Prokaryotic Expression of Coat Protein Gene of Grapevine Berry Inner Necrosis Virus and Preparation of Its Polyclonal Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Plant Material
2.2. Prokaryotic Expression and Purification of CPGINV Protein
2.3. Production of the Polyclonal Antibody Against the His-CPGINV Protein
2.4. RT-PCR and Western Blot Analysis
2.5. Dot Blot, ELISA Quantification, and Data Analysis
3. Results
3.1. Cloning, Evaluation, and Purification of the GINV CP Gene
3.2. Evaluation of Prepared Anti-Body Titer for CPGINV
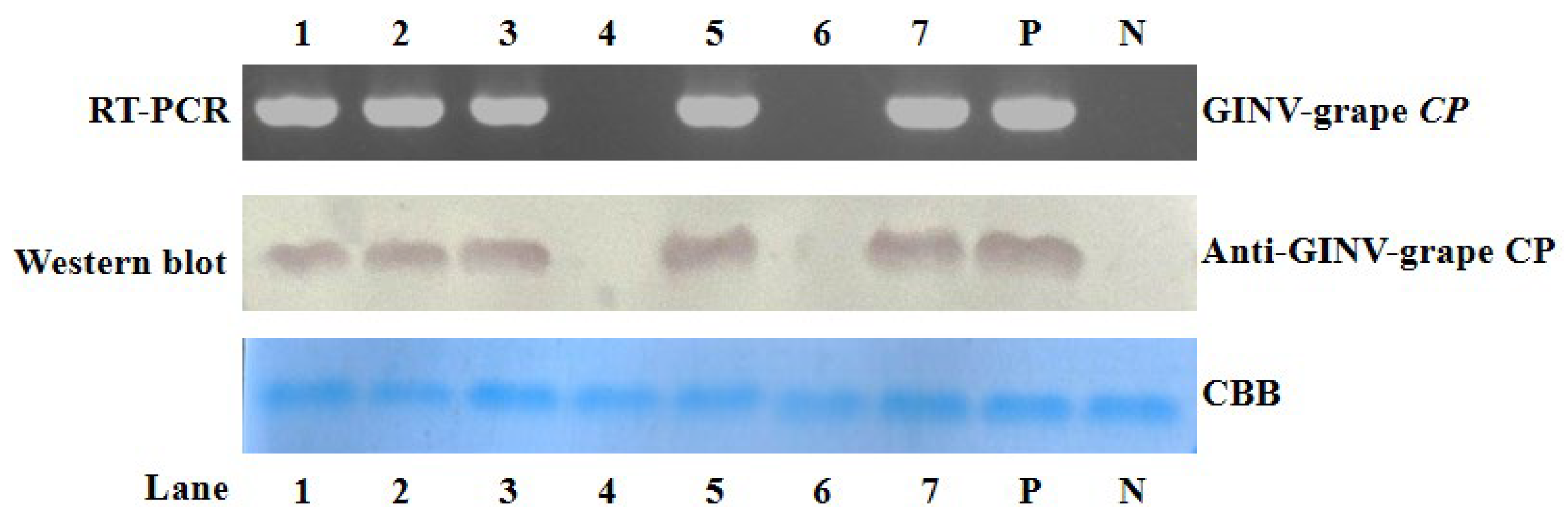
3.3. RT-PCR and Western Blot Detection of GINV Infection in Grape Plants
3.4. Serological Detection of GINV by Dot Blot and ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X.D.; Hu, G.J.; Chen, S.L.; Ren, F.; Du, N.F.; Zhan, L.; Zhang, Z.P.; Dong, Y.F. Research progress of viruses associated with grapevine fanleaf degeneration disease in China. China Fruits 2023, 9, 1–5. (In Chinese) [Google Scholar]
- Sebastian, M.; Laetitia, V.; Gerard, A.B.; Shaheen, Z.N.; Thierry, W. First report of grapevine Pinot gris virus (GPGV) and grapevine rupestris stem pitting-associated virus (GRSPaV) in grapevine in Belgium. Plant Dis. 2020, 104, 1879. [Google Scholar]
- Vinogradova, S.; Porotikova, E.; Navrotskaya, E.; Galbacs, Z.N.; Massart, S.; Varallyay, E. The first virome of a Russian vineyard. Plants 2023, 12, 3292. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Jones, T.; McHenry, D.; Bush, E.; Oliver, C.; Kawaguchi, A.; Nita, A.; Katori, M. A NitroPure Nitrocellulose membrane-based grapevine virus sampling kit: Development and deployment to survey Japanese vineyards and nurseries. Viruses 2023, 15, 2102. [Google Scholar] [CrossRef]
- M’rabet Samaali, B.; Loulou, A.; MougouHamdane, A.; Kallel, S. Acquisition and transmission of grapevine fanleaf virus (GFLV) by Xiphinema index and Xiphinema italiae (Longidoridae). J. Helminthol. 2024, 98, e26. [Google Scholar] [CrossRef]
- Yu, S.Z.; Kan, Q.; Huang, H.Q.; Wang, J.Y.; Xie, Y.S.; Li, H.W.; Zhang, X.Q.; Liu, C.; Cheng, Y.Q. Grapevine cultivar Shine Muscat in China: Occurrence of viruses and attempts to produce certified propagation material. J. Plant Pathol. 2023, 105, 1609–1616. [Google Scholar] [CrossRef]
- Zhao, X.L.; Qi, W.; Luo, W.F.; Zhang, Y.J.; Zheng, Z.L.; Deng, C.L. Establishment of droplet digital RT-PCR assay for detection of grapevine Pinot gris virus. J. Plant Prot. 2020, 47, 457–458. (In Chinese) [Google Scholar]
- Fan, X.D.; Zhang, Z.P.; Ren, F.; Hu, G.J.; Li, Z.N.; Zhang, S.N.; Dong, Y.F. Complete genome sequence analysis of grapevine berry inner necrosis virus type 1 isolate. Acta Phytopathol. Sin. 2018, 48, 423–427. (In Chinese) [Google Scholar]
- Yoshikawa, N.; Iida, H.; Goto, S.; Magome, H.; Takahashi, T.; Terai, Y. Grapevine berry inner necrosis, a new trichovirus: Comparative studies with several known trichoviruses. Arch. Virol. 1997, 142, 1351–1363. [Google Scholar] [CrossRef]
- Fan, X.D.; Hong, N.; Zhang, Z.P.; Yang, Z.K.; Ren, F.; Hu, G.J.; Li, Z.N.; Zhou, J.; Dong, Y.F.; Wang, G.P. Identification of a divergent variant of grapevine berry inner necrosis virus in grapevines showing chlorotic mottling and ring spot symptoms. Arch. Virol. 2016, 161, 2025–2027. [Google Scholar] [CrossRef]
- Fan, X.D.; Zhang, Z.P.; Ren, F.; Hu, G.J.; Zhang, M.Y.; Li, C.; Zhang, B.D.; Dong, Y.F. Construction and screening of a yeast two-hybrid cDNA library from Beta grapevine infected by grapevine berry inner necrosis virus. Acta Phytopathol. Sin. 2021, 51, 210–216. (In Chinese) [Google Scholar]
- Wu, J.Y.; Zhang, Y.; Zhou, X.P.; Qian, Y.J. Three sensitive and reliable serological assays for detection of potato virus A in potato plants. J. Integr. Agric. 2021, 20, 2966–2975. [Google Scholar] [CrossRef]
- Zhang, M.H.; Chen, R.; Zhou, X.P.; Wu, J.X. Monoclonal antibody-based serological detection methods for wheat dwarf virus. Virol. Sin. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Xie, Y.; Jiao, X.Y.; Zhou, X.P.; Liu, H.; Ni, Y.Q.; Wu, J.X. Highly sensitive serological methods for detecting tomato yellow leaf curl virus in tomato plants and whiteflies. Virol J. 2013, 10, 142. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Xing, F.; Wang, H.Q.; Li, S.F. Prokaryotic expression and preparation of antiserum of coat protein gene of apple necrotic mosaic virus. J. Southwest Univ. 2024, 46, 63–69. (In Chinese) [Google Scholar]
- Hua, Y.H.; Feng, C.W.; Gu, T.X.; Chen, H.Y.; Liu, D.X.; Xu, K.; Zhang, K. Development of polyclonal antibodies and a serological-based reverse-transcription loop-mediated isothermal amplification (S-RT-LAMP) assay for rice black-streaked dwarf virus detection in both rice and small brown planthopper. Viruses 2023, 15, 2127. [Google Scholar] [CrossRef]
- He, Z.; Dong, T.T.; Chen, W.; Wang, T.L.; Gan, H.F.; Li, L.J. Antiserum preparation of recombinant sweet potato latent virus-lotus (SPLV-lotus) coat protein and application for virus-infected lotus plant detection. Plant Pathol. J. 2020, 36, 651–657. [Google Scholar] [CrossRef]
- Gu, T.X.; Feng, C.W.; Hua, Y.H.; Liu, D.X.; Chen, H.Y.; He, Z.; Xu, K.; Zhang, K. Molecular characterization and pathogenicity of an infectious cDNA clone of youcai mosaic virus on Solanum nigrum. Int. J. Mol. Sci. 2024, 25, 1620. [Google Scholar] [CrossRef]
- Fang, X.X.; Jia, Z.X.; Yu, T.Q.; Rui, P.H.; Zheng, H.Y.; Lu, Y.W.; Peng, J.J.; Rao, S.F.; Wu, J.; Chen, J.P.; et al. FATTY ACID DESATURASE4 enhances plant RNA virus replication and undergoes host vacuolar ATPase-mediated degradation. Plant Physiol. 2024, 196, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Li, Z.P.; Lin, H.B.; Cheng, Y.; Wang, H.Q.; Jiang, Z.M.; Ji, Z.X.; Huang, Z.J.; Chen, H.Y.; Wei, T.Y. A phytoplasma effector destabilizes chloroplastic glutamine synthetase inducing chlorotic leaves that attract leafhopper vectors. Proc. Natl. Acad. Sci. USA 2024, 12, e2402911121. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Li, X.Y.; Zhang, B.; Zhang, L.; Sun, P.P.; Li, Z.N. Advances in vitivirus research members of the genus vitivirus. China fruits 2021, 08, 12–18. (In Chinese) [Google Scholar]
- Zhang, Y.; Yuan, Q.Y.; Ren, F.; Hu, G.J.; Fan, X.D.; Dong, Y.F. Establishment of RT-qPCR detection technology for GINV and its spatial and temporal distribution in different grape rootstocks. Sci. Agric. Sin. 2024, 57, 2771–2780. (In Chinese) [Google Scholar]
- Wu, J.X.; Ni, Y.Q.; Liu, H.; Ding, M.; Zhou, X.P. Monoclonal antibody-based serological assays and immunocapture-RT-PCR for detecting Rice dwarf virus in field rice plants and leafhopper vectors. J. Virol. Methods 2014, 195, 134–140. [Google Scholar] [CrossRef]
- Yardimci, N.; Kilic, H.C.; Demir, Y. Detection of PVY, PVX, PVS, PVA, and PLRV on different potato varieties in Turkey using DAS-ELISA. J. Agr. Sci. Tech. Iran 2015, 17, 757–764. [Google Scholar]
- Katwal, V.S.; Handa, A.; Thakur, P.D.; Tomar, M. Prevalence and serological detection of apple viruses in Himachal Pradesh. Plant Pathol. J. 2016, 15, 40–48. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Hong, J.; Wang, X.F.; Zhou, C.Y.; Zhuo, X.P.; Wu, J.X. Monoclonal antibody-based serological methods for detecting Citrus tristeza virus in citrus groves. Virol. Sin. 2016, 31, 324–330. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, Y.L.; Guo, L.L.; Shen, J.; Hu, H.L.; Zhou, R.J. Transcriptome analysis of Crimson seedless grapevine (Vitis vinifera L.) infected by grapevine berry inner necrosis virus. Curr. Res. Virol. Sci. 2022, 3, 100024. [Google Scholar] [CrossRef]
- Gopi, K.; Kumar, S.S.; Kadappa, S.H.; Nitika, G.; Abdeen, K.Z.; Kumar, S.S.; Kumar, B.V. Diverse spectra of virus infection identified through high throughput sequencing in nursery plants of two Indian grapevine cultivars. Physiol. Mol. Plant Pathol. 2023, 128, 102135. [Google Scholar]
- Feng, C.W.; Hua, Y.H.; Liu, D.X.; Chen, H.Y.; Wu, M.J.; Hua, J.; Zhang, K. Establishment of a serology- and molecular-combined detection system for Youcai mosaic virus and its application in various host plants. Agronomy 2024, 14, 1900. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Wang, Z.; Wang, N.; Zhao, H.; Qin, L.; Jiang, R.; Yuan, M.; Chen, X.; He, Z. Prokaryotic Expression of Coat Protein Gene of Grapevine Berry Inner Necrosis Virus and Preparation of Its Polyclonal Antibody. Agronomy 2024, 14, 2594. https://doi.org/10.3390/agronomy14112594
Deng X, Wang Z, Wang N, Zhao H, Qin L, Jiang R, Yuan M, Chen X, He Z. Prokaryotic Expression of Coat Protein Gene of Grapevine Berry Inner Necrosis Virus and Preparation of Its Polyclonal Antibody. Agronomy. 2024; 14(11):2594. https://doi.org/10.3390/agronomy14112594
Chicago/Turabian StyleDeng, Xiaolong, Zhilei Wang, Nian Wang, Haiting Zhao, Lang Qin, Runzhou Jiang, Meng Yuan, Xijun Chen, and Zhen He. 2024. "Prokaryotic Expression of Coat Protein Gene of Grapevine Berry Inner Necrosis Virus and Preparation of Its Polyclonal Antibody" Agronomy 14, no. 11: 2594. https://doi.org/10.3390/agronomy14112594
APA StyleDeng, X., Wang, Z., Wang, N., Zhao, H., Qin, L., Jiang, R., Yuan, M., Chen, X., & He, Z. (2024). Prokaryotic Expression of Coat Protein Gene of Grapevine Berry Inner Necrosis Virus and Preparation of Its Polyclonal Antibody. Agronomy, 14(11), 2594. https://doi.org/10.3390/agronomy14112594




