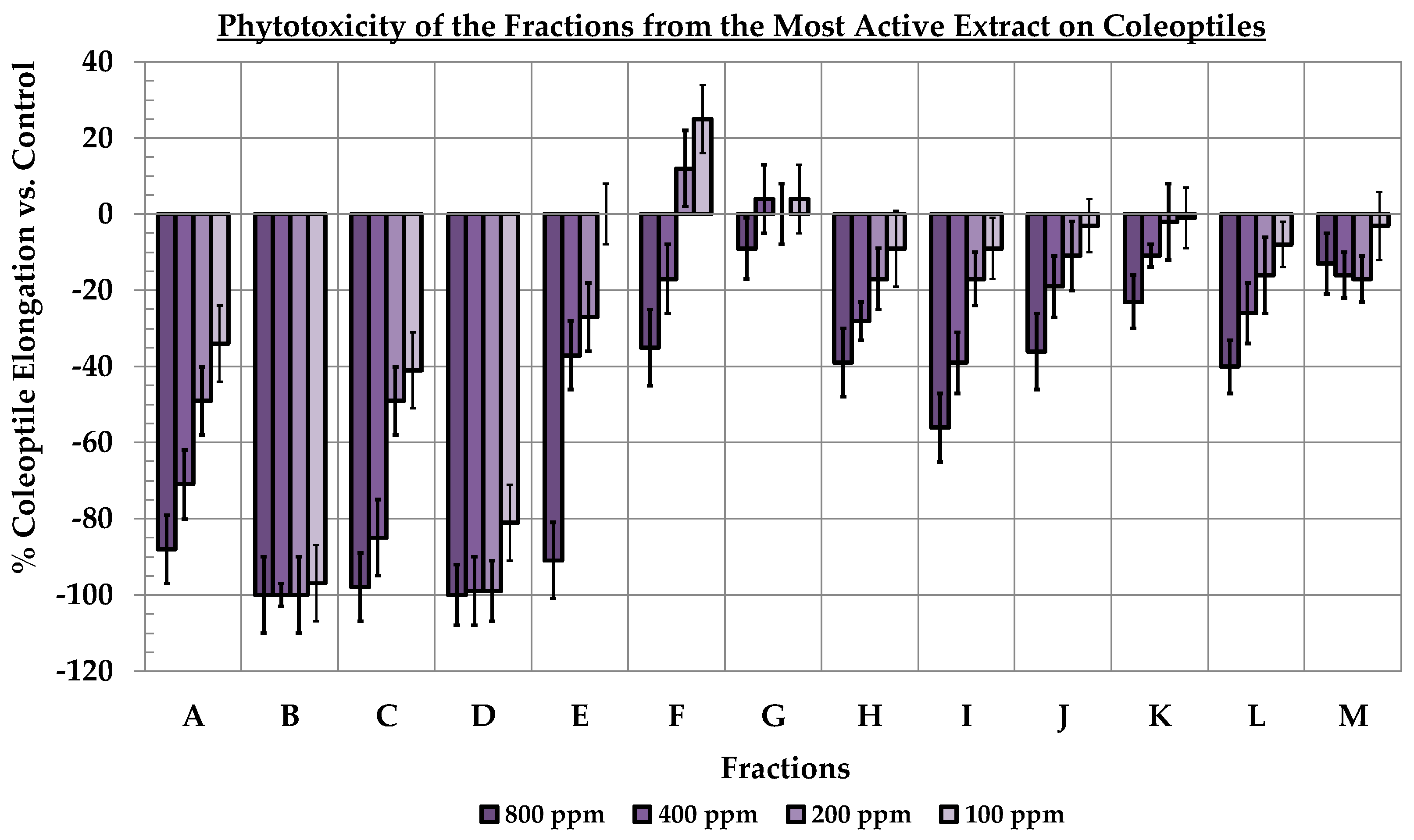
3.2. Bio-Guided Purification of Metabolites from the Most Phytotoxic Ginger Extract
3.3. Study of the Phytotoxicity of the Isolated Compounds
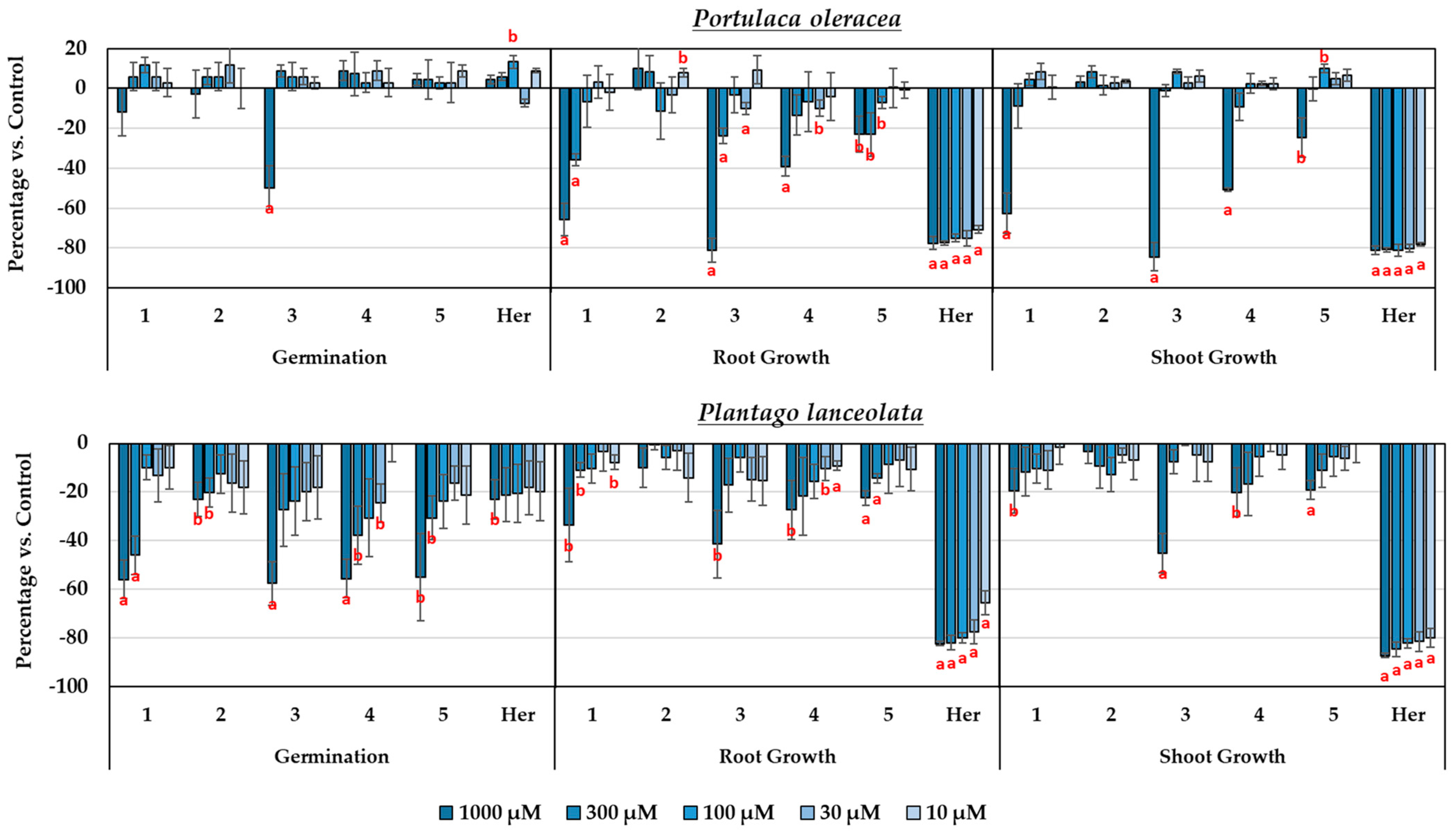
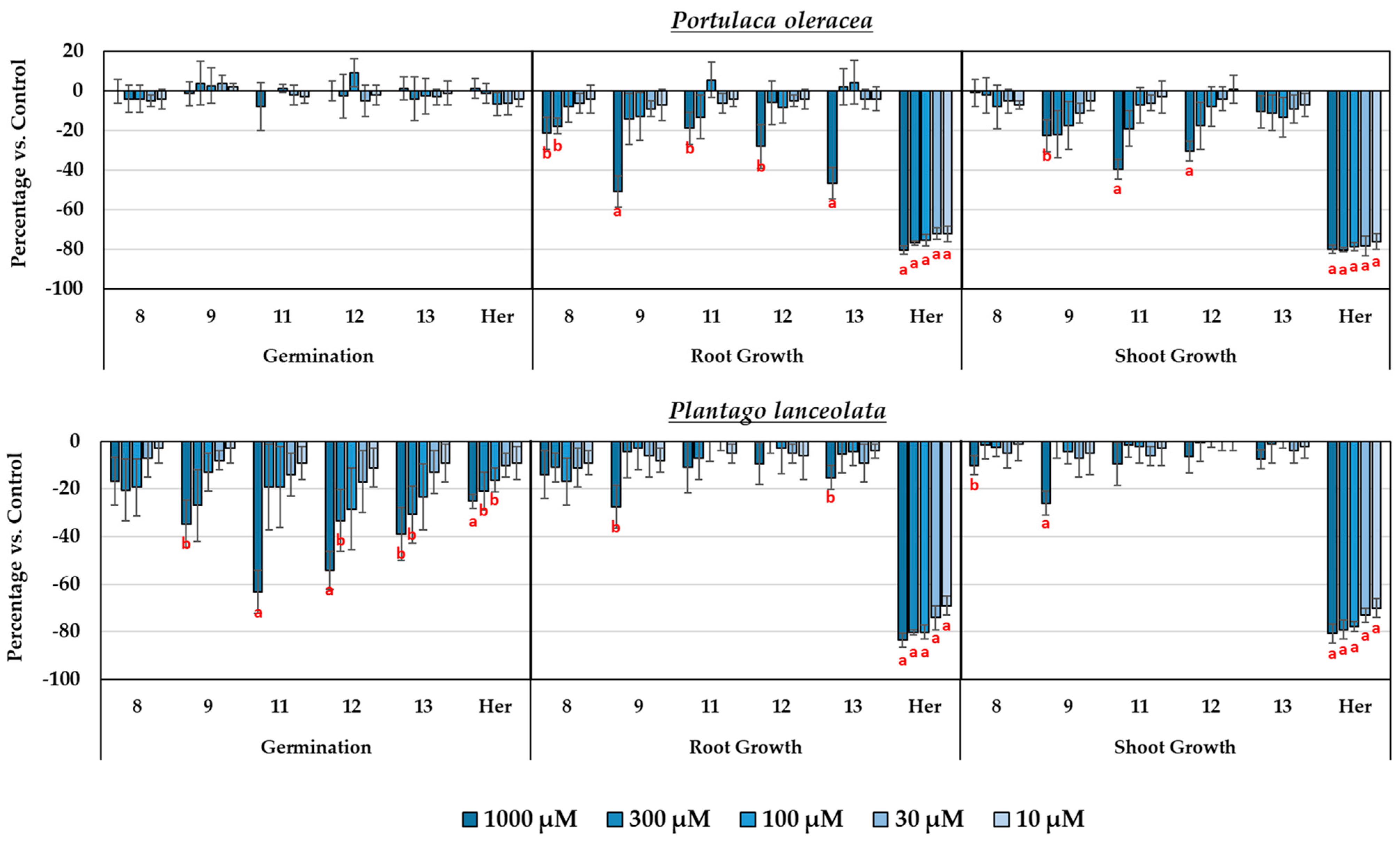
3.4. Synthesis and Study of the Phytotoxicity of Synthetic Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, N.K. Agricultural sustainability and food security. Environ. Sustain. 2018, 1, 217–219. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Iturburu, F.G.; Calderon, G.; Oyesqui, L.A.; De Gerónimo, E.; Aparicio, V.C. Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere 2021, 263, 128061. [Google Scholar] [CrossRef]
- Das, T.K.; Behera, B.; Nath, C.P.; Ghosh, S.; Sen, S.; Raj, R.; Ghosh, S.; Sharma, A.R.; Yaduraju, N.T.; Nalia, A.; et al. Herbicides use in crop production: An analysis of cost-benefit, non-target toxicities and environmental risks. Crop Prot. 2024, 181, 106691. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Assessment of allelopathic compounds to develop new natural herbicides: A review. Allelopath. J. 2021, 52, 21–40. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. The search for new herbicide mechanisms of action: Is there a ‘holy grail’? Pest Manag. Sci. 2022, 78, 1303–1313. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Suman, U.; Divya, Y.; Ram, C.; Naveen, A. Evaluation of antibacterial and phytochemical properties of different spice extracts. Afr. J. Microbiol. Res. 2018, 12, 27–37. [Google Scholar] [CrossRef]
- Mallik, S.; Sharangi, A.B.; Sarkar, T. Phytochemicals of coriander, cumin, fenugreek, fennel and black cumin: A preliminary study. Natl. Acad. Sci. Lett. 2020, 43, 477–480. [Google Scholar] [CrossRef]
- Yadav, D.; Gaurav, H.; Yadav, R.; Waris, R.; Afzal, K.; Shukla, A.C. A comprehensive review on soft rot disease management in ginger (Zingiber officinale) for enhancing its pharmaceutical and industrial values. Heliyon 2023, 9, e18337. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, D.Q.; Xiong, W.; Zhang, P.Z.; Ma, G.Q.; Liu, R.H.; Yao, X.L. A new pyridine alkaloid from Zingiberis rhizoma. Nat. Prod. Res. 2017, 31, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Ginger and turmeric essential oils for weed control and food crop protection. Plants 2019, 8, 59. [Google Scholar] [CrossRef]
- Al Harun, M.A.Y.; Johnson, J.; Uddin, M.N.; Robinson, R.W. Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 2015, 10, e0139992. [Google Scholar]
- Araniti, F.; Miras-Moreno, B.; Lucini, L.; Landi, M.; Abenavoli, M.R. Metabolomic, proteomic and physiological insights into the potential mode of action of thymol, a phytotoxic natural monoterpenoid phenol. Plant Physiol. Biochem. 2020, 153, 141–153. [Google Scholar] [CrossRef]
- Castaldi, S.; Zorrilla, J.G.; Petrillo, C.; Russo, M.T.; Ambrosino, P.; Masi, M.; Cimmino, A.; Isticato, R. Alternaria alternata isolated from infected pears (Pyrus communis) in Italy produces non-host toxins and hydrolytic enzymes as infection mechanisms and exhibits competitive exclusion against Botrytis cinerea in co-infected host fruits. J. Fungi 2023, 9, 326. [Google Scholar] [CrossRef]
- Goggin, D.E.; Powles, S.B.; Steadman, K.J. Understanding Lolium rigidum seeds: The key to managing a problem weed? Agronomy 2012, 2, 222–239. [Google Scholar] [CrossRef]
- Hanley, M.E. Seedling defoliation, plant growth and flowering potential in native-and invasive-range Plantago lanceolata populations. Weed Res. 2012, 52, 252–259. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Vraka, C.; Nics, L.; Wagner, K.H.; Hacker, M.; Wadsak, W.; Mitterhauser, M. LogP, a yesterday’s value? Nucl. Med. Biol. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- PerkinElmer. Available online: http://perkinelmer.com (accessed on 10 September 2024).
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.H.; Badmaev, V.; Ho, C.T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hosokawa, S.; Ono, Y.; Kubodera, N. Practical and facile route to a functional intermediate from stigmasterol for the synthesis of 1α-hydroxyvitamin D5 and related compounds. Heterocycles 2016, 93, 101–113. [Google Scholar]
- Andreu, I.; Neshchadin, D.; Rico, E.; Griesser, M.; Samadi, A.; Morera, I.M.; Gescheidt, G.; Miranda, M.A. Probing lipid peroxidation by using linoleic acid and benzophenone. Chem.–Eur. J. 2011, 17, 10089–10096. [Google Scholar] [CrossRef]
- Kuo, P.C.; Cherng, C.Y.; Jeng, J.F.; Damu, A.G.; Teng, C.M.; Lee, E.J.; Wu, T.S. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch. Pharm. Res. 2005, 28, 518–528. [Google Scholar] [CrossRef]
- Yamauchi, K.; Natsume, M.; Yamaguchi, K.; Batubara, I.; Mitsunaga, T. Structure-activity relationship for vanilloid compounds from extract of Zingiber officinale var rubrum rhizomes: Effect on extracellular melanogenesis inhibitory activity. Med. Chem. Res. 2019, 28, 1402–1412. [Google Scholar] [CrossRef]
- Kumar, N.V.; Murthy, P.S.; Manjunatha, J.R.; Bettadaiah, B.K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef]
- Galal, A.M. Antimicrobial activity of 6-paradol and related compounds. Int. J. Pharmacogn. 1996, 34, 64–69. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Innangi, M.; Cala Peralta, A.; Soriano, G.; Russo, M.T.; Masi, M.; Fernández-Aparicio, M.; Cimmino, A. Sesquiterpene lactones isolated from Centaurea cineraria L. subsp. cineraria inhibit the radicle growth of broomrape weeds. Plants 2024, 13, 178. [Google Scholar] [CrossRef]
- El-Naggar, M.H.; Mira, A.; Bar, F.M.A.; Shimizu, K.; Amer, M.M.; Badria, F.A. Synthesis, docking, cytotoxicity, and LTA4H inhibitory activity of new gingerol derivatives as potential colorectal cancer therapy. Bioorg. Med. Chem. 2017, 25, 1277–1285. [Google Scholar] [CrossRef]
- Mejías, F.J.; Durán, A.G.; Zorrilla, J.G.; Varela, R.M.; Molinillo, J.M.G.; Valdivia, M.M.; Macías, F.A. Acyl derivatives of eudesmanolides to boost their bioactivity: An explanation of behavior in the cell membrane using a molecular dynamics approach. ChemMedChem 2021, 16, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Facile synthesis of anhydrojudaicin and 11,13-dehydroanhydrojudaicin, two eudesmanolide-skeleton lactones with potential allelopathic activity. Phytochem. Lett. 2019, 31, 229–236. [Google Scholar] [CrossRef]
- Han, J.S.; Lee, S.; Kim, H.Y.; Lee, C.H. MS-based metabolite profiling of aboveground and root components of Zingiber mioga and officinale. Molecules 2015, 20, 16170–16185. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Portal-Gonzalez, N.; Wang, X.; Li, J.; Li, H.; Portieles, R.; Borras-Hidalgo, O.; He, W.; Santos-Bermudez, R. Insights into the microbial assembly and metabolites associated with ginger (Zingiber officinale L. Roscoe) microbial niches and agricultural environments. Sci. Total Environ. 2024, 947, 174395. [Google Scholar] [CrossRef] [PubMed]
- Han, C.M.; Pan, K.W.; Wu, N.; Wang, J.C.; Li, W. Allelopathic effect of ginger on seed germination and seedling growth of soybean and chive. Sci. Hortic. 2008, 116, 330–336. [Google Scholar] [CrossRef]
- Bhattarai, S.; Duke, C.C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Zhang, N.; Udenigwe, C.C.; Zhang, Y.; Fu, Y. Preparation, pungency and bioactivity of gingerols from ginger (Zingiber officinale Roscoe): A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2708–2733. [Google Scholar] [CrossRef]
- Roli, O.I.; Adetunji, C.O.; Mishra, R.R.; Adetunji, J.B.; Mishra, P.; Fatoki, T.H. Rediscovering medicinal activity and food significance of shogaol (4, 6, 8, 10, and 12): Comprehensive review. In Innovations in Food Technology; Mishra, P., Mishra, R.R., Adetunji, C.O., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Cárdenas, D.M.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Masi, M.; Macías, F.A. Bioprospection of phytotoxic plant-derived eudesmanolides and guaianolides for the control of Amaranthus viridis, Echinochloa crus-galli, and Lolium perenne weeds. J. Agric. Food Chem. 2024, 72, 1797–1810. [Google Scholar] [CrossRef]
- Tice, C.M. Selecting the right compounds for screening: Does Lipinski’s rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag. Sci. 2001, 57, 3–16. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, J.S.; Chen, L.H.; Peng, W.W.; Cai, B.C. Simultaneous determination of five gingerols in raw and processed ginger by HPLC. Chin. Pharm. J. 2012, 47, 471–474. [Google Scholar]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef] [PubMed]
- Tramontini, M.; Angiolini, L. Mannich Bases—Chemistry and Uses; Rees, C.W., Ed.; Imperial College of Science, Technology and Medicine: London, UK, 1994. [Google Scholar]
- Stupnicka-Rodzynkiewicz, E.; Dabkowska, T.; Stoklosa, A.; Hura, T.; Dubert, F.; Lepiarczyk, A. The effect of selected phenolic compounds on the initial growth of four weed species. J. Plant Dis. Prot. 2006, 20, 479. [Google Scholar]




| Extraction Method and Solvent | Amount of Ground Ginger Roots (g) | Extract Weight (g) | Isolation Yield (%) | IC50 in the Wheat Coleoptile Bioassay (ppm) |
|---|---|---|---|---|
| M; Ethyl acetate | 1.0047 | 0.0539 | 5.36 | 197.30 |
| M; Methanol | 1.0166 | 0.1063 | 10.5 | 274.00 |
| S; Ethyl acetate S; Methanol | 1.0510 | 0.0508 | 4.83 | 41.15 |
| 1.0044 | 0.1035 | 10.3 | 459.50 | |
| E; Ethyl acetate | 1.0166 | 0.0101 | 0.99 | 86.39 |
| E; Methanol | 0.0962 | 9.46 | >800 |
| Compound | 1 | 2 | 3 | 4 | 6 | 7 |
|---|---|---|---|---|---|---|
| IC50 (μM) | 63.79 | 413.90 | 389.00 | 158.00 | 742.20 | >1000 |
| Clog P | 3.91 | 6.03 | 2.94 | 4.00 | 10.45 | 7.30 |
| Compound | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|
| IC50 (μM) | 694.4 | 103.2 | >1000 | 40.03 | 71.87 | 65.93 |
| Clog P | 1.07 | 3.71 | 6.17 | 4.70 | 3.82 | 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorrilla, J.G.; Rial, C.; Martínez-González, M.I.; Molinillo, J.M.G.; Macías, F.A.; Varela, R.M. Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control. Agronomy 2024, 14, 2353. https://doi.org/10.3390/agronomy14102353
Zorrilla JG, Rial C, Martínez-González MI, Molinillo JMG, Macías FA, Varela RM. Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control. Agronomy. 2024; 14(10):2353. https://doi.org/10.3390/agronomy14102353
Chicago/Turabian StyleZorrilla, Jesús G., Carlos Rial, Miriam I. Martínez-González, José M. G. Molinillo, Francisco A. Macías, and Rosa M. Varela. 2024. "Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control" Agronomy 14, no. 10: 2353. https://doi.org/10.3390/agronomy14102353
APA StyleZorrilla, J. G., Rial, C., Martínez-González, M. I., Molinillo, J. M. G., Macías, F. A., & Varela, R. M. (2024). Ginger Phytotoxicity: Potential Efficacy of Extracts, Metabolites and Derivatives for Weed Control. Agronomy, 14(10), 2353. https://doi.org/10.3390/agronomy14102353









