Multispecies Trichoderma in Combination with Hydrolyzed Lignin Improve Tomato Growth, Yield, and Nutritional Quality of Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tests of Lignin Effect on Tomato Germination
2.2. Trichoderma spp.–Lignin Compatibility Tests
2.3. Effect of Trichoderma–Lignin Formulations on Tomato Growth
2.4. Field Experiments: Effect of Trichoderma–Lignin on Tomato Yield and Fruit Quality
2.5. Statistical Analyses
3. Results
3.1. Effect of Lignin on Tomato Germination
3.2. Trichoderma–Lignin Compatibility
3.3. Effect of Trichoderma–Lignin Formulations on Tomato Growth
3.4. Field Experiments: Effect of Trichoderma–Lignin on Tomato Yield and Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, L. Chemical Fertilizer Rate, Use Efficiency and Reduction of Cereal Crops in China, 1998–2018. J. Geogr. Sci. 2022, 32, 65–78. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Herder, G.D.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The Roots of a New Green Revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Fedoroff, N. V Food in a Future of 10 Billion. Agric. Food Secur. 2015, 4, 11. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The Persistent Threat of Emerging Plant Disease Pandemics to Global Food Security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial Biofertilizers: Bioresources and Eco-Friendly Technologies for Agricultural and Environmental Sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant Growth Promoting Rhizobacteria: Constraints in Bioformulation, Commercialization, and Future Strategies. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–116. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhatt, K.; Sharma, A.; Zhang, W.; Mishra, S.; Chen, S. Biotechnological Basis of Microbial Consortia for the Removal of Pesticides from the Environment. Crit. Rev. Biotechnol. 2021, 41, 317–338. [Google Scholar] [CrossRef]
- Tabacchioni, S.; Passato, S.; Ambrosino, P.; Huang, L.; Caldara, M.; Cantale, C.; Hett, J.; Del Fiore, A.; Fiore, A.; Schlüter, A.; et al. Identification of Beneficial Microbial Consortia and Bioactive Compounds with Potential as Plant Biostimulants for a Sustainable Agriculture. Microorganisms 2021, 9, 426. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as Biocontrol Agent against Pests: New Uses for a Mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Liu-Xu, L.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Scalschi, L.; Llorens, E. Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae 2024, 10, 621. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma Harzianum Rifai 1295-22. Appl Environ. Microbiol 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Popa, V.I.; Dumitru, M.; Volf, I.; Anghel, N. Lignin and Polyphenols as Allelochemicals. Ind. Crops Prod. 2008, 27, 144–149. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. Physiological and Biochemical Responses Induced by Spruce Bark Aqueous Extract and Deuterium Depleted Water with Synergistic Action in Sunflower (Helianthus annuus L.) Plants. Ind. Crops Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Sanjuán, J.; Nápoles, M.C.; Pérez-Mendoza, D.; Lorite, M.J.; Rodríguez-Navarro, D.N. Microbials for Agriculture: Why Do They Call Them Biostimulants When They Mean Probiotics? Microorganisms 2023, 11, 153. [Google Scholar] [CrossRef]
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical Pesticides for an Eco-Friendly and Sustainable Agriculture: New Challenges and Prospects. In Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2022; pp. 69–96. [Google Scholar]
- Regnault-Roger, C.; Philogène, B.J.R. Past and Current Prospects for the Use of Botanicals and Plant Allelochemicals in Integrated Pest Management. Pharm. Biol. 2008, 46, 41–52. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic Biomass for Bioethanol Production: Current Perspectives, Potential Issues and Future Prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Sethupathy, S.; Murillo Morales, G.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin Valorization: Status, Challenges and Opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 225–241. [Google Scholar]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular Economy Aspects of Lignin: Towards a Lignocellulose Biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. Bioresources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing Organic Substances Stimulate Phenylpropanoid Metabolism in Zea mays. J. Plant Nutr. Soil. Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Altissimo, A. Review: Long-Term Research Activity on the Biostimulant Properties of Natural Origin Compounds. Acta Hortic. 2013, 1009, 181–187. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Benites-Alfaro, O.E.; Pompelli, M.F. GerminaR: An R Package for Germination Analysis with the Interactive Web Application “GerminaQuant for R”. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma Harzianum, 6-Pentyl-α-Pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Bakker, M.G.; Manter, D.K.; Sheflin, A.M.; Weir, T.L.; Vivanco, J.M. Harnessing the Rhizosphere Microbiome through Plant Breeding and Agricultural Management. Plant Soil. 2012, 360, 1–13. [Google Scholar] [CrossRef]
- Singh, P.K.; Vijay, K. Biological Control of Fusarium Wilt of Chrysanthemum with Trichoderma and Botanicals. J. Agric. Technol. 2011, 7, 1603–1613. [Google Scholar]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic Properties of Lignin Components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Zúñiga-Partida, V.; Ramírez-Cano, F.; Hahn, N.-C.; Faix, O. Conversion of Technical Lignins into Slow-Release Nitrogenous Fertilizers by Ammoxidation in Liquid Phase. Bioresour. Technol. 1994, 49, 121–128. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological Responses to Humic Substances as Plant Growth Promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic Substances Stimulate Maize Nitrogen Assimilation and Amino Acid Metabolism at Physiological and Molecular Level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
- Kesba, H.H.; El-Beltagi, H.S. Biochemical Changes in Grape Rootstocks Resulted from Humic Acid Treatments in Relation to Nematode Infection. Asian Pac. J. Trop. Biomed. 2012, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost Humic Acids as an Ecological Pathway to Protect Rice Plant against Oxidative Stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Gerig, T.M.; Blum, U. Effects of Mixtures of Four Phenolic Acids on Leaf Area Expansion of Cucumber Seedlings Grown in Portsmouth B1 Soil Materials. J. Chem. Ecol. 1991, 17, 29–40. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and Effect of Interaction Phytotoxic Compounds from Exudates of Cistus Ladanifer Leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic Potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Williams, R.D.; Hoagland, R.E. The Effects of Naturally Occurring Phenolic Compounds on Seed Germination. Weed Sci. 1982, 30, 206–212. [Google Scholar] [CrossRef]
- Almaghrabi, O.A. Control of Wild Oat (Avena Fatua) Using Some Phenolic Compounds I—Germination and Some Growth Parameters. Saudi J. Biol. Sci. 2012, 19, 17–24. [Google Scholar] [CrossRef]
- Kuiters, A.T. Effects of Phenolic Acids on Germination and Early Growth of Herbaceous Woodland Plants. J. Chem. Ecol. 1989, 15, 467–479. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; Gonzalez, L. Effect of Phenolic Compounds on the Germination of Six Weeds Species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of Commercial Lignosulfonate-Humate on Zea mays L. Metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like Bioactivity on Emergence and Early Growth of Maize (Zea mays L.) of Water-Soluble Lignins Isolated from Biomass for Energy. Plant Soil. 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The Use of Organic Biostimulants in Hot Pepper Plants to Help Low Input Sustainable Agriculture. Chem. Biol. Technol. Agric. 2015, 2, 11. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-Induced Acidification Is an Early Trigger for Changes in Arabidopsis Root Growth and Determines Fungal Phytostimulation. Front. Plant Sci. 2017, 8, 822. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth Stimulation and Fruit Yield Improvement of Greenhouse Tomato Plants by Inoculation with Pseudomonas Putida or Trichoderma Atroviride: Possible Role of Indole Acetic Acid (IAA). Soil. Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Uresti-Rivera, E.E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flores, S. Colonization of Arabidopsis Roots by Trichoderma Atroviride Promotes Growth and Enhances Systemic Disease Resistance through Jasmonic Acid/Ethylene and Salicylic Acid Pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering Microbial Consortia: A New Frontier in Synthetic Biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Eriksson, K.-E.L. Laccase Is Essential for Lignin Degradation by the White-Rot Fungus Pycnoporus cinnabarinus. FEBS Lett. 1997, 407, 89–92. [Google Scholar] [CrossRef]
- ten Have, R.; Teunissen, P.J.M. Oxidative Mechanisms Involved in Lignin Degradation by White-Rot Fungi. Chem. Rev. 2001, 101, 3397–3414. [Google Scholar] [CrossRef]
- Dabhi, B.; Vyas, R.; Shelat, H. Biodegradation of Lignin by Fungal Cultures. J. Pharmacogn. Phytochem. 2017, 6, 1840–1842. [Google Scholar]
- Heinzkill, M.; Bech, L.; Halkier, T.; Schneider, P.; Anke, T. Characterization of Laccases and Peroxidases from Wood-Rotting Fungi (Family coprinaceae). Appl. Environ. Microbiol. 1998, 64, 1601–1606. [Google Scholar] [CrossRef]
- Gayazov, R.; Rodakiewicz-Nowak, J. Semi-Continuous Production of Laccase ByPhlebia Radiata in Different Culture Media. Folia Microbiol. 1996, 41, 480–484. [Google Scholar] [CrossRef]
- Assavanig, A.; Amornikitticharoen, B.; Ekpaisal, N.; Meevootisom, V.; Flegel, T. Isolation, Characterization and Function of Laccase from Trichoderma. Appl. Microbiol. Biotechnol. 1992, 38, 198–202. [Google Scholar] [CrossRef]
- Hölker, U.; Dohse, J.; Höfer, M. Extracellular Laccases in AscomycetesTrichoderma Atroviride AndTrichoderma Harzianum. Folia Microbiol. 2002, 47, 423–427. [Google Scholar] [CrossRef]
- Fusco, G.M.; Burato, A.; Pentangelo, A.; Cardarelli, M.; Nicastro, R.; Carillo, P.; Parisi, M. Can Microbial Consortium Applications Affect Yield and Quality of Conventionally Managed Processing Tomato? Plants 2022, 12, 14. [Google Scholar] [CrossRef]
- Zanor, M.I.; Rambla, J.-L.; Chaïb, J.; Steppa, A.; Medina, A.; Granell, A.; Fernie, A.R.; Causse, M. Metabolic Characterization of Loci Affecting Sensory Attributes in Tomato Allows an Assessment of the Influence of the Levels of Primary Metabolites and Volatile Organic Contents. J. Exp. Bot. 2009, 60, 2139–2154. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A.; Mosca, G. Essential Amino Acids: Master Regulators of Nutrition and Environmental Footprint? Sci. Rep. 2016, 6, 26074. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E. Differential Expression of Maize Chitinases in the Presence or Absence of Trichoderma Harzianum Strain T22 and Indications of a Novel Exo- Endo-Heterodimeric Chitinase Activity. BMC Plant Biol. 2010, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma Harzianum on Microelement Concentrations and Increased Growth of Cucumber Plants. Plant Soil. 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Martinez, V.; Nuñez, J.M.; Ortiz, A.; Cerda, A. Changes in Amino Acid and Organic Acid Composition in Tomato and Cucumber Plants in Relation to Salinity and Nitrogen Nutrition. J. Plant Nutr. 1994, 17, 1359–1368. [Google Scholar] [CrossRef]
- Lam, H.M.; Peng, S.S.Y.; Coruzzi, G.M. Metabolic Regulation of the Gene Encoding Glutamine-Dependent Asparagine Synthetase in Arabidopsis Thaliana. Plant Physiol. 1994, 106, 1347–1357. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen Transfer in the Arbuscular Mycorrhizal Symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Ruzicka, D.R.; Hausmann, N.T.; Barrios-Masias, F.H.; Jackson, L.E.; Schachtman, D.P. Transcriptomic and Metabolic Responses of Mycorrhizal Roots to Nitrogen Patches under Field Conditions. Plant Soil. 2012, 350, 145–162. [Google Scholar] [CrossRef]
- Gallego, P.P.; Whotton, L.; Picton, S.; Grierson, D.; Gray, J.E. A Role for Glutamate Decarboxylase during Tomato Ripening: The Characterisation of a CDNA Encoding a Putative Glutamate Decarboxylase with a Calmodulin-Binding Site. Plant Mol. Biol. 1995, 27, 1143–1151. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L.; Cai, Y.; Huang, Y.; Zheng, X.; Yu, T. L-Glutamate Treatment Enhances Disease Resistance of Tomato Fruit by Inducing the Expression of Glutamate Receptors and the Accumulation of Amino Acids. Food Chem. 2019, 293, 263–270. [Google Scholar] [CrossRef]
- Yang, J.; Sun, C.; Fu, D.; Yu, T. Test for l -Glutamate Inhibition of Growth of Alternaria Alternata by Inducing Resistance in Tomato Fruit. Food Chem. 2017, 230, 145–153. [Google Scholar] [CrossRef]
- Bellisle, F. Glutamate and the UMAMI Taste: Sensory, Metabolic, Nutritional and Behavioural Considerations. A Review of the Literature Published in the Last 10 Years. Neurosci. Biobehav. Rev. 1999, 23, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ninomiya, K. Umami and Food Palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli, P.R.; Regitano-d’Arce, M.A.B.; Palma, E.R. Physicochemical Characteristics and Fatty Acid Composition of Tomato Seed Oils from Processing Wastes. Sci. Agric. 1993, 50, 117–120. [Google Scholar] [CrossRef]

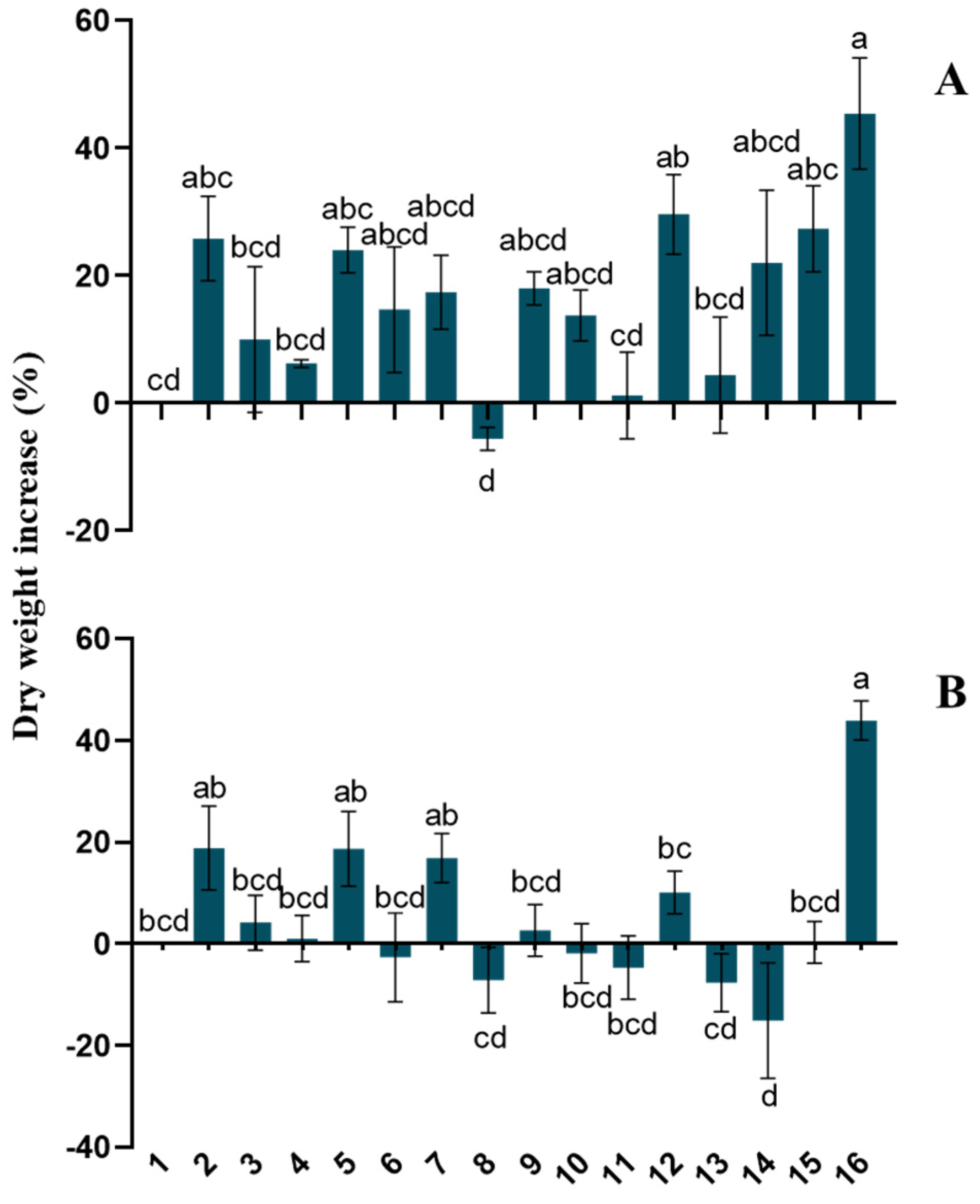

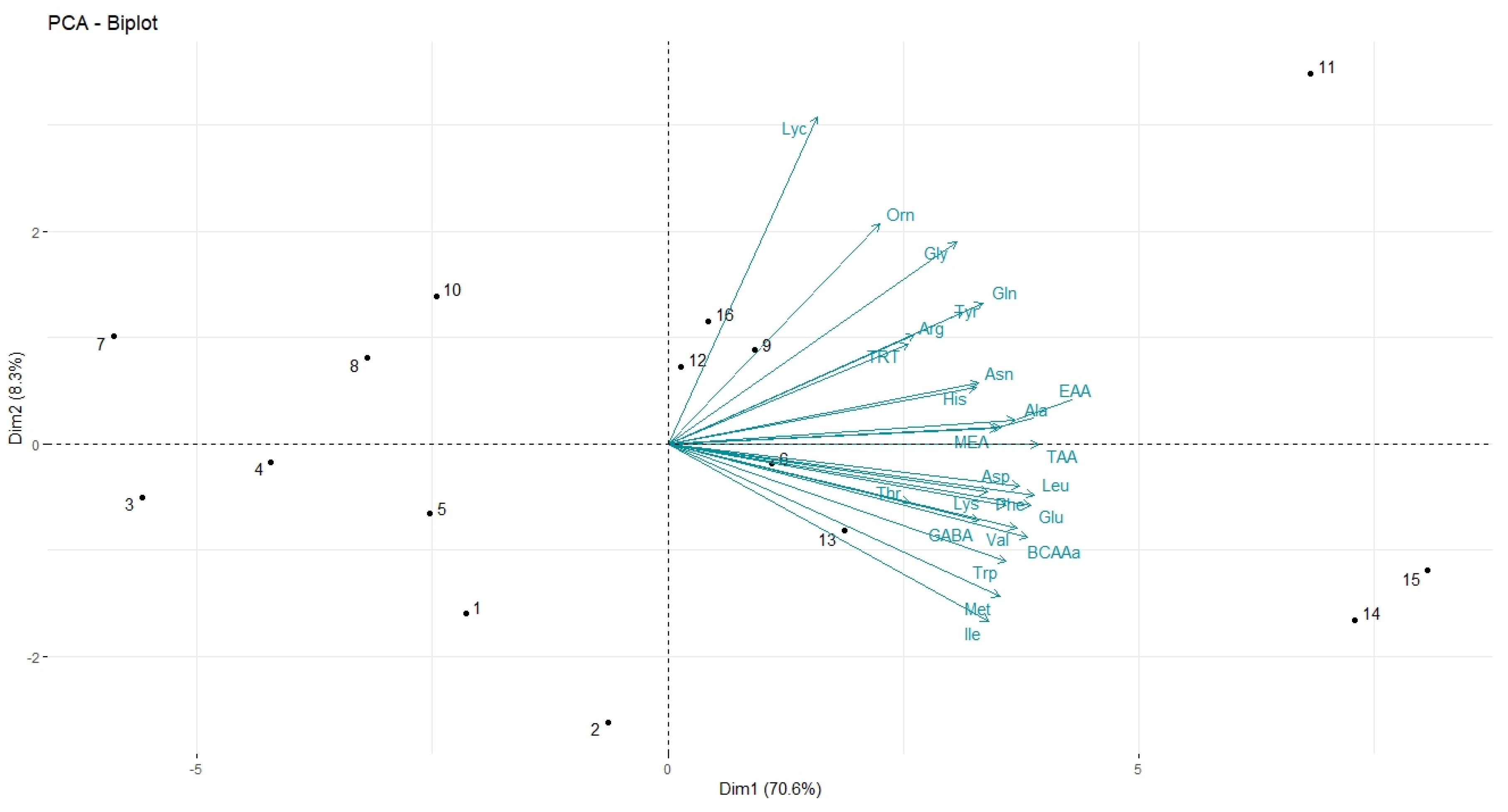
| Treatment | Concentration |
|---|---|
| Control (water) | |
| T. virens GV41 | 4 × 106 sp mL−1 |
| T. asperellum | 4 × 106 sp mL−1 |
| T. atroviride | 4 × 106 sp mL−1 |
| Lignin | 1% |
| T. virens GV41. + lignin | 4 × 106 sp mL−1 + lignin |
| T. asperellum + lignin | 4 × 106 sp mL−1 + lignin |
| T. atroviride + lignin | 4 × 106 sp mL−1 + lignin |
| T asperellum + T. atroviride + lignin | 4 × 106 sp mL−1 + lignin |
| T. asperellum + T. virens GV41 + lignin | 4 × 106 sp mL−1 + lignin |
| T. atroviride + T. virens GV41 + lignin | 4 × 106 sp mL−1 + lignin |
| T. asperellum + T. atroviride | 4 × 106 sp mL−1 (1:1) |
| T. asperellum + T. virens GV41 | 4 × 106 sp mL−1 (1:1) |
| T. atroviride + T. virens GV41 | 4 × 106 sp mL−1 (1:1) |
| T. asperellum + T. atroviride + T. virens GV41 | 4 × 106 sp mL−1 (1:1:1) |
| T. virens GV41 + T. asperellum + T. atroviride + lignin | 4 × 106 sp mL−1 + lignin |
| Lignin Concentrations | D1 | D4 | D5 | D7 | D10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (water) | 0 ± 0 | a | 17.78 ± 16.78 | c | 46.67 ± 6.67 | a | 51.11 ± 7.7 | f | 91.11 ± 10.18 | a |
| 0.01% | 0 ± 0 | a | 51.11 ± 7.7 | ab | 60 ± 13.33 | a | 88.89 ± 10.18 | ab | 97.78 ± 3.85 | a |
| 0.1% | 0 ± 0 | a | 40 ± 6.67 | abc | 51.11 ± 10.18 | a | 86.67 ± 6.67 | bc | 97.78 ± 3.85 | a |
| 0.3% | 0 ± 0 | a | 66.67 ± 6.67 | a | 86.67 ± 13.33 | a | 100 ± 0 | a | 100 ± 0 | a |
| 0.5% | 0 ± 0 | a | 26.67 ± 11.55 | c | 55.56 ± 10.18 | a | 73.33 ± 6.67 | de | 88.89 ± 7.7 | a |
| 1% | 0 ± 0 | a | 24.44 ± 3.85 | c | 55.56 ± 15.4 | a | 75.56 ± 10.18 | cd | 93.33 ± 6.67 | a |
| 2% | 0 ± 0 | a | 31.11 ± 10.18 | bc | 53.33 ± 6.67 | a | 64.44 ± 3.85 | ef | 80 ± 6.67 | a |
| Treatment | CFU/g (a) | CFU/g (b) |
|---|---|---|
| Water | 0 | 0 |
| T. virens | 3.24 × 106 ± 0.24 | 5.24 × 106 ± 0.38 |
| T. asperellum | 3.74 × 106 ± 0.21 | 6.64 × 106 ± 0.17 |
| T. atroviride | 4.21 × 106 ± 0.34 | 8.01 × 106 ± 0.18 |
| Solargo™ | 0 | 0 |
| T. virens + lignin | 4.88 × 106 ± 0.52 | 3.74 × 106 ± 0.44 |
| T. asperellum + lignin | 3.62 × 106 ± 0.31 | 8.58 × 106 ± 0.31 |
| T. atroviride + lignin | 4.26 × 106 ± 0.26 | 9.63 × 106 ± 0.18 |
| T. asperellum + T. atroviride + lignin | 5.02 × 106 ± 0.17 | 1.03 × 107 ± 0.03 |
| T. asperellum + T. virens + lignin | 6.04 × 106 ± 0.24 | 1.12 × 107 ± 0.15 |
| T. atroviride + T. virens + lignin | 3.42 × 106 ± 0.34 | 7.24 × 106 ± 0.63 |
| T. asperellum + T. atroviride | 4.73 × 106 ± 0.23 | 7.42 × 106 ± 0.33 |
| T. asperellum + T. virens | 2.1 × 106 ± 0.27 | 1.69 × 107 ± 0.28 |
| T. atroviride + T. virens | 4.8 × 106 ± 0.63 | 8 × 106 ± 0.17 |
| T. asperellum + T. atroviride + T. virens | 4.24 × 106 ± 0.19 | 1.31 × 107 ± 0.18 |
| T. virens + T. asperellum + T. atroviride + lignin | 4.32 × 106 ± 0.31 | 7.68 × 106 ± 0.27 |
| Code | SI | pH | EC | TSS | DM (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.28 ± 0.0761 | abc | 4.04 ± 0.0306 | ab | 4.51 ± 0.2442 | abc | 6.40 ± 0.4583 | a | 5.934 ± 0.0062 | a |
| 2 | 1.3 ± 0.0778 | ab | 4.07 ± 0.0702 | ab | 4.50 ± 0.1914 | abc | 6.40 ± 0.7 | a | 6.167 ± 0.0061 | a |
| 3 | 1.27 ± 0.0903 | abc | 3.99 ± 0.0361 | ab | 4.55 ± 0.1026 | abc | 6.26 ± 0.6506 | a | 6.091 ± 0.011 | a |
| 4 | 1.25 ± 0.0689 | bc | 4.04 ± 0.0608 | ab | 4.39 ± 0.581 | bc | 6.40 ± 0.9165 | a | 6.070 ± 0.0132 | a |
| 5 | 1.23 ± 0.0738 | c | 4.07 ± 0.0757 | ab | 4.45 ± 0.4341 | bc | 6.57 ± 1.0017 | a | 5.975 ± 0.0119 | a |
| 6 | 1.32 ± 0.0813 | ab | 4.11 ± 0.1266 | ab | 4.39 ± 0.2003 | bc | 6.20 ± 0.2646 | a | 5.779 ± 0.0028 | a |
| 7 | 1.3 ± 0.057 | abc | 4.09 ± 0.1 | ab | 4.55 ± 0.1908 | abc | 6.23 ± 0.7767 | a | 5.720 ± 0.0109 | a |
| 8 | 1.29 ± 0.0761 | abc | 4.07 ± 0.0961 | ab | 4.65 ± 0.3315 | ab | 6.17 ± 0.4041 | a | 5.699± 0.0073 | a |
| 9 | 1.32 ± 0.0761 | a | 4.28 ± 0.4508 | a | 4.42 ± 0.1823 | bc | 6.60 ± 0.4583 | a | 5.674 ± 0.0047 | a |
| 10 | 1.27 ± 0.1038 | abc | 4.35 ± 0.3889 | a | 5.01 ± 0.193 | a | 6.70 ± 0.6557 | a | 5.602 ± 0.0061 | a |
| 11 | 1.27 ± 0.0974 | abc | 4.03 ± 0.02 | ab | 4.70 ± 0.3672 | ab | 6.53 ± 0.3055 | a | 5.583 ± 0.0023 | a |
| 12 | 1.31 ± 0.1112 | ab | 3.87 ± 0.2804 | b | 4.35 ± 0.2227 | bc | 6.37 ± 0.7371 | a | 5.571 ± 0.003 | a |
| 13 | 1.29 ± 0.0574 | abc | 4.06 ± 0.0208 | ab | 4.29 ± 0.1361 | bc | 6.53 ± 0.5508 | a | 5.510 ± 0.0074 | a |
| 14 | 1.3 ± 0.05 | ab | 4.08 ± 0.0493 | ab | 4.43 ± 0.1007 | bc | 6.50 ± 0.5568 | a | 5.476 ± 0.0025 | a |
| 15 | 1.31 ± 0.07 | ab | 3.82 ± 0.3032 | b | 4.29 ± 0.0945 | bc | 6.13 ± 0.2082 | a | 5.378 ± 0.0023 | a |
| 16 | 1.31 ± 0.0686 | ab | 4.05 ± 0.0252 | ab | 4.08 ± 0.3331 | c | 6.20 ± 0.2646 | a | 5.873 ± 0.0058 | a |
| TRT | Lycopene | TAAs | GABA | MEA | Orn | EAAs | BCAAs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.03 ± 0.18 | cde | 425.45 ± 96.23 | def | 33.54 ± 6.72 | cde | 1.22 ± 0.36 | cdef | 1.74 ± 0.58 | d | 44.37 ± 10.92 | bcd | 6.99 ± 1.84 | cdefg |
| 2 | 0.79 ± 0.16 | ef | 423.07 ± 68.85 | def | 22.54 ± 3.46 | efg | 1.12 ± 0.26 | def | 1.74 ± 0.35 | d | 44.11 ± 8.33 | bcd | 7.64 ± 1.44 | bcdefg |
| 3 | 0.72 ± 0.084 | f | 459.74 ± 124.89 | def | 34.05 ± 4.30 | cde | 1.42 ± 0.38 | cdef | 1.59 ± 0.39 | d | 47.05 ± 10.41 | bcd | 9.25 ± 2.00 | abcd |
| 4 | 0.72 ± 0.20 | f | 300.73 ± 77.62 | f | 18.44 ± 3.10 | fg | 0.77 ± 0.19 | f | 1.17 ± 0.33 | d | 33.58 ± 5.47 | cd | 5.28 ± 1.15 | fg |
| 5 | 0.941 ± 0.075 | def | 370.23 ± 86.22 | ef | 36.98 ± 9.56 | bcd | 1.39 ± 0.27 | cdef | 1.36 ± 0.32 | d | 37.44 ± 10.71 | bcd | 5.74 ± 1.36 | efg |
| 6 | 1.04 ± 0.24 | cde | 285.51 ± 11.82 | f | 12.58 ± 0.81 | g | 1.02 ± 0.13 | ef | 1.32 ± 0.16 | d | 33.58 ± 1.63 | d | 4.94 ± 0.25 | g |
| 7 | 1.15 ± 0.27 | cde | 366.40 ± 8.74 | def | 18.30 ± 2.14 | fg | 1.42 ± 0.28 | cdef | 1.98 ± 0.36 | d | 45.81 ± 2.31 | bcd | 6.24 ± 0.67 | defg |
| 8 | 1.21 ± 0.09 | bcd | 494.05 ± 125.82 | bc | 25.71±3.67 | def | 2.50±0.53 | ab | 1.93±0.31 | d | 64.85±14.13 | b | 8.22±2.11 | bcdef |
| 9 | 1.45 ± 0.17 | ab | 405.69 ± 89.26 | cde | 33.54 ± 6.25 | cde | 1.33 ± 0.36 | cdef | 1.33 ± 0.20 | d | 53.60 ± 9.52 | bcd | 6.50 ± 1.73 | defg |
| 10 | 1.52 ± 0.10 | a | 726.72 ± 115.23 | ab | 43.39 ± 10.35 | abc | 2.46 ± 0.66 | ab | 6.90 ± 1.67 | a | 80.21 ± 13.84 | a | 10.46 ± 1.52 | ab |
| 11 | 0.93 ± 0.16 | def | 455.88 ± 53.85 | def | 30.32 ± 3.60 | cdef | 1.67 ± 0.44 | cde | 4.93 ± 0.01 | b | 42.48 ± 5.37 | bcd | 8.51 ± 1.54 | bcdef |
| 12 | 1.04 ± 0.06 | cde | 509.53 ± 105.93 | def | 37.79 ± 8.24 | bcd | 2.59 ± 0.58 | ab | 4.20 ± 0.60 | bc | 47.06 ± 7.05 | b | 10.11 ± 2.16 | abc |
| 13 | 1.02 ± 0.05 | cde | 726.22 ± 124.58 | a | 52.86 ± 16.25 | a | 3.10 ± 0.76 | a | 1.38 ± 0.30 | d | 93.41 ± 12.50 | a | 11.96 ± 2.32 | a |
| 14 | 1.16 ± 0.09 | cd | 755.55 ± 156.36 | a | 48.83 ± 6.67 | ab | 2.44 ± 0.31 | ab | 3.49 ± 0.81 | c | 92.95 ± 8.68 | a | 12.11 ± 1.97 | a |
| 15 | 1.26 ± 0.15 | abc | 488.44 ± 88.67 | bc | 28.46 ± 3.70 | def | 1.84 ± 0.33 | bcd | 3.77 ± 0.35 | c | 65.67 ± 11.70 | bc | 8.05 ± 0.87 | bcdefg |
| 16 | 1.01 ± 0.07 | cdef | 512.18 ± 110.75 | cd | 32.40 ± 5.99 | cde | 1.97 ± 0.19 | bc | 1.96 ± 0.68 | d | 58.53 ± 10.45 | b | 8.64 ± 2.26 | bcde |
| TRT | Ala | Arg | Asn | Asp | Gln | Glu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.93 ± 1.87 | bcd | 17.24 ± 3.41 | efg | 14.89 ± 3.79 | e | 27.69 ± 7.70 | bc | 41.25 ± 14.09 | def | 235.41 ± 48.98 | bcd |
| 2 | 7.37 ± 0.90 | bcd | 14.90 ± 4.54 | fg | 17.65 ± 4.35 | e | 31.65 ± 9.98 | abc | 39.58 ± 12.10 | def | 239.56 ± 29.91 | bcd |
| 3 | 6.41 ± 1.23 | cd | 18.37 ± 4.00 | def | 24.14 ± 4.48 | de | 34.23 ± 10.87 | ab | 51.12 ± 14.10 | bcdef | 244.03 ± 80.58 | bcd |
| 4 | 4.52 ± 1.14 | d | 11.47 ± 2.95 | gh | 13.62 ± 2.58 | e | 22.64 ± 5.56 | bc | 32.26 ± 5.45 | ef | 157.17 ± 53.68 | d |
| 5 | 6.29 ± 1.71 | cd | 16.22 ± 4.45 | efg | 14.63 ± 5.06 | e | 26.12 ± 5.61 | bc | 31.84 ± 8.29 | ef | 199.62 ± 45.21 | cd |
| 6 | 5.46 ± 2.05 | cd | 14.46 ± 4.43 | fg | 15.05 ± 5.17 | e | 18.22 ± 8.75 | c | 29.15 ± 15.49 | f | 153.88 ± 58.26 | d |
| 7 | 8.00 ± 1.31 | bcd | 20.06 ± 0.20 | cdef | 14.66 ± 0.47 | e | 26.04 ± 3.84 | bc | 30.01 ± 2.71 | f | 203.53 ± 8.89 | cd |
| 8 | 8.82 ± 2.63 | bc | 30.40 ± 5.14 | b | 21.70 ± 5.60 | de | 35.51 ± 11.68 | ab | 52.55 ± 18.05 | bcdef | 257.83 ± 69.80 | bcd |
| 9 | 5.62 ± 1.45 | cd | 25.41 ± 6.22 | bcd | 15.02 ± 1.40 | e | 24.77 ± 5.58 | bc | 41.91 ± 11.03 | def | 211.72 ± 57.13 | cd |
| 10 | 10.99 ± 1.99 | ab | 37.96 ± 5.95 | a | 41.70 ± 5.36 | b | 46.88 ± 9.72 | a | 122.52 ± 25.25 | a | 338.11 ± 44.31 | ab |
| 11 | 8.23 ± 2.82 | bcd | 5.78 ± 0.62 | h | 30.46 ± 2.96 | cd | 29.60 ± 3.04 | bc | 68.35 ± 7.06 | bc | 215.15 ± 29.54 | cd |
| 12 | 6.16 ± 1.72 | cd | 5.42 ± 1.86 | h | 22.51 ± 3.37 | de | 31.56 ± 5.65 | abc | 61.57 ± 14.83 | bcd | 274.85 ± 72.78 | bc |
| 13 | 10.79 ± 3.83 | ab | 38.93 ± 7.57 | a | 28.14 ± 4.99 | d | 45.64 ± 7.19 | a | 70.41 ± 17.67 | bc | 389.70 ± 57.87 | a |
| 14 | 13.93 ± 4.00 | a | 30.64 ± 6.26 | b | 51.68 ± 14.66 | a | 46.27 ± 16.35 | a | 73.33 ± 9.99 | b | 395.57 ± 105.33 | a |
| 15 | 7.11 ± 1.17 | bcd | 27.59 ± 4.91 | bc | 39.14 ± 8.24 | bc | 26.13 ± 3.26 | bc | 47.38 ± 9.44 | cdef | 250.00 ± 55.27 | bcd |
| 16 | 8.68 ± 1.75 | bcd | 23.34 ± 2.67 | bcde | 23.15 ± 3.65 | de | 36.87 ± 7.18 | ab | 56.04 ± 16.21 | bcde | 271.71 ± 64.45 | bc |
| His | Ile | Leu | Lys | Met | Phe | |||||||
| 1 | 5.09 ± 1.25 | d | 2.06 ± 0.44 | def | 3.73 ± 1.29 | cde | 2.09 ± 0.66 | de | 0.54 ± 0.15 | bcde | 8.51 ± 2.65 | cde |
| 2 | 6.96 ± 1.94 | cd | 2.58 ± 0.59 | abcde | 3.47 ± 0.95 | cde | 1.86 ± 0.42 | de | 0.53 ± 0.11 | bcde | 8.30 ± 1.50 | cde |
| 3 | 5.55 ± 1.55 | d | 3.12 ± 0.92 | abc | 4.35 ± 0.94 | bcde | 1.85 ± 0.62 | de | 0.79 ± 0.10 | ab | 7.73 ± 1.92 | de |
| 4 | 4.99 ± 0.59 | d | 1.75 ± 0.29 | ef | 2.61 ± 0.83 | e | 2.80 ± 0.06 | cde | 0.39 ± 0.07 | e | 6.52 ± 1.25 | e |
| 5 | 4.33 ± 1.30 | d | 1.74 ± 0.38 | ef | 3.00 ± 0.82 | de | 1.71 ± 0.86 | de | 0.44 ± 0.12 | de | 6.48 ± 1.92 | e |
| 6 | 4.89 ± 1.64 | d | 1.41 ± 0.49 | f | 2.66 ± 1.33 | e | 1.51 ± 0.72 | e | 0.37 ± 0.19 | e | 5.23 ± 3.40 | e |
| 7 | 7.63 ± 1.68 | bcd | 1.71 ± 0.13 | ef | 3.29 ± 0.29 | de | 1.82 ± 0.14 | de | 0.48 ± 0.05 | cde | 6.73 ± 0.86 | e |
| 8 | 9.34 ± 1.68 | abc | 2.43 ± 0.67 | bcde | 4.24 ± 0.98 | bcde | 2.26 ± 0.49 | de | 0.56 ± 0.11 | bcde | 9.62 ± 3.33 | bcde |
| 9 | 9.88 ± 2.40 | abc | 1.82 ± 0.36 | ef | 3.58 ± 1.18 | cde | 2.05 ± 0.62 | de | 0.55 ± 0.18 | bcde | 6.58 ± 1.11 | e |
| 10 | 10.76 ± 2.59 | ab | 2.83 ± 0.40 | abcd | 5.63 ± 0.79 | ab | 3.66 ± 0.39 | c | 0.72 ± 0.12 | abc | 11.94 ± 2.77 | abcd |
| 11 | 5.75 ± 0.87 | d | 2.27 ± 0.57 | bcdef | 4.59 ± 0.59 | bcd | 3.03 ± 0.24 | cd | 0.56 ± 0.10 | bcde | 7.95 ± 0.59 | cde |
| 12 | 7.59 ± 1.42 | bcd | 2.99 ± 0.72 | abcd | 5.31 ± 1.17 | abc | 3.93 ± 0.84 | bc | 0.76 ± 0.27 | ab | 8.43 ± 2.14 | cde |
| 13 | 12.54 ± 2.28 | a | 3.21 ± 0.58 | ab | 6.56 ± 1.17 | a | 5.00 ± 1.08 | ab | 0.92 ± 0.21 | a | 14.33 ± 4.31 | a |
| 14 | 11.45 ± 3.52 | a | 3.46 ± 0.58 | a | 6.58 ± 1.03 | a | 5.26 ± 1.64 | a | 0.90 ± 0.15 | a | 13.59 ± 3.34 | ab |
| 15 | 6.48 ± 1.92 | cd | 2.18 ± 0.25 | cdef | 4.35 ± 0.42 | bcde | 3.03 ± 0.23 | cd | 0.55 ± 0.08 | bcde | 7.26 ± 1.16 | e |
| 16 | 7.39 ± 1.37 | bcd | 2.44 ± 0.46 | bcde | 4.66 ± 1.58 | bcd | 2.35 ± 0.79 | de | 0.68 ± 0.23 | abcd | 12.20 ± 3.06 | abc |
| Thr | Trp | Tyr | Val | Gly | ||||||||
| 1 | 2.70 ± 0.87 | c | 1.22 ± 0.38 | bcd | 2.31 ± 0.69 | cdef | 1.20 ± 0.27 | cdef | 3.33 ± 0.85 | ef | ||
| 2 | 2.55 ± 0.76 | c | 1.36 ± 0.17 | abc | 2.52 ± 0.67 | bcdef | 1.59 ± 0.23 | abcd | 2.03 ± 0.70 | fg | ||
| 3 | 2.11 ± 0.46 | c | 1.41 ± 0.26 | abc | 3.05 ± 0.62 | bcde | 1.77 ± 0.44 | abc | 0.73 ± 0.13 | g | ||
| 4 | 1.62 ± 0.50 | c | 0.52 ± 0.09 | e | 1.82 ± 0.22 | ef | 0.93 ± 0.14 | ef | 2.35 ± 0.14 | efg | ||
| 5 | 1.94 ± 0.59 | c | 0.59 ± 0.13 | e | 1.78 ± 0.44 | ef | 1.00 ± 0.23 | def | 2.83 ± 0.59 | efg | ||
| 6 | 1.62 ± 0.72 | c | 0.57 ± 0.42 | e | 1.66 ± 0.80 | f | 0.88 ± 0.33 | f | 4.10 ± 1.58 | def | ||
| 7 | 1.94 ± 0.23 | c | 0.90 ± 0.28 | cde | 1.94 ± 0.23 | def | 1.24 ± 0.25 | cdef | 4.58 ± 0.43 | de | ||
| 8 | 3.07 ± 1.10 | c | 1.38 ± 0.26 | abc | 3.57 ± 0.86 | bc | 1.55 ± 0.47 | abcde | 8.75 ± 2.21 | b | ||
| 9 | 1.93 ± 0.45 | c | 0.70 ± 0.22 | de | 2.23 ± 0.74 | cdef | 1.11 ± 0.22 | def | 2.52 ± 0.71 | efg | ||
| 10 | 3.14 ± 0.55 | c | 1.58 ± 0.50 | ab | 6.31 ± 1.55 | a | 2.00 ± 0.38 | ab | 15.20 ± 3.03 | a | ||
| 11 | 9.82 ± 1.43 | b | 1.08 ± 0.28 | bcde | 3.33 ± 0.19 | bc | 1.64 ± 0.43 | abcd | 10.56 ± 0.73 | b | ||
| 12 | 9.30 ± 2.40 | b | 1.52 ± 0.37 | abc | 2.90 ± 0.68 | bcdef | 1.81 ± 0.30 | abc | 8.39 ± 1.04 | bc | ||
| 13 | 7.79 ± 6.21 | b | 1.94 ± 0.56 | a | 3.68 ± 1.11 | b | 2.18 ± 0.62 | a | 10.34 ± 2.15 | b | ||
| 14 | 17.06 ± 3.92 | a | 1.94 ± 0.39 | a | 3.49 ± 0.48 | bc | 2.08 ± 0.46 | ab | 8.46 ± 1.57 | bc | ||
| 15 | 11.40 ± 2.53 | b | 1.31 ± 0.34 | bc | 1.95 ± 0.29 | def | 1.52 ± 0.20 | bcdef | 6.33 ± 1.44 | cd | ||
| 16 | 2.71 ± 0.52 | c | 1.22 ± 0.36 | bcd | 3.19 ± 0.68 | bcd | 1.54 ± 0.23 | abcde | 6.24 ± 0.48 | cd | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzuise, S.; Manganiello, G.; Vincenzo, C.; Carillo, P.; Macchia, V.; Pietarinen, S.; Fusco, G.M.; Nicastro, R.; Lorito, M.; Woo, S.L. Multispecies Trichoderma in Combination with Hydrolyzed Lignin Improve Tomato Growth, Yield, and Nutritional Quality of Fruits. Agronomy 2024, 14, 2449. https://doi.org/10.3390/agronomy14102449
Lanzuise S, Manganiello G, Vincenzo C, Carillo P, Macchia V, Pietarinen S, Fusco GM, Nicastro R, Lorito M, Woo SL. Multispecies Trichoderma in Combination with Hydrolyzed Lignin Improve Tomato Growth, Yield, and Nutritional Quality of Fruits. Agronomy. 2024; 14(10):2449. https://doi.org/10.3390/agronomy14102449
Chicago/Turabian StyleLanzuise, Stefania, Gelsomina Manganiello, Cono Vincenzo, Petronia Carillo, Vito Macchia, Suvi Pietarinen, Giovanna Marta Fusco, Rosalinda Nicastro, Matteo Lorito, and Sheridan Lois Woo. 2024. "Multispecies Trichoderma in Combination with Hydrolyzed Lignin Improve Tomato Growth, Yield, and Nutritional Quality of Fruits" Agronomy 14, no. 10: 2449. https://doi.org/10.3390/agronomy14102449
APA StyleLanzuise, S., Manganiello, G., Vincenzo, C., Carillo, P., Macchia, V., Pietarinen, S., Fusco, G. M., Nicastro, R., Lorito, M., & Woo, S. L. (2024). Multispecies Trichoderma in Combination with Hydrolyzed Lignin Improve Tomato Growth, Yield, and Nutritional Quality of Fruits. Agronomy, 14(10), 2449. https://doi.org/10.3390/agronomy14102449









