A New Approach to Differentiate the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Variety
Abstract
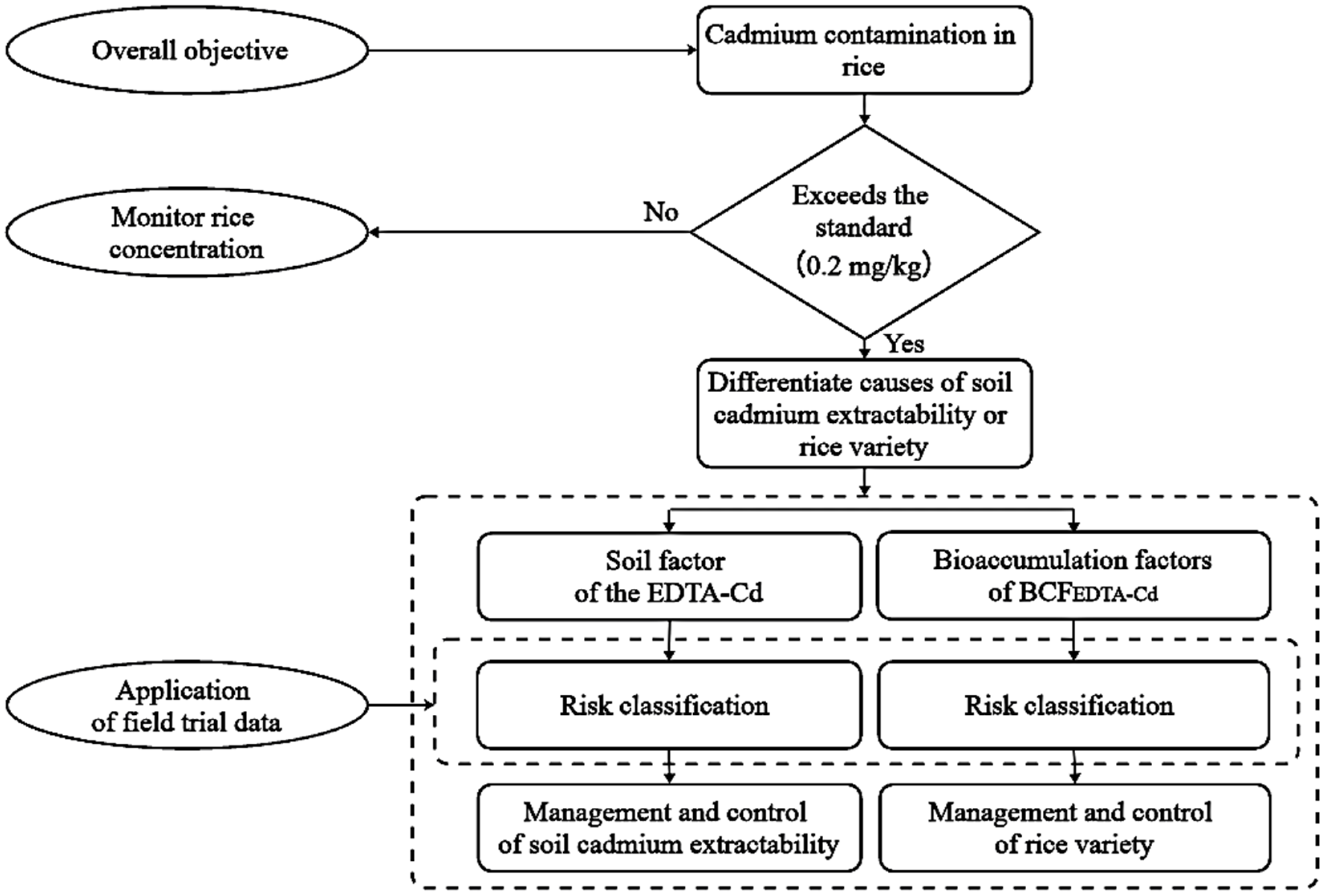
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analyses
2.2.1. Soil Factors
2.2.2. Bioaccumulation Factors
2.2.3. Transfer Factors
2.2.4. Normalization of the BCF Data
2.2.5. SSD Curve Construction
2.2.6. The Conversion of EDTA-Cd and DTPA-Cd
2.2.7. Analysis of Paddy Field Data
2.2.8. Determining the Respective Contributions of the Causes of Rice Cd Contamination
2.2.9. Statistics
3. Results
3.1. Soil Factor of the EDTA-Cd
3.1.1. Risk Classification of Soil-Cd
3.1.2. Risk Classification of EDTA-Cd
3.1.3. Risk Classification of EDTA-Cd (%)
3.2. Bioaccumulation Factor of Rice Grains
Classification of Bioaccumulation Factors of Rice Based on EDTA-Cd
3.3. Comparison of Soil Factors and Extractable Bioaccumulation Factors at Measured Rice Concentrations
3.4. Applying Model by Using Experimental Field Data
| Scenario | a Cdgrain (mg/kg) | pH | b EDTA-Cd (mg/kg) | c BCFEDTA-Cd | d Measures (Primacy) | Reference |
|---|---|---|---|---|---|---|
| 1 | 0.04 Ultra-low | 5.26 | 0.26 Low | Ultra-low | [55] | |
| 2 | 0.30 Moderate | 5.60 | 0.77 Moderate | Moderate | A = B | [56] |
| 3 | 0.38 Moderate | 8.17 | 1.48 Moderate | High | A < B | [57] |
| 4 | 0.40 High | 5.00 | 0.86 Ultra-high | Moderate | A > B | [58] |
| 5 | 0.81 Ultra-high | 6.57 | 0.16 Ultra-low | Ultra-high | [59] |
4. Discussion
4.1. Risk Assessment of EDTA-Cd in Soil
4.2. Risk Assessment of BCFEDTA-Cd
4.3. Differentiating the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, Q.Y.; Liu, Z.; Shao, W.Y.; Tian, J.; Luo, H.P.; Ni, F.; Shan, Y.X. Probabilistic risk assessment of heavy metals in urban farmland soils of a typical oasis city in northwest China. Sci. Total Environ. 2022, 833, 155096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, D.M.; Zhong, T.Y.; Zhang, X.M.; Cheng, M.; Li, X.H. Assessment of cadmium (Cd) concentration in arable soil in China. Environ. Sci. Pollut. Res. 2015, 22, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Zhang, Q.C.; Yan, C.A.; Tang, G.Y.; Zhang, M.Y.; Ma, L.Q.; Gu, R.H.; Xiang, P. Heavy metal (loid) s in agriculture soils, rice, and wheat across China: Status assessment and spatiotemporal analysis. Sci. Total Environ. 2023, 882, 163361. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of China, and State Administration for Market Regulation of China: Beijing, China, 2018. (In Chinese)
- Teng, Y.G.; Wu, J.; Lu, S.J.; Wang, Y.Y.; Jiao, X.D.; Song, L.T. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef]
- Duan, M.M.; Wang, S.; Huang, D.Y.; Zhu, Q.H.; Liu, S.L.; Zhang, Q.; Zhu, H.H.; Xu, C. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef]
- Mu, Y.; Cui, J.X.; Liu, A.D.; Wang, S.; Shi, Q.J.; Wang, J.; Wei, S.Q.; Zhang, J.Z. Interactions and quantification of multiple influencing factors on cadmium accumulation in soil-rice systems at a large region. Sci. Total Environ. 2023, 881, 163392. [Google Scholar] [CrossRef]
- Huang, L.K.; Wang, Q.; Zhou, Q.Y.; Ma, L.Y.; Wu, Y.J.; Liu, Q.Z.; Wang, S.; Feng, Y. Cadmium uptake from soil and transport by leafy vegetables: A meta-analysis. Environ. Pollut. 2020, 264, 114677. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Wu, W.G.; Han, F.X.; Li, J.X.; Ye, W.L.; Fu, H.H.; Yan, Y.H.; Ma, Y.H.; Wang, Q. Agronomic management and rice varieties controlling Cd bioaccumulation in rice. Int. J. Environ. Res. Public Health 2019, 16, 2376. [Google Scholar] [CrossRef]
- NaziaTahir; Ullah, A.; Tahir, A.; Rashid, H.U.; Rehman, T.U.; Danish, S.; Hussain, B.; Akca, H. Strategies for reducing Cd concentration in paddy soil for rice safety. J. Clean. Prod. 2021, 316, 128116. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Mao, J.; Tan, J.; Zhong, K.; Chen, J.X.; Huang, D.; Gu, X.Y.; Zhang, C.L. Heavy metal contamination, accumulation, and risk assessment in a paddy field near Pb-Zn mine, in Guangxi Province, China. J. Soils Sediments 2023, 23, 1345–1355. [Google Scholar] [CrossRef]
- Li, J.H.; Nie, D.T.; Liu, M.N.; Mao, X.Y.; Liao, Z.W.; Chen, X. Comparison of Cd bioavailability determination methods and the risk control value of Cd for typical Cd-contaminated paddy soils in Guangdong. J. Agric. Resour. Environ. 2021, 38, 1094–1101. (In Chinese) [Google Scholar]
- Li, F.S.; Han, M.; Xiong, D.Q.; Lu, G.L.; Liu, F. Efficiency of some extractants for available heavy metals from several typical soils. J. Agro-Environ. Sci. 2003, 22, 704–706. (In Chinese) [Google Scholar]
- Yi, L.; Zhang, Z.Q.; Shen, F.; Liu, H. Impact of different extraction conditions and different extracts on heavy metals from several typical soils. J. Northwest Agric. Sci. 2012, 21, 156–160. (In Chinese) [Google Scholar]
- Qian, E.; Zhao, Y.J.; Liu, X.W.; Li, Z.T.; Zhang, C.C.; Sun, Y.; Zhou, Q.W.; Liang, X.F.; Wang, H.H. Screening and evaluation of soil cadmium extraction methods for predicting cadmium accumulation in rice. J. Agro-Environ. Sci. 2020, 39, 1000–1009. (In Chinese) [Google Scholar]
- Wang, Y.F.; Su, Y.; Lu, S. Predicting accumulation of Cd in rice (Oryza sativa L.) and soil threshold concentration of Cd for rice safe production. Sci. Total Environ. 2020, 738, 139805. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Kong, F.Y.; Lu, S.G. Prediction model for Cd accumulation of rice (Oryza sativa L.) based on extractable Cd in soils and prediction for high Cd-risk regions of southern Zhejiang Province, China. Environ. Sci. Pollut. Res. 2023, 30, 15964–15974. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Soubasakou, G.; Cavoura, O.; Damikouka, I. Phytoremediation of cadmium-contaminated soils: A review of new cadmium hyperaccumulators and factors affecting their efficiency. Bull. Environ. Contam. Toxicol. 2022, 109, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.L.; Guo, Z.H.; Peng, C.; Xiao, X.Y.; He, Y.L. Factors influencing the effectiveness of liming on cadmium reduction in rice: A meta-analysis and decision tree analysis. Sci. Total Environ. 2021, 779, 146477. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Wang, L.L.; Wang, Y.H.; Li, H.S.; Chen, G.K. Soil properties and cultivars determine heavy metal accumulation in rice grain and cultivars respond differently to Cd stress. Environ. Sci. Pollut. Res. 2019, 26, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Fan, G.P.; Liu, C.; Zhou, D.M. Predicting soil available cadmium by machine learning based on soil properties. J. Hazard. Mater. 2023, 460, 132327. [Google Scholar] [CrossRef]
- Li, K.; Cao, C.L.; Ma, Y.B.; Su, D.C.; Li, J.M. Identification of cadmium bioaccumulation in rice (Oryza sativa L.) by the soil-plant transfer model and species sensitivity distribution. Sci. Total Environ. 2019, 692, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, X.; Zhou, Y.B.; Zhou, Q.F.; Wang, K.; Yang, Y.Z. Factors affecting cadmium accumulation in rice and strategies for minimization. Chin. J. Rice Sci. 2021, 35, 342. (In Chinese) [Google Scholar]
- Du, Z.L.; Lin, D.S.; Li, H.F.; Li, Y.; Chen, H.A.; Dou, W.Q.; Qin, L.; An, Y. Bibliometric analysis of the influencing factors, derivation, and application of heavy metal thresholds in soil. Int. J. Environ. Res. Public Health 2022, 19, 6561. [Google Scholar] [CrossRef]
- Gao, J.T.; Ye, X.X.; Wang, X.Y.; Jiang, Y.J.; Li, D.C.; Ma, Y.B.; Sun, B. Derivation and validation of thresholds of cadmium, chromium, lead, mercury and arsenic for safe rice production in paddy soil. Ecotoxicol. Environ. Saf. 2021, 220, 112404. [Google Scholar] [CrossRef]
- Mu, D.M.; Zheng, S.N.; Lin, D.S.; Xu, Y.M.; Dong, R.Y.; Pei, P.G.; Sun, Y.B. Derivation and validation of soil cadmium thresholds for the safe farmland production of vegetables in high geological background area. Sci. Total Environ. 2023, 873, 162171. [Google Scholar] [CrossRef]
- Li, X.Z.; Du, J.Y.; Sun, L.; Zhang, Y.; Feng, Y.H.; Zheng, L.P.; Wang, G.Q.; Huang, X.H. Derivation of soil criteria of cadmium for safe rice production applying soil–plant transfer model and species sensitivity distribution. Int. J. Environ. Res. Public Health 2022, 19, 8854. [Google Scholar] [CrossRef]
- Zou, M.M.; Zhou, S.L.; Zhou, Y.J.; Jia, Z.Y.; Guo, T.W.; Wang, J.X. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.T.; Zhou, T.; Li, Z.; Hu, P.J.; Luo, Y.M.; Christie, P.; Wu, L.H. Prediction models for rice cadmium accumulation in Chinese paddy fields and the implications in deducing soil thresholds based on food safety standards. Environ. Pollut. 2020, 258, 113879. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Q.; Lu, X.Z.; Sun, R.; Huang, C.L.; Kang, Z.J.; Xu, M.X.; Wei, Y.C.; Cai, Z.H. Spatio-temporal variation prediction on Cd content in the rice grains from Northern Zhejiang Plain during 2014–2019 based on high-precision soil geochemical data. J. Geogr. Sci. 2023, 33, 413–426. [Google Scholar] [CrossRef]
- NY 147-1988; Good Quality and Edible Rice Grains. Ministry of Agriculture, the People’s Republic of China: Beijing, China, 1988. (In Chinese)
- GB 5009.15-2014; National Food Safety Standard-Determination of Cadmium in Food. National Health and Family Planning Commission: Beijing, China, 2015. (In Chinese)
- Luo, Q.H.; Bai, B.; Xie, Y.H.; Yao, D.P.; Zhang, D.M.; Chen, Z.; Zhuang, W.; Deng, Q.Y.; Xiao, Y.H.; Wu, J. Effects of Cd uptake, translocation and redistribution in different hybrid rice varieties on grain Cd concentration. Ecotoxicol. Environ. Saf. 2022, 240, 113683. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Li, K.; Jiang, B.; Li, J.M.; Ma, Y.B. Derivation and validation of soil total and extractable cadmium criteria for safe vegetable production. J. Integr. Agric. 2023, 22, 3792–3803. [Google Scholar] [CrossRef]
- Ye, X.X.; Li, H.Y.; Ma, Y.B.; Wu, L.; Sun, B. The bioaccumulation of Cd in rice grains in paddy soils as affected and predicted by soil properties. J. Soils Sediments 2014, 14, 1407–1416. [Google Scholar] [CrossRef]
- Fox, D.R.; van Dam, R.A.; Fisher, R.; Batley, G.E.; Tillmanns, A.R.; Thorley, J.; Schwarz, C.J.; Spry, D.J.; McTavish, K. Recent developments in species sensitivity distribution modeling. Environ. Toxicol. Chem. 2021, 40, 293–308. [Google Scholar] [CrossRef]
- Xu, F.L.; Li, Y.L.; Wang, Y.; He, W.; Kong, X.Z.; Qin, N.; Liu, W.X.; Wu, W.J.; Jorgensen, S.E. Key issues for the development and application of the species sensitivity distribution (SSD) model for ecological risk assessment. Ecol. Indic. 2015, 54, 227–237. [Google Scholar] [CrossRef]
- Kwak, S.K.; Kim, J.H. Statistical data preparation: Management of missing values and outliers. Korean J. Anesthesiol. 2017, 70, 407–411. [Google Scholar] [CrossRef]
- Zhang, L.X.; Ren, Z.H.; Chen, B.; Gong, P.; Xu, B.; Fu, H.H. A prolonged artificial nighttime-light dataset of China (1984–2020). Sci. Data 2024, 11, 414. [Google Scholar] [CrossRef]
- Liu, Z.W. Risk Control and Management Index System of Cadmium Contaminated Paddy Field. Master’s Thesis, Macau University of Science and Technology, Macau, China, 2022. (In Chinese). [Google Scholar]
- Li, L.J.; Jiang, B.; Wan, Y.N.; Li, J.M.; Ma, Y.B. Integrating bioavailability and aging in the criteria derivation of cadmium for the safe production of rice in paddy soils. Ecotoxicol. Environ. Saf. 2021, 219, 112356. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.Q.; Qin, Q.; Lu, W.G.; Xue, Y.; Sun, L.J.; Song, K. Effects of long-term application of organic manure on contents of total and available cadmium in greenhouse soil. Acta Pedol. Sin. 2021, 58, 1486–1495. [Google Scholar]
- Chen, H.P.; Zhang, W.W.; Yang, X.P.; Wang, P.; McGrath, S.P.; Zhao, F.J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef]
- Hu, Y.A.; Cheng, H.F.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, F.Y.; Lu, S.G. Remediation effect and mechanism of inorganic passivators on cadmium contaminated acidic paddy soil. Chin. J. Environ. Sci. 2022, 43, 4679–4686. (In Chinese) [Google Scholar]
- Mubeen, S.; Ni, W.J.; He, C.T.; Yang, Z.Y. Agricultural strategies to reduce cadmium accumulation in crops for food safety. Agriculture 2023, 13, 471. [Google Scholar] [CrossRef]
- Xue, T.; Liao, X.Y.; Li, H.Y.; Xie, Y.H.; Wei, W.; Chen, J.; Liu, Z.B.; Ji, X.H. Remediation of Cd contaminated paddy fields by intercropping of the high-and low-Cd-accumulating rice cultivars. Sci. Total Environ. 2023, 878, 163133. [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, X.; Yu, L.; Wang, T.Q.; Wang, J.N.; Liu, T.T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Li, C. Effects of Soil Conditioners on Remediation of Heavy Metal Cadmium Pollution Meta-Analysis and Empirical Research. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2022. (In Chinese). [Google Scholar]
- Wan, Y.N.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H.F. Heavy metals in agricultural soils: Sources, influencing factors, and remediation strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Liao, P.; Xiong, Q.Q.; Deng, X.Y.; Gao, H.; Wei, H.Y.; Dai, Q.G.; Pan, X.H.; Zeng, Y.J.; et al. Effects of biochar on the dynamic immobilization of Cd and Cu and rice accumulation in soils with different acidity levels. J. Clean. Prod. 2022, 372, 133730. [Google Scholar] [CrossRef]
- Sheng, H.; Gu, Y.; Yin, Z.R.; Xue, Y.; Zhou, P.; Thompson, M.L. Consistent inter–annual reduction of rice cadmium in 5-year biannual organic amendment. Sci. Total Environ. 2022, 807, 151026. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, M. Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol. Environ. Saf. 2010, 73, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L.; Xu, Y.X.; Chen, D.; Xiao, W.D.; Ye, X.Z. Effects of different passivators on Cd absorption in rice. J. Zhejiang Agric. Sci. 2023, 64, 282–285. (In Chinese) [Google Scholar]
- Zhang, Y.S.; Jiang, C.C.; Xiao, H.; Wu, Y.J.; Yang, X.F.; Wang, Z.Q.; Zheng, H.P.; Ao, H.J. Preliminary study on the effects of soil modifier on available Cd in soil and cadmium content in brown rice. China Rice 2019, 25, 53–57. [Google Scholar]
- Zhuang, S.K.; Lu, X.W. Environmental risk evaluation and source identification of heavy metal(loid)s in agricultural soil of Shangdan Valley, Northwest China. Sustainability 2020, 12, 5806. [Google Scholar] [CrossRef]
- Fei, X.F.; Lou, Z.H.; Xiao, R.; Ren, Z.Q.; Lv, X.N. Source analysis and source-oriented risk assessment of heavy metal pollution in agricultural soils of different cultivated land qualities. J. Clean. Prod. 2022, 341, 130942. [Google Scholar] [CrossRef]
- Wan, M.X.; Hu, W.Y.; Wang, H.F.; Tian, K.; Huang, B. Comprehensive assessment of heavy metal risk in soil-crop systems along the Yangtze River in Nanjing, Southeast China. Sci. Total Environ. 2021, 780, 146567. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhang, Y.; Yang, J.S.; Wang, H.Y.; Li, Y.L.; Shi, Y.; Li, D.C.; Holm, P.E.; Ou, Q.; Hu, W.Y. Quantitative source apportionment, risk assessment and distribution of heavy metals in agricultural soils from southern Shandong Peninsula of China. Sci. Total Environ. 2021, 767, 144879. [Google Scholar] [CrossRef]
- Qin, L.Y.; Yu, L.; Wang, M.; Sun, X.Y.; Wang, J.; Liu, J.X.; Wang, Y.; White, J.C.; Chen, S.B. The environmental risk threshold (HC5) for Cd remediation in Chinese agricultural soils. J. Environ. Manag. 2024, 362, 121316. [Google Scholar] [CrossRef]


| Sample Size | EDTA-Cd (mg/kg) | |||
|---|---|---|---|---|
| Mean | Standard Error | Maximum | Minimum | |
| 36 | 0.4193 | 0.0271 | 0.7251 | 0.2286 |
| Variables | Minimum | Maximum | Mean | Median | SD | |
|---|---|---|---|---|---|---|
| Soil–rice | Soil pH | 4.52 | 7.86 | 5.56 | 5.44 | 0.51 |
| (n = 1003) | Cdsoil (mg/kg) | 0.105 | 1.195 | 0.368 | 0.333 | 0.163 |
| CdEDTA (mg/kg) | 0.063 | 0.998 | 0.278 | 0.255 | 0.135 | |
| Cdgrain (mg/kg) | 0.011 | 2.096 | 0.429 | 0.379 | 0.257 | |
| BCFEDTA-Cd | 0.057 | 3.694 | 1.660 | 1.569 | 0.811 | |
| Risk | Ultra-Low | Low | Moderate | High | Ultra-High |
|---|---|---|---|---|---|
| Rice Cd content (mg/kg) | <0.2 | 0.2–0.3 | 0.3–0.4 | 0.4–0.5 | >0.5 |
| Grade | Risk | Soil-Cd Content (mg/kg) | ||||
|---|---|---|---|---|---|---|
| pH ≤ 4.5 | 4.5 < pH ≤ 5.5 | 5.5 < pH ≤ 6.5 | 6.5 < pH ≤ 7.5 | pH > 7.5 | ||
| 1 | Ultra-low | <0.19 | <0.31 | <0.49 | <0.79 | <1.26 |
| 2 | Low | 0.19–0.38 | 0.31–0.61 | 0.49–0.99 | 0.79–1.58 | 1.26–2.53 |
| 3 | Moderate | 0.38–0.58 | 0.61–0.92 | 0.99–1.48 | 1.58–2.38 | 2.53–3.81 |
| 4 | High | 0.58–0.77 | 0.92–1.23 | 1.48–1.97 | 2.38–3.17 | 3.81–5.07 |
| 5 | Ultra-high | >0.77 | >1.23 | >1.97 | >3.17 | >5.07 |
| Grade | Risk | EDTA-Cd Content (mg/kg) | ||||
|---|---|---|---|---|---|---|
| pH ≤ 4.5 | 4.5 < pH ≤ 5.5 | 5.5 < pH ≤ 6.5 | 6.5 < pH ≤ 7.5 | pH > 7.5 | ||
| 1 | Ultra-low | <0.11 | <0.18 | <0.29 | <0.46 | <0.73 |
| 2 | Low | 0.11–0.22 | 0.18–0.36 | 0.29–0.57 | 0.46–0.92 | 0.73–1.47 |
| 3 | Moderate | 0.22–0.33 | 0.36–0.53 | 0.57–0.86 | 0.92–1.37 | 1.47–2.20 |
| 4 | High | 0.33–0.44 | 0.53–0.71 | 0.86–1.14 | 1.37–1.83 | 2.20–2.93 |
| 5 | Ultra-high | >0.44 | >0.71 | >1.14 | >1.83 | >2.93 |
| EDTA-Cd (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Min (%) | Max (%) | Mean (%) | Median (%) | Percentile (%) Percentage (%) | |||
| 1003 | 32 | 98 | 74 | 76 | 5 | 25 | 75 | 95 |
| 51 | 67 | 83 | 93 | |||||
| Grade | Risk | pH ≤ 5.5 | 5.5 < pH ≤ 6.5 | 6.5 < pH ≤ 7.5 | pH > 7.5 |
|---|---|---|---|---|---|
| 1 | Ultra-low | <0.24 | <0.17 | <0.12 | <0.09 |
| 2 | Low | 0.24–0.38 | 0.17–0.28 | 0.12–0.21 | 0.09–0.14 |
| 3 | Moderate | 0.38–0.57 | 0.28–0.42 | 0.21–0.29 | 0.14–0.21 |
| 4 | High | 0.57–1.40 | 0.42–1.00 | 0.29–0.73 | 0.21–0.52 |
| 5 | Ultra-high | >1.40 | >1.00 | >0.73 | >0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Li, K.; Li, J.; Wu, Y.; Ma, Y. A New Approach to Differentiate the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Variety. Agronomy 2024, 14, 2519. https://doi.org/10.3390/agronomy14112519
Li E, Li K, Li J, Wu Y, Ma Y. A New Approach to Differentiate the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Variety. Agronomy. 2024; 14(11):2519. https://doi.org/10.3390/agronomy14112519
Chicago/Turabian StyleLi, Erdange, Kun Li, Jumei Li, Yang Wu, and Yibing Ma. 2024. "A New Approach to Differentiate the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Variety" Agronomy 14, no. 11: 2519. https://doi.org/10.3390/agronomy14112519
APA StyleLi, E., Li, K., Li, J., Wu, Y., & Ma, Y. (2024). A New Approach to Differentiate the Causes of Excessive Cadmium in Rice: Soil Cadmium Extractability or Rice Variety. Agronomy, 14(11), 2519. https://doi.org/10.3390/agronomy14112519






