Tillage and Its Effect on Agricultural Soils: A Quality Index Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study and Sampling Area
2.2. Sample Preparation
2.3. Physicochemical Characterization
2.4. Statistical Analysis
2.5. Development of the SQI
3. Results and Discussion
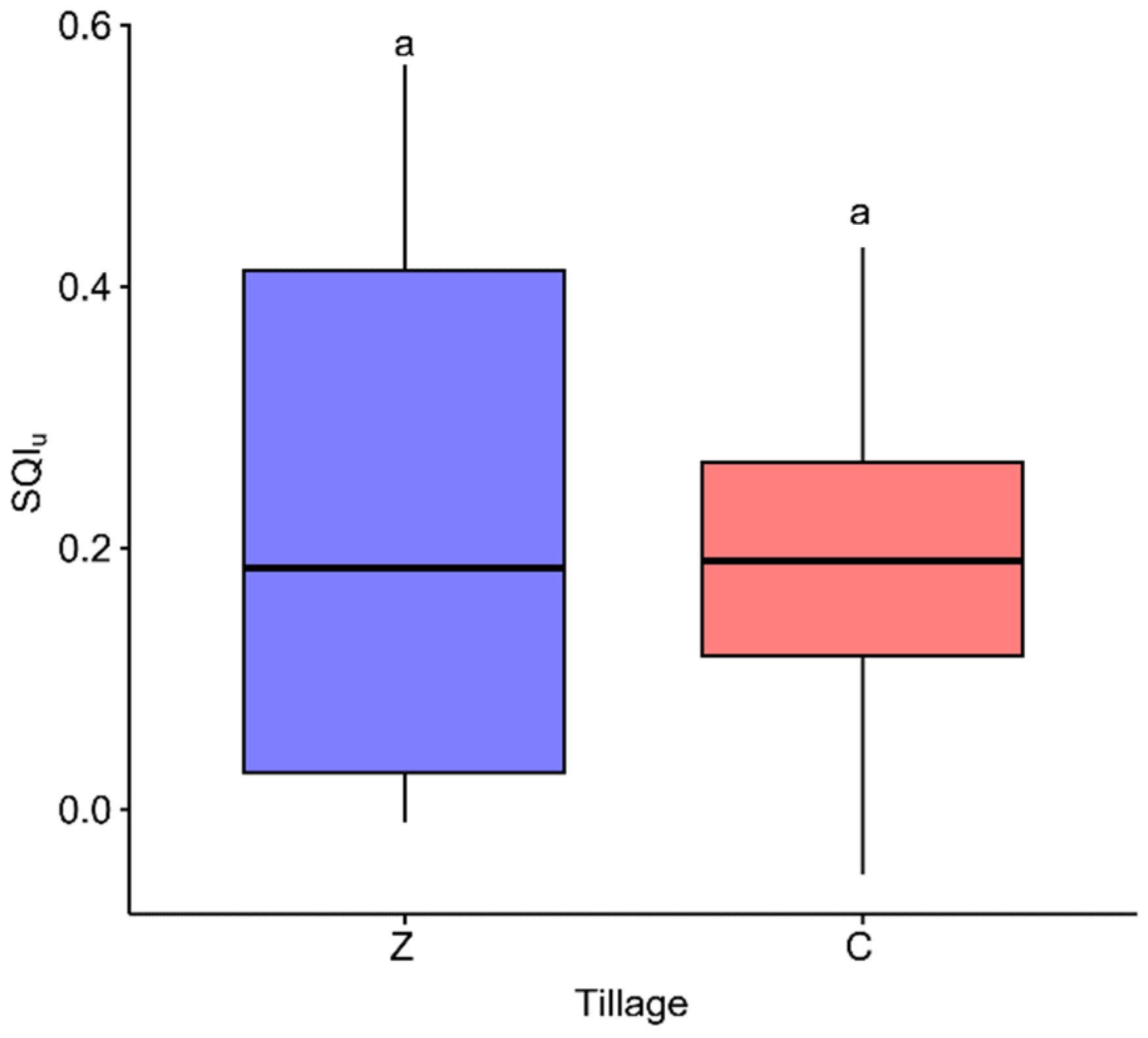
3.1. Analysis of Variance of Indicators
3.2. PCA
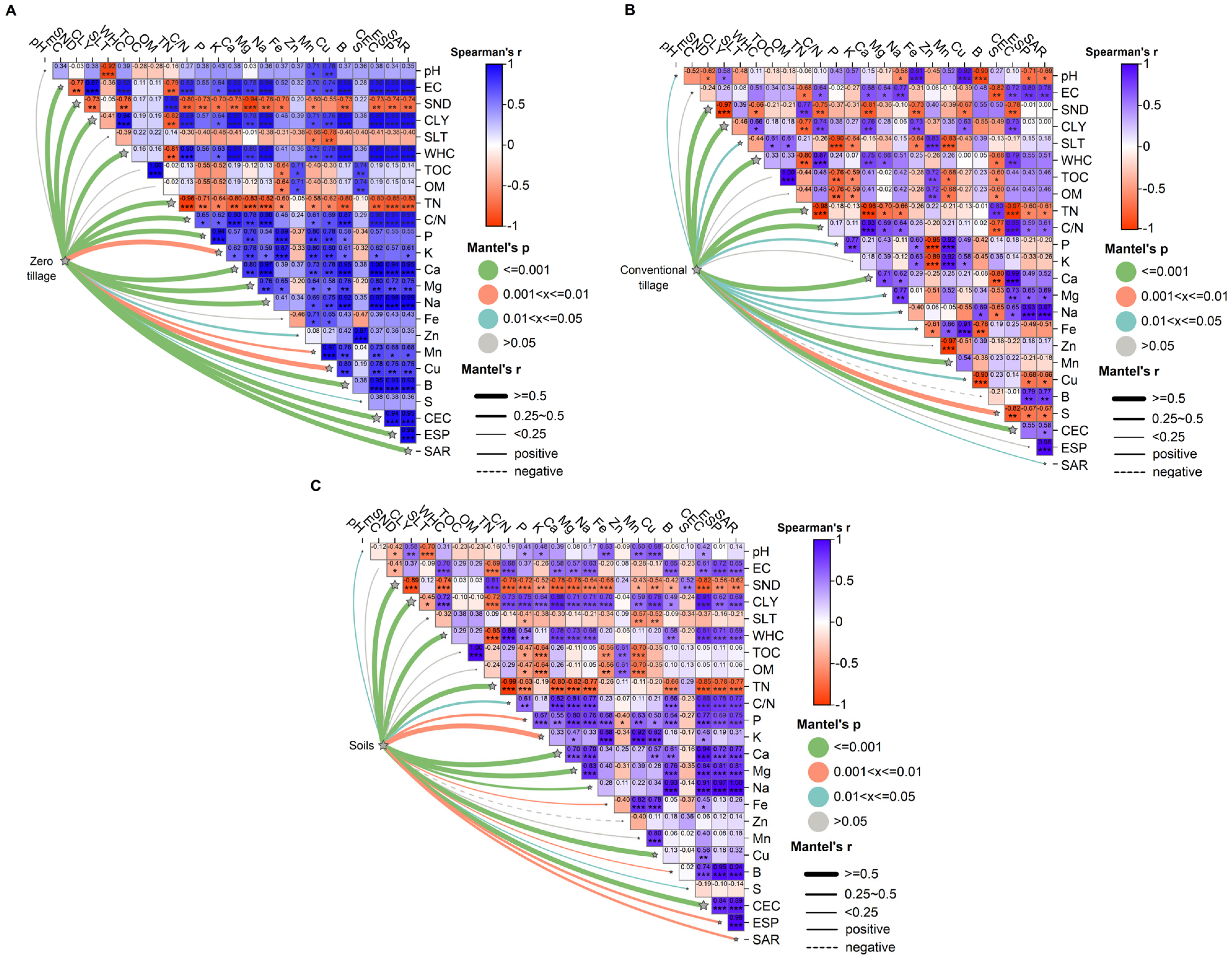
3.2.1. Correlation Matrix and Mantel Test
3.2.2. Obtaining PCs
3.2.3. Key Indicators for the Development of the SQI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Denton-Thompson, S.M.; Sayer, E.J. Micronutrients in Food Production: What Can We Learn from Natural Ecosystems? Soil Syst. 2022, 6, 8. [Google Scholar] [CrossRef]
- Nunes, M.R.; Van Es, H.M.; Schindelbeck, R.; Ristow, A.J.; Ryan, M. No-till and Cropping System Diversification Improve Soil Health and Crop Yield. Geoderma 2018, 328, 30–43. [Google Scholar] [CrossRef]
- Bedolla-Rivera, H.I.; Xochilt Negrete-Rodríguez, M.D.L.L.; Medina-Herrera, M.D.R.; Gámez-Vázquez, F.P.; Álvarez-Bernal, D.; Samaniego-Hernández, M.; Gámez-Vázquez, A.J.; Conde-Barajas, E. Development of a Soil Quality Index for Soils under Different Agricultural Management Conditions in the Central Lowlands of Mexico: Physicochemical, Biological and Ecophysiological Indicators. Sustainability 2020, 12, 9754. [Google Scholar] [CrossRef]
- Adak, S.; Bandyopadhyay, K.; Purakayastha, T.J.; Sen, S.; Sahoo, R.N.; Shrivastava, M.; Krishnan, P. Impact of Contrasting Tillage, Residue Mulch and Nitrogen Management on Soil Quality and System Productivity under Maize-Wheat Rotation in the North-Western Indo-Gangetic Plains. Front. Sustain. Food Syst. 2023, 7, 1230207. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Taghizadeh-Mehrjardi, R.; Eskandari, S. Assessing and Monitoring the Soil Quality of Forested and Agricultural Areas Using Soil-Quality Indices and Digital Soil-Mapping in a Semi-Arid Environment. Arch. Agron. Soil Sci. 2018, 64, 696–707. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Zhang, L.; Li, Q.; Zhou, D. Selecting the Minimum Data Set and Quantitative Soil Quality Indexing of Alkaline Soils under Different Land Uses in Northeastern China. Sci. Total Environ. 2018, 616–617, 564–571. [Google Scholar] [CrossRef]
- Chang, T.; Feng, G.; Paul, V.; Adeli, A.; Brooks, J.P.; Jenkins, J.N. Soil Health Assessment for Different Tillage and Cropping Systems to Determine Sustainable Management Practices in a Humid Region. Soil Tillage Res. 2023, 233, 105796. [Google Scholar] [CrossRef]
- Mandujano Bueno, A.; Paredes Melesio, R.; Alamilla Gómez, M.d.P. Guía para la Producción de Granos Básicos Maíz, Fijol, Trigo y Sorgo en Guanajuato; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias [INIFAP]: Coyoacán, Mexico, 2013. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, [USDA]. Soil Survey Manual; United Department of Agriculture: Washington, DC, USA, 1951. [Google Scholar]
- Thomas, G.W. Soil pH and Soil Acidity. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 475–490. ISBN 978-0-89118-866-7. [Google Scholar]
- Hendrickx, J.M.H.; Das, B.; Corwin, D.L.; Wraith, J.M.; Kachanoski, R.G. Relationship between Soil Water Solute Concentration and Apparent Soil Electrical Conductivity. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 2002; Volume 4, pp. 1275–1282. [Google Scholar]
- Hopkins, D.W.; Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemestry. J. Appl. Ecol. 1996, 33, 178. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sönmez, M. The Role of Organic/Bio–Fertilizer Amendment on Aggregate Stability and Organic Carbon Content in Different Aggregate Scales. Soil Tillage Res. 2017, 168, 118–124. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Chemical Methods; The Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 1085–1121. [Google Scholar]
- Bettinelli, M.; Baroni, U. A Microwave Oven Digestion Method for the Determination of Metals in Sewage Sludges by ICP-AES and GFAAS. Int. J. Environ. Anal. Chem. 1991, 43, 33–40. [Google Scholar] [CrossRef]
- Cottenie, A. Soil and Plant Testing as a Basis of Fertilizer Recommendations; FAO Soils Bulletin; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980; pp. 64–65. [Google Scholar]
- Secretaría de Medio Ambiente y Recursos Naturales [SEMARNAT] NOM-021-RECNAT-2002. 2003. Available online: http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69255.pdf (accessed on 20 November 2024).
- R Core Team. R: Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Mahajan, G.; Das, B.; Morajkar, S.; Desai, A.; Murgaokar, D.; Kulkarni, R.; Sale, R.; Patel, K. Soil Quality Assessment of Coastal Salt-Affected Acid Soils of India. Environ. Sci. Pollut. Res. 2020, 27, 26221–26238. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rojas, M.; Erickson, T.E.; Dixon, K.W.; Merritt, D.J. Soil Quality Indicators to Assess Functionality of Restored Soils in Degraded Semiarid Ecosystems. Restor. Ecol. 2016, 24, S43–S52. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R. A Keystone Microbial Enzyme for Nitrogen Control of Soil Carbon Storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do All the Numbers Mean? CSIRO Publishing: Collingwood, VIC, Australia, 2007; ISBN 978-0-643-09225-9. [Google Scholar]
- Leogrande, R.; Vitti, C. Use of Organic Amendments to Reclaim Saline and Sodic Soils: A Review. Arid Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Ma, R.; Chen, M.; Wei, W.; Ding, X. Carbon Sequestration under Different Organic Amendments in Saline-Alkaline Soils. CATENA 2021, 196, 104882. [Google Scholar] [CrossRef]
- Zanor, G.; López-Pérez, M.; Martínez-Yáñez, R.; Ramírez-Santoyo, L.; Gutiérrez-Vargas, S.; León-Galván, M.F. Mejoramiento de Las Propiedades Físicas y Químicas de Un Suelo Agrícola Mezclado Con Lombricompostas de Dos Efluentes de Biodigestor. Ing. Investig. Tecnol. 2018, 19, e036. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of Nitrogen Addition on Activities of Soil Nitrogen Acquisition enzymes: A Meta-Analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The Influence of Organic Amendment Source on Carbon and Nitrogen Mineralization in Different Soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Naeem, M.; Ansari, A.A.; Gill, S.S. (Eds.) Contaminants in Agriculture: Sources, Impacts and Management; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-41551-8. [Google Scholar]
- Wong, V.N.L.; Greene, R.S.B.; Dalal, R.C.; Murphy, B.W. Soil Carbon Dynamics in Saline and Sodic Soils: A Review: Soil Carbon Dynamics in Saline and Sodic Soils. Soil Use Manag. 2010, 26, 2–11. [Google Scholar] [CrossRef]
- Zungu, N.S.; Egbewale, S.O.; Olaniran, A.O.; Pérez-Fernández, M.; Magadlela, A. Soil Nutrition, Microbial Composition and Associated Soil Enzyme Activities in KwaZulu-Natal Grasslands and Savannah Ecosystems Soils. Appl. Soil Ecol. 2020, 155, 103663. [Google Scholar] [CrossRef]
- Poeplau, C.; Riefling, T.; Schiedung, M.; Anlauf, R. Land Use and Soil Property Effects on Aggregate Stability Assessed by Three Different Slaking Methods. Eur. J. Soil Sci. 2024, 75, e13549. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Usowicz, B.; Lipiec, J. Spatial Variability of Saturated Hydraulic Conductivity and Its Links with Other Soil Properties at the Regional Scale. Sci. Rep. 2021, 11, 8293. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, I.; Bedolla-Rivera, H.I.; Negrete-Rodríguez, M.D.L.L.X.; Castillo, O.S.; Álvarez-Bernal, D.; Conde-Barajas, E. Mineralization of Microalgal Carbon and Nitrogen in Sodic Soils. Rev. Int. Contam. Ambient. 2024, 40, 149–166. [Google Scholar] [CrossRef]
- Sánchez-Guzmán, A.; Bedolla-Rivera, H.I.; Conde-Barajas, E.; Negrete-Rodríguez, M.D.L.L.X.; Lastiri-Hernández, M.A.; Gámez-Vázquez, F.P.; Álvarez-Bernal, D. Corn Cropping Systems in Agricultural Soils from the Bajio Region of Guanajuato: Soil Quality Indexes (SQIs). Appl. Sci. 2024, 14, 2858. [Google Scholar] [CrossRef]
- Dugan, I.; Pereira, P.; Kisic, I.; Matisic, M.; Bogunovic, I. Analyzing the Influence of Conservation Tillage and Manure on Soil Parameter Modulations in Croplands. Plants 2024, 13, 607. [Google Scholar] [CrossRef]
- Rocco, S.; Munkholm, L.J.; Jensen, J.L. Long-Term Soil Quality and C Stock Effects of Tillage and Cover Cropping in a Conservation Agriculture System. Soil Tillage Res. 2024, 241, 106129. [Google Scholar] [CrossRef]

| Municipality | Soils | Crop | Type of Irrigation | Fertilization (NPK) | Tillage | Localization |
|---|---|---|---|---|---|---|
| Comonfort | Cm2 | Alfalfa | Furrow | 34-34-34 | Zero | 20°40′45.79″ N, 100°45′14.99″ W |
| Celaya | Ce1 | Alfalfa | Furrow | 34-34-34 | Zero | 20°35′42.76″ N, 100°44′43.96″ W |
| Ce2 | Jicama | Furrow | 00-00-00 | Conventional | 20°39′56.41″ N, 100°47′00.48″ W | |
| Cortazar | Co1 | Corn | Furrow | 240-40-00 | Conventional | 20°27′14.61″ N, 101°01′34.44″ W |
| Salamanca | Sa5 | Chickpea | Residual moisture | 16-46-00 | Zero | 20°37′46.45″ N, 101°11′23.09″ W |
| Sa6 | Corn | Furrow | 240-40-00 | Conventional | 20°35′42.42″ N, 101°12′37.97″ W | |
| Valle de Santiago | Va1 | Sorghum | Furrow | 240-40-00 | Conventional | 20°26′11.07″ N, 101°08′21.51″ W |
| Va2 | Corn | Furrow | 240-40-00 | Zero | 20°27′54.79″ N, 101°11′05.97″ W |
| Quality | Very High | High | Moderate | Low | Very Low |
|---|---|---|---|---|---|
| Scale | 0.80–1.00 | 0.60–0.79 | 0.40–0.59 | 0.20–0.39 | 0.00–0.19 |
| Class | 1 | 2 | 3 | 4 | 5 |
| Indicators | General, N = 24 | Ce1, N = 3 | Ce2, N = 3 | Cm2, N = 3 | Co1, N = 3 | Sa5, N = 3 | Sa6, N = 3 | Va1, N = 3 | Va2, N = 3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 6.56 (0.31) | 6.68 ab (0.08) | 6.95 a (0.11) | 6.19 b (0.03) | 6.31 ab (0.03) | 6.90 ab (0.07) | 6.80 ab (0.14) | 6.21 ab (0.01) | 6.43 ab (0.04) | 0.003 |
| EC | 0.27 (0.16) | 0.23 ab (0.01) | 0.15 b (0.01) | 0.25 ab (0.01) | 0.16 ab (0.01) | 0.33 a (0.04) | 0.47 a (0.42) | 0.29 ab (0.04) | 0.28 ab (0.01) | 0.009 |
| SND | 28.00 (15) | 62.00 a (2) | 20.00 ab (1) | 32.00 ab (1) | 34.00 ab (0) | 20.00 ab (3) | 13.00 b (1) | 25.00 ab (3) | 17.00 ab (0) | 0.002 |
| CLY | 46.00 (16) | 17.00 b (1) | 55.00 ab (0) | 27.00 ab (2) | 42.00 ab (1) | 62.00 ab (3) | 67.00 a (2) | 46.00 ab (1) | 54.00 ab (0) | 0.002 |
| SLT | 26.00 (7) | 21.00 ab (2) | 25.00 ab (1) | 41.00 a (2) | 24.00 ab (0) | 18.00 b (1) | 19.00 ab (1) | 29.00 ab (3) | 29.00 ab (1) | 0.003 |
| WHC | 112.00 (16) | 98.00 ab (1) | 100.00 ab (10) | 108.00 ab (0) | 92.00 b (1) | 134.00 a (5) | 133.00 a (1) | 109.00 ab (3) | 124.00 ab (3) | 0.005 |
| TOC | 1.74 (0.44) | 1.48 ab (0.06) | 1.65 ab (0.21) | 2.66 a (0.20) | 1.29 b (0.06) | 1.92 ab (0.09) | 1.45 ab (0.11) | 2.02 ab (0.12) | 1.47 ab (0.02) | 0.005 |
| OM | 3.00 (0.76) | 2.55 ab (0.10) | 2.84 ab (0.36) | 4.59 a (0.34) | 2.22 b (0.10) | 3.31 ab (0.15) | 2.49 ab (0.19) | 3.48 ab (0.20) | 2.54 ab (0.03) | 0.005 |
| TN | 0.82 (0.58) | 1.66 a (0.08) | 1.14 ab (0.04) | 0.91 ab (0.11) | 1.55 ab (0.34) | 0.22 b (0.04) | 0.19 b (0.13) | 0.67 ab (0.20) | 0.24 b (0.12) | 0.003 |
| C/N | 4.92 (6.17) | 0.90 b (0.01) | 1.45 ab (0.17) | 2.98 ab (0.49) | 0.86 ab (0.20) | 9.13 a (1.92) | 13.80 a (13.98) | 3.21 ab (0.94) | 7.03 ab (2.87) | 0.003 |
| P | 0.15 (0.11) | 0.04 ab (0.00) | 0.10 ab (0.00) | 0.03 b (0.00) | 0.19 ab (0.00) | 0.21 ab (0.00) | 0.23 ab (0.03) | 0.05 ab (0.00) | 0.34 a (0.01) | 0.002 |
| K | 1.31 (0.32) | 0.88 ab (0.04) | 1.51 ab (0.01) | 0.75 b (0.03) | 1.58 ab (0.08) | 1.43 ab (0.04) | 1.62 a (0.04) | 1.24 ab (0.03) | 1.47 ab (0.03) | 0.003 |
| Ca | 23.00 (7) | 11.00 b (0) | 23.00 ab (1) | 18.00 ab (0) | 17.00 ab (1) | 34.00 a (1) | 28.00 ab (1) | 24.00 ab (1) | 23.00 ab (1) | 0.003 |
| Mg | 7.08 (2.64) | 1.88 b (0.03) | 4.39 ab (0.05) | 6.64 ab (0.20) | 7.77 ab (0.34) | 8.12 ab (0.13) | 10.01 a (0.48) | 7.97 ab (0.10) | 9.87 a (0.33) | 0.002 |
| Na | 1.62 (1.54) | 0.18 b (0.01) | 0.40 ab (0.01) | 0.36 b (0.00) | 0.82 ab (0.03) | 4.69 a (0.10) | 1.56 ab (0.04) | 1.62 ab (0.06) | 3.30 ab (0.15) | 0.002 |
| S | 0.09 (0.03) | 0.09 ab (0.00) | 0.09 ab (0.00) | 0.10 ab (0.00) | 0.12 ab (0.00) | 0.15 a (0.01) | 0.06 ab (0.01) | 0.06 ab (0.00) | 0.05 b (0.00) | 0.003 |
| Fe | 0.08 (0.05) | 0.04 ab (0.02) | 0.15 a (0.00) | 0.02 b (0.00) | 0.09 ab (0.00) | 0.05 ab (0.00) | 0.16 a (0.01) | 0.04 ab (0.00) | 0.12 ab (0.00) | 0.003 |
| Zn | 0.002 (0.0020) | 0.001 b (0.0002) | 0.002 ab (0.0001) | 0.001 b (0.0002) | 0.001 b (0.0001) | 0.004 ab (0.0003) | 0.001 b (0.0000) | 0.006 a (0.0006) | 0.001 b (0.0001) | 0.002 |
| Mn | 0.018 (0.011) | 0.012 ab (0.001) | 0.018 ab (0.002) | 0.009 b (0.001) | 0.021 ab (0.001) | 0.016 ab (0.001) | 0.044 a (0.002) | 0.009 b (0.001) | 0.015 ab (0.001) | 0.002 |
| Cu | 0.003 ab (0.0010) | 0.001 b (0.0001) | 0.004 a (0.0002) | 0.001 b (0.0000) | 0.003 ab (0.0000) | 0.003 ab (0.0001) | 0.004 a (0.0001) | 0.003 ab (0.0001) | 0.002 ab (0.0001) | 0.002 |
| B | 0.05 (0.04) | 0.00 b (0.00) | 0.01 ab (0.00) | 0.02 ab (0.00) | 0.02 ab (0.00) | 0.12 a (0.00) | 0.02 ab (0.00) | 0.08 ab (0.00) | 0.11 (0.00) | 0.002 |
| CEC | 33.00 (10) | 14.00 b (1) | 30.00 ab (1) | 26.00 ab (1) | 27.00 ab (1) | 49.00 a (1) | 42.00 a (2) | 35.00 ab (1) | 38.00 ab (1) | 0.002 |
| ESP | 4.20 (3.16) | 1.23 b (0.09) | 1.35 ab (0.03) | 1.37 ab (0.02) | 2.98 ab (0.01) | 9.63 a (0.10) | 3.75 ab (0.11) | 4.60 ab (0.03) | 8.70 ab (0.15) | 0.002 |
| SAR | 0.27 (0.24) | 0.05 b (0.00) | 0.08 ab (0.00) | 0.07 b (0.00) | 0.16 ab (0.00) | 0.72 a (0.01) | 0.25 ab (0.00) | 0.28 ab (0.00) | 0.57 ab (0.02) | 0.002 |
| Indicator | PC1 | PC2 |
|---|---|---|
| C/N | 0.61 | --- |
| SND | −0.80 | --- |
| ESP | 0.89 | --- |
| Fe | --- | −0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrete-Rodríguez, M.d.l.L.X.; Conde-Barajas, E.; Silva-Martínez, G.A.; Acosta-García, G.; Ramírez-Medina, H.; Tristán-Flores, F.E.; Bedolla-Rivera, H.I. Tillage and Its Effect on Agricultural Soils: A Quality Index Approach. Agronomy 2024, 14, 2793. https://doi.org/10.3390/agronomy14122793
Negrete-Rodríguez MdlLX, Conde-Barajas E, Silva-Martínez GA, Acosta-García G, Ramírez-Medina H, Tristán-Flores FE, Bedolla-Rivera HI. Tillage and Its Effect on Agricultural Soils: A Quality Index Approach. Agronomy. 2024; 14(12):2793. https://doi.org/10.3390/agronomy14122793
Chicago/Turabian StyleNegrete-Rodríguez, María de la Luz Xochilt, Eloy Conde-Barajas, Guillermo Antonio Silva-Martínez, Gerardo Acosta-García, Humberto Ramírez-Medina, Fabiola Estefanía Tristán-Flores, and Héctor Iván Bedolla-Rivera. 2024. "Tillage and Its Effect on Agricultural Soils: A Quality Index Approach" Agronomy 14, no. 12: 2793. https://doi.org/10.3390/agronomy14122793
APA StyleNegrete-Rodríguez, M. d. l. L. X., Conde-Barajas, E., Silva-Martínez, G. A., Acosta-García, G., Ramírez-Medina, H., Tristán-Flores, F. E., & Bedolla-Rivera, H. I. (2024). Tillage and Its Effect on Agricultural Soils: A Quality Index Approach. Agronomy, 14(12), 2793. https://doi.org/10.3390/agronomy14122793









