Cutting-Edge Strategies to Enhance Bioactive Compound Production in Plants: Potential Value of Integration of Elicitation, Metabolic Engineering, and Green Nanotechnology
Abstract
:1. Introduction
2. Elicitation Techniques
2.1. Physical and Chemical Factors for Secondary Metabolites Production
2.2. Recent Studies and Advancements
3. Metabolic Engineering Approaches
4. Biosynthesized Metallic and Metallic Oxide Nanoparticles in Plant Metabolite Production
4.1. Nanotechnology and Nanomaterial (NM) Classification
4.2. Metallic and Metallic Oxide Nanoparticles
Synthesis Methods of Metallic and Metallic Oxide Nanoparticles
4.3. Mechanisms of Action, Applications and Case Studies
4.3.1. Absorption Pathways of Nanoparticles in Plants
4.3.2. Nanoparticles to Enhance Metabolite Synthesis
- Silver nanoparticles (Ag NPs) have demonstrated the ability to increase the production of phenolic compounds and flavonoids in plants such as fenugreek, thereby enhancing their antioxidant capacity and pharmacological properties [170,199]. Research has shown that Ag NPs can interact with plant cells, inducing oxidative stress and activating defense mechanisms [247]. This activation leads to the upregulation of genes involved in the biosynthesis of phenolic compounds and flavonoids, which are key secondary metabolites with significant antioxidant properties [59,248]. Ag NPs enhanced tolerance to saline stress by reducing the salt ion content in plants and improving antioxidant enzyme activity, resulting in increased yield and photosynthetic activity [249].
- Titanium dioxide nanoparticles (TiO2 NPs) can enhance the synthesis of secondary metabolites under stress conditions, such as UV radiation, by increasing the production of antioxidants and other protective compounds [34,250]. When plants are exposed to TiO2 NPs, these nanoparticles can interact with cellular components, leading to the generation of reactive oxygen species (ROS). This controlled oxidative stress can act as a signal that triggers the plant’s defense mechanisms, resulting in the upregulation of pathways involved in the production of secondary metabolites [250]. Under UV radiation, TiO2 NPs can further amplify the plant’s stress response, promoting the synthesis of antioxidants and other protective compounds. These secondary metabolites, such as phenolics and flavonoids, play crucial roles in mitigating oxidative damage and enhancing the plant’s overall resilience to environmental stress [251,252]. In maize crops, hydroponic exposure to TiO2 NPs increased cadmium absorption and phytotoxicity, resulting in reduced chlorophyll content and dry weight in plants. However, the foliar application of TiO2 NPs helped reduce cadmium content in the shoots, thereby mitigating phytotoxicity [253].
- Iron oxide nanoparticles (Fe3O4 NPs) in hydroponic cultures have demonstrated the ability to enhance plant productivity and growth, which is associated with an increase in the synthesis of secondary metabolites. In hydroponic systems, the introduction of Fe3O4 NPs can improve nutrient availability and uptake by plants. These nanoparticles can interact with root systems, enhancing the absorption of essential nutrients and thereby promoting overall plant health and vigor. This improved nutrient status can stimulate metabolic activities within the plant, leading to increased growth rates and higher biomass production [152]. Fe3O4 NPs can induce stress responses that activate the biosynthesis of secondary metabolites. These metabolites, such as phenolic compounds, flavonoids, and other antioxidants, play vital roles in plant defense and adaptation to environmental challenges. The presence of Fe3O4 NPs can enhance the production of these compounds, contributing to the plant’s resilience and overall quality [169,171,254]. The exposure of alfalfa crops to iron nanoparticles (Fe NPs) resulted in increased root length and chlorophyll content due to interactions with hydroxyl radicals that loosened the cell wall [255].
- Cerium oxide nanoparticles (CeO2 NPs) in soybean cultivated under varying soil moisture conditions, both fresh and dry weight increased without significantly affecting the total chlorophyll content. CeO2 NPs may enhance photosynthetic efficiency by improving water and nutrient availability, leading to better growth and biomass production. The impact of nanoparticles on photosynthesis was dependent on soil moisture levels. The findings highlight the importance of considering environmental factors such as soil moisture when assessing the effects of nanoparticles on plant growth and photosynthesis [256].
- The foliar application of zinc oxide nanoparticles (ZnO NPs) in cowpea and okra crops under salinity conditions improved growth parameters compared to plants not treated with nanoparticles. This improvement is attributed to the release of zinc, a vital micronutrient involved in various plant metabolic processes. The improved growth parameters observed in ZnO-treated cowpea and okra plants include increased biomass, better root and shoot development, and higher overall vigor. This indicates that ZnO nanoparticles can mitigate the adverse effects of salinity on plant growth [172,257].
4.3.3. Negative Impact of Nanoparticles on Plant Cells
4.4. Toxicological Concerns, Risk Mitigation, and Guidelines for Safety Applications
5. Integration of Elicitation, Metabolic Engineering, and Biosynthesized Nanoparticles to Bioactive Compound Production in Plants
6. Future Directions and Applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barone, R.P.; Knittel, D.K.; Ooka, J.K.; Porter, L.N.; Smith, N.T.; Owens, D.K. The production of plant natural products beneficial to humanity by metabolic engineering. Curr. Plant Biol. 2020, 24, 100121. [Google Scholar] [CrossRef]
- Awere, C.O.; Rakkammal, K.; Mwaura, M.M.; Anadebe, V.C.; Ramesh, M. Hairy-root technology: A metabolic engineering tool and specialized metabolite pathway elucidation and production of secondary metabolites. A review. Results Eng. 2024, 23, 102697. [Google Scholar] [CrossRef]
- Cardillo, A.B.; Perassolo, M.; Giulietti, A.M.; Rodriguez Talou, J. Cyclodextrins: A tool in plant cell and organ culture bioprocesses for the production of secondary metabolites. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 146, 1–19. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. LED Technology Applied to Plant Development for Promoting the Accumulation of Bioactive Compounds: A Review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef]
- de Bruijn, W.J.C.; Levisson, M.; Beekwilder, J.; van Berkel, W.J.H.; Vincken, J.P. Plant Aromatic Prenyltransferases: Tools for Microbial Cell Factories. Trends Biotechnol. 2020, 38, 917–934. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering Considerations to Produce Bioactive Compounds from Plant Cell Suspension Culture in Bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Rosegrant, M.W.; Tokgoz, S.; Bhandary, P. The New Normal? A Tighter Global Agricultural Supply and Demand Relation and Its Implications for Food Security. Am. J. Agric. Econ. 2013, 95, 303–309. [Google Scholar] [CrossRef]
- Sheikh Mohammad Fakhrul, I.; Zahurul, K. World’s Demand for Food and Water: The Consequences of Climate Change. In Desalination; Mohammad Hossein Davood Abadi, F., Vahid, V., Amir Hooshang, T., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 4. [Google Scholar]
- Tian, X.; Engel, B.A.; Qian, H.; Hua, E.; Sun, S.; Wang, Y. Will reaching the maximum achievable yield potential meet future global food demand? J. Clean. Prod. 2021, 294, 126285. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Perez-Matas, E.; Escrich, A.; Cusido, R.M.; Palazon, J.; Bonfill, M. Biotic Elicitors in Adventitious and Hairy Root Cultures: A Review from 2010 to 2022. Molecules 2022, 27, 5253. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Oh, D.-H. Elicitation: A new perspective into plant chemo-diversity and functional property. Crit. Rev. Food Sci. Nutr. 2023, 63, 4522–4540. [Google Scholar] [CrossRef]
- Huang, L.; Ho, C.-T.; Wang, Y. Biosynthetic pathways and metabolic engineering of spice flavors. Crit. Rev. Food Sci. Nutr. 2021, 61, 2047–2060. [Google Scholar] [CrossRef]
- Ullah, N.; Shahzad, K.; Wang, M. The Role of Metabolic Engineering Technologies for the Production of Fatty Acids in Yeast. Biology 2021, 10, 632. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.d.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.d.L.; Torres-Pacheco, I. Plant Hormesis Management with Biostimulants of Biotic Origin in Agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green Synthesized Metal Oxide Nanoparticles Mediate Growth Regulation and Physiology of Crop Plants under Drought Stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Rangabhashiyam, S.; Saini, A.; Saini, R.V.; Le, Q.V.; Nadda, A.K.; Le, T.-T.; et al. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Thanh Hai, N.T.; Viet, N.M.; Nguyen, V.-H. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ. Res. 2022, 203, 111858. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Zain, M.; Ma, H.; Ur Rahman, S.; Nuruzzaman, M.; Chaudhary, S.; Azeem, I.; Mehmood, F.; Duan, A.; Sun, C. Nanotechnology in precision agriculture: Advancing towards sustainable crop production. Plant Physiol. Biochem. 2024, 206, 108244. [Google Scholar] [CrossRef]
- Alvarado, A.M.; Aguirre-Becerra, H.; Vázquez-Hernández, M.C.; Magaña-Lopez, E.; Parola-Contreras, I.; Caicedo-Lopez, L.H.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Influence of Elicitors and Eustressors on the Production of Plant Secondary Metabolites. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Akhtar, M.S., Swamy, M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; pp. 333–388. [Google Scholar]
- Godínez-Mendoza, P.L.; Hurtado-Zuñiga, A.; Siboney-Montante, V.; Guzman-Cruz, R.; Guevara-González, R.G. Eustressors to Improve Plant Secondary Metabolites Production: Insect Frass and Physical Factors as Examples Applied in Agriculture and Horticulture. In Molecular Dynamics of Plant Stress and Its Management; Shahid, M., Gaur, R., Eds.; Springer Nature: Singapore, 2024; pp. 25–49. [Google Scholar]
- Páramo, L.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Esquivel, K. Medicago sativa L. Plant Response against Possible Eustressors (Fe, Ag, Cu)-TiO2: Evaluation of Physiological Parameters, Total Phenol Content, and Flavonoid Quantification. Plants 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Long, T.A. Many needles in a haystack: Cell-type specific abiotic stress responses. Curr. Opin. Plant Biol. 2011, 14, 325–331. [Google Scholar] [CrossRef]
- Berrios, L.; Rentsch, J.D. Linking Reactive Oxygen Species (ROS) to Abiotic and Biotic Feedbacks in Plant Microbiomes: The Dose Makes the Poison. Int. J. Mol. Sci. 2022, 23, 4402. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, Y.Z.; Abdel-Rahman, S.S.A.; Soliman, W.S.; Salaheldin, S. Growth, yield, and quality of roselle (Hibiscus sabdariffa L.) plants as affected by nano zinc and bio-stimulant treatments. Hortic. Environ. Biotechnol. 2021, 62, 879–890. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Munzoor Hasan, S.M.; Angers, P.; Arul, J. UV-B radiation hormesis in broccoli florets: Glucosinolates and hydroxy-cinnamates are enhanced by UV-B in florets during storage. Postharvest Biol. Technol. 2020, 168, 111278. [Google Scholar] [CrossRef]
- Neugart, S.; Bumke-Vogt, C. Flavonoid Glycosides in Brassica Species Respond to UV-B Depending on Exposure Time and Adaptation Time. Molecules 2021, 26, 494. [Google Scholar] [CrossRef]
- Sidibé, A.; Charles, M.T.; Lucier, J.-F.; Xu, Y.; Beaulieu, C. Preharvest UV-C Hormesis Induces Key Genes Associated With Homeostasis, Growth and Defense in Lettuce Inoculated with Xanthomonas campestris pv. vitians. Front. Plant Sci. 2022, 12, 793989. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.T.D.; Puthur, J.T. UV-B priming enhances specific secondary metabolites in Oryza sativa (L.) empowering to encounter diverse abiotic stresses. Plant Growth Regul. 2020, 92, 169–180. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (LED) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, L.; Chen, M.; Zhang, L.; Lu, Q.; Wei, J.; Duan, X. Hormesis Responses of Growth and Photosynthetic Characteristics in Lonicera japonica Thunb. to Cadmium Stress: Whether Electric Field Can Improve or Not? Plants 2023, 12, 933. [Google Scholar] [CrossRef]
- Bhandawat, A.; Jayaswall, K.; Sharma, H.; Roy, J. Sound as a stimulus in associative learning for heat stress in Arabidopsis. Commun. Integr. Biol. 2020, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.; Guevara-Gonzalez, R.G.; Andrade, J.E.; Esquivel-Delgado, A.; Perez-Matzumoto, A.E.; Torres-Pacheco, I.; Contreras-Medina, L.M. Effect of hydric stress-related acoustic emission on transcriptional and biochemical changes associated with a water deficit in Capsicum annuum L. Plant Physiol. Biochem. 2021, 165, 251–264. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Feregrino-Pérez, A.A.; Esquivel, K.; Perez-Garcia, C.E.; Vazquez-Hernandez, M.C.; Mariana-Alvarado, A. Nanomaterials as an alternative to increase plant resistance to abiotic stresses. Front. Plant Sci. 2022, 13, 1023636. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef] [PubMed]
- Humbal, A.; Pathak, B. Influence of exogenous elicitors on the production of secondary metabolite in plants: A review (“VSI: Secondary metabolites”). Plant Stress. 2023, 8, 100166. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal Nanoparticles in Agriculture: A Review of Possible Use. Coatings 2022, 12, 1586. [Google Scholar] [CrossRef]
- Kirova, E.; Geneva, M.; Petrova, M.; Miladinova-Georgieva, K.; Sichanova, M. Employment of nanoparticles for improvement of plant growth and development. Botanica 2022, 28, 113–132. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.u.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Dasari, R.; Gopu, C.; Vankudoth, S.; Dharavath, S.; Taduri, S. Enhancement of production of pharmaceutically important anti-cancerous compound; cucurbitacin E via elicitation and precursor feeding of in vitro culture of Citrullus colocynthis (L.) Schard. Vegetos 2020, 33, 323–334. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, M.; Kumar, A.; Pandey, K.D. 12—Biosynthesis of nanoparticles and applications in agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 199–217. [Google Scholar]
- Uddin, M.; Bhat, U.H.; Singh, S.; Singh, S.; Chishti, A.S.; Khan, M.M.A. Combined application of SiO2 and TiO2 nanoparticles enhances growth characters, physiological attributes and essential oil production of Coleus aromatics Benth. Heliyon 2023, 9, e21646. [Google Scholar] [CrossRef]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019, 9, 10372. [Google Scholar] [CrossRef]
- Nokandeh, S.; Ramezani, M.; Gerami, M. The physiological and biochemical responses to engineered green graphene/metal nanocomposites in Stevia rebaudiana. J. Plant Biochem. Biotechnol. 2021, 30, 579–585. [Google Scholar] [CrossRef]
- Magaña-López, E.; Palos-Barba, V.; Zuverza-Mena, N.; Vázquez-Hernández, M.C.; White, J.C.; Nava-Mendoza, R.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R.G. Nanostructured mesoporous silica materials induce hormesis on chili pepper (Capsicum annuum L.) under greenhouse conditions. Heliyon 2022, 8, e09049. [Google Scholar] [CrossRef]
- Guzmán-Báez, G.A.; Trejo-Téllez, L.I.; Ramírez-Olvera, S.M.; Salinas-Ruíz, J.; Bello-Bello, J.J.; Alcántar-González, G.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Silver Nanoparticles Increase Nitrogen, Phosphorus, and Potassium Concentrations in Leaves and Stimulate Root Length and Number of Roots in Tomato Seedlings in a Hormetic Manner. Dose-Response 2021, 19, 15593258211044576. [Google Scholar] [CrossRef]
- Chahardoli, A.; Sharifan, H.; Karimi, N.; Kakavand, S.N. Uptake, translocation, phytotoxicity, and hormetic effects of titanium dioxide nanoparticles (TiO2NPs) in Nigella arvensis L. Sci. Total Environ. 2022, 806, 151222. [Google Scholar] [CrossRef]
- Yadegari, M. Study of phytohormones effects on UV-B stress seeds of thyme species. J. Herb. Drugs 2017, 8, 109–115. [Google Scholar] [CrossRef]
- Volkova, P.Y.; Clement, G.; Makarenko, E.S.; Kazakova, E.A.; Bitarishvili, S.V.; Lychenkova, M.A. Metabolic Profiling of γ-Irradiated Barley Plants Identifies Reallocation of Nitrogen Metabolism and Metabolic Stress Response. Dose-Response 2020, 18, 1559325820914186. [Google Scholar] [CrossRef]
- Orlando, M.; Trivellini, A.; Puccinelli, M.; Ferrante, A.; Incrocci, L.; Mensuali-Sodi, A. Increasing the functional quality of Crocus sativus L. by-product (tepals) by controlling spectral composition. Hortic. Environ. Biotechnol. 2022, 63, 363–373. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Zare Mehrjerdi, M.; Dianati Daylami, S.; Serek, M.; Woltering, E.; Li, T. Blue Light Improves Vase Life of Carnation Cut Flowers Through Its Effect on the Antioxidant Defense System. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Shabrangy, A. Using Magnetic Fields to Enhance the Seed Germination, Growth, and Yield of Plants. In Plant Functional Genomics: Methods and Protocols; Maghuly, F., Ed.; Springer US: New York, NY, USA, 2024; Volume 2, pp. 375–395. [Google Scholar]
- Afzal, I.; Saleem, S.; Skalicky, M.; Javed, T.; Bakhtavar, M.A.; ul Haq, Z.; Kamran, M.; Shahid, M.; Sohail Saddiq, M.; Afzal, A.; et al. Magnetic Field Treatments Improves Sunflower Yield by Inducing Physiological and Biochemical Modulations in Seeds. Molecules 2021, 26, 2022. [Google Scholar] [CrossRef]
- Paponov, I.A.; Fliegmann, J.; Narayana, R.; Maffei, M.E. Differential root and shoot magnetoresponses in Arabidopsis thaliana. Sci. Rep. 2021, 11, 9195. [Google Scholar] [CrossRef]
- Rodrigo-Moreno, A.; Bazihizina, N.; Azzarello, E.; Masi, E.; Tran, D.; Bouteau, F.; Baluska, F.; Mancuso, S. Root phonotropism: Early signalling events following sound perception in Arabidopsis roots. Plant Sci. 2017, 264, 9–15. [Google Scholar] [CrossRef]
- Javed, R.; Khan, B.; Sharafat, U.; Bilal, M.; Galagedara, L.; Abbey, L.; Cheema, M. Dynamic interplay of metal and metal oxide nanoparticles with plants: Influencing factors, action mechanisms, and assessment of stimulatory and inhibitory effects. Ecotoxicol. Environ. Saf. 2024, 271, 115992. [Google Scholar] [CrossRef]
- Venegas-Molina, J.; Proietti, S.; Pollier, J.; Orozco-Freire, W.; Ramirez-Villacis, D.; Leon-Reyes, A. Induced tolerance to abiotic and biotic stresses of broccoli and Arabidopsis after treatment with elicitor molecules. Sci. Rep. 2020, 10, 10319. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011, 62, 4087–4100. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019, 196, 111509. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and Plant Growth Regulators on In Vitro Proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef]
- Hectors, K.; Prinsen, E.; De Coen, W.; Jansen, M.A.K.; Guisez, Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 2007, 175, 255–270. [Google Scholar] [CrossRef]
- Aksakal, O.; Tabay, D.; Esringu, A.; Icoglu Aksakal, F.; Esim, N. Effect of proline on biochemical and molecular mechanisms in lettuce (Lactuca sativa L.) exposed to UV-B radiation. Photochem. Photobiol. Sci. 2017, 16, 246–254. [Google Scholar] [CrossRef]
- Yadav, D.; Tanveer, A.; Malviya, N.; Yadav, S. Chapter 1—Overview and Principles of Bioengineering: The Drivers of Omics Technologies. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–23. [Google Scholar]
- Appolloni, E.; Paucek, I.; Pennisi, G.; Manfrini, L.; Gabarrell, X.; Gianquinto, G.; Orsini, F. Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity. Agronomy 2023, 13, 671. [Google Scholar] [CrossRef]
- Tiwari, V.; Kamara, I.; Ratner, K.; Many, Y.; Lukyanov, V.; Ziv, C.; Gilad, Z.; Esquira, I.; Charuvi, D. Daytime or Edge-of-Daytime Intra-Canopy Illumination Improves the Fruit Set of Bell Pepper at Passive Conditions in the Winter. Plants 2022, 11, 424. [Google Scholar] [CrossRef]
- Liu, W.; Zha, L.; Zhang, Y. Growth and Nutrient Element Content of Hydroponic Lettuce are Modified by LED Continuous Lighting of Different Intensities and Spectral Qualities. Agronomy 2020, 10, 1678. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of Pre-Sowing Magnetic Field Treatment on Enzymes and Phytohormones in Pea (Pisum sativum L.) Seeds and Seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Sun, P.; Zheng, F.; Wang, K.; Zhong, M.; Wu, D.; Zhu, H. Electro- and Magneto-Modulated Ion Transport through Graphene Oxide Membranes. Sci. Rep. 2014, 4, 6798. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Ghanati, F.; Abdolmaleki, P.; Payez, A. Antioxidant capacity of parsley cells (Petroselinum crispum L.) in relation to iron-induced ferritin levels and static magnetic field. Electromagn. Biol. Med. 2013, 32, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Del Stabile, F.; Marsili, V.; Forti, L.; Arru, L. Is There a Role for Sound in Plants? Plants 2022, 11, 2391. [Google Scholar] [CrossRef]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Cho, J.-I.; Park, S.-H.; Kim, K.-H.; Lee, S.; Kwon, T.-R.; Park, S.-C.; Siddiqui, Z.S. Sound frequencies induce drought tolerance in rice plant. Pak. J. Bot. 2014, 46, 2015–2020. [Google Scholar]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Garcia-Mier, L.; Jimenez-Garcia, S.N.; Contreras-Medina, L.M. Effects of acoustic waves on plants: An agricultural, ecological, molecular and biochemical perspective. Sci. Hortic. 2018, 235, 340–348. [Google Scholar] [CrossRef]
- Telewski, F.W. A unified hypothesis of mechanoperception in plants. Am. J. Bot. 2006, 93, 1466–1476. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28. [Google Scholar] [CrossRef]
- Moola, A.K.; Senthil Kumar, T.; Ranjitha Kumari, B.D. Enhancement of Celastrol compound by silver nanoparticles and acetosyringone in Celastrus paniculatus Willd. through adventitious and hairy root culture. J. Plant Biochem. Biotechnol. 2022, 31, 429–434. [Google Scholar] [CrossRef]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO(2) Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Xia, L.; Huang, H.; Feng, W.; Chen, Y. Silica nanoparticles boost plant resistance against pathogens. Sci. Bull. 2021, 66, 1151–1153. [Google Scholar] [CrossRef]
- Miteu, G.D.; Emmanuel, A.A.; Addeh, I.; Ojeokun, O.; Olayinka, T.; Godwin, J.S.; Adeyemo, O.I.; Benneth, E.O. Nanoscience and technology as a pivot for sustainable agriculture and its One Health approach awareness. Sci. One Health 2023, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Jadid, N.; Widodo, A.F.; Ermavitalini, D.; Sa’adah, N.N.; Gunawan, S.; Nisa, C. The medicinal Umbelliferae plant Fennel (Foeniculum vulgare Mill.): Cultivation, traditional uses, phytopharmacological properties, and application in animal husbandry. Arab. J. Chem. 2023, 16, 104541. [Google Scholar] [CrossRef]
- Jadid, N.; Ramadani, M.R.N.; Widodo, A.F.; Sa’adah, N.N.; Ermavitalini, D.; Rahmawati, M.; Sari, S.A.; Soleha, I.D.; Mas’ud, F. In silico characterization of GbPAL, GbCHS, GbDFR and GbANS structural genes involved in the biosynthesis of flavonoids in Gynura bicolor DC. S. Afr. J. Bot. 2024, 165, 428–442. [Google Scholar] [CrossRef]
- Rohela, G.K.; Saini, P.; Aziz, D.; Rafiq, S.; Jogam, P.; Zhang, B. Nanoparticles as elicitors and stimulators for plant tissue culture, transgenics, and genome editing: A comprehensive review. Ind. Crops Prod. 2024, 222, 120097. [Google Scholar] [CrossRef]
- Kruszka, D.; Selvakesavan, R.K.; Kachlicki, P.; Franklin, G. Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind. Crops Prod. 2022, 178, 114561. [Google Scholar] [CrossRef]
- Yeow, L.C.; Chew, B.L.; Sreeramanan, S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol. Rep. 2020, 27, e00497. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.T.; Moe, M.M.; Kim, Y.J.; Bang, K.S. Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr. Molecules 2019, 24, 4336. [Google Scholar] [CrossRef] [PubMed]
- Poornananda, M.N.; Jameel, M.A.K. Abiotic and Biotic Elicitors–Role in Secondary Metabolites Production through In Vitro Culture of Medicinal Plants. In Abiotic and Biotic Stress in Plants; Arun, K.S., Chitra, S., Eds.; IntechOpen: Rijeka, Croatia, 2016; Chapter 10. [Google Scholar]
- Golovatskaya, I.F.; Karnachuk, R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2015, 62, 727–740. [Google Scholar] [CrossRef]
- Veits, M.; Khait, I.; Obolski, U.; Zinger, E.; Boonman, A.; Goldshtein, A.; Saban, K.; Seltzer, R.; Ben-Dor, U.; Estlein, P.; et al. Flowers respond to pollinator sound within minutes by increasing nectar sugar concentration. Ecol. Lett. 2019, 22, 1483–1492. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef]
- Appel, H.; Cocroft, R. Plant ecoacoustics: A sensory ecology approach. Trends Ecol. Evol. 2023, 38, 623–630. [Google Scholar] [CrossRef]
- Mann, V.; Harker, M.; Pecker, I.; Hirschberg, J. Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotechnol. 2000, 18, 888–892. [Google Scholar] [CrossRef]
- Sato, F.; Hashimoto, T.; Hachiya, A.; Tamura, K.; Choi, K.B.; Morishige, T.; Fujimoto, H.; Yamada, Y. Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 367–372. [Google Scholar] [CrossRef]
- Vom Endt, D.; Kijne, J.W.; Memelink, J. Transcription factors controlling plant secondary metabolism: What regulates the regulators? Phytochemistry 2002, 61, 107–114. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, D.; Purwar, S.; Kaushik, P.; Sharma, V.; Kumar, H.; Rai, A.; Singh, C.M.; Kamaluddin; Dubey, R.B. Applications of Molecular Markers for Developing Abiotic-Stress-Resilient Oilseed Crops. Life 2023, 13, 88. [Google Scholar] [CrossRef]
- Sinaga, A.O.Y.; Marpaung, D.S.S. Abiotic stress-induced gene expression in pineapple as a potential genetic marker. Adv. Agrochem. 2024, 3, 133–142. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Z.; Hu, Z.; Wang, T.; Teng, L.; Dai, T.; Liu, P.; Hao, J.; Liu, X. Utilizing metabolomic approach to study the mode of action of fungicides and corresponding resistance in plant pathogens. Adv. Agrochem. 2024, 3, 197–205. [Google Scholar] [CrossRef]
- Padilla, Y.G.; Miras-Moreno, B.; Gisbert-Mullor, R.; Lucini, L.; López-Galarza, S.; Calatayud, Á. Leaves and roots metabolomic signatures underlying rootstock-mediated water stress tolerance in grafted pepper plants. Plant Stress 2024, 13, 100542. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Xia, Y.; Xu, J.; Li, Q.; Zhang, C.; Li, M. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020, 103, 304–320. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Metabolomics for assessing safety and quality of plant-derived food. Food Res. Int. 2013, 54, 1172–1183. [Google Scholar] [CrossRef]
- Oh, S.; Lu, C. Vertical farming—Smart urban agriculture for enhancing resilience and sustainability in food security. J. Hortic. Sci. Biotechnol. 2023, 98, 133–140. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Impact of Abiotic Elicitors on In vitro Production of Plant Secondary Metabolites: A Review. J. Adv. Res. Biotech. 2016, 1, 7. [Google Scholar]
- Zhao, J.L.; Zou, L.; Zhang, C.Q.; Li, Y.Y.; Peng, L.X.; Xiang, D.B.; Zhao, G. Efficient production of flavonoids in Fagopyrum tataricum hairy root cultures with yeast polysaccharide elicitation and medium renewal process. Pharmacogn. Mag. 2014, 10, 234–240. [Google Scholar] [CrossRef]
- Ming, Q.; Su, C.; Zheng, C.; Jia, M.; Zhang, Q.; Zhang, H.; Rahman, K.; Han, T.; Qin, L. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag+, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Qin, Q.-P.; Wang, Z.-Y.; Liu, J.; Fu, Y.-J. Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crops Prod. 2018, 124, 28–35. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Naghavi, M.R. Production and gene expression of morphinan alkaloids in hairy root culture of Papaver orientale L. using abiotic elicitors. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Liu, R.; Bassalo, M.C.; Zeitoun, R.I.; Gill, R.T. Genome scale engineering techniques for metabolic engineering. Metab. Eng. 2015, 32, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, K.; Martínez-Chávez, L.A.; Rosales-Pérez, A.; Hernández-Rangel, R. Chapter 1 Nanomaterials: Classification, synthesis methods, and physicochemical characterization. In Engineered Nanoparticles in Agriculture; Walter de Gruyter GmbH: Berlin, Germany, 2023; pp. 1–58. [Google Scholar] [CrossRef]
- Bratovcic, A.; Hikal, W.M.; Mehdizadeh, M.; Hussein, A.H.S.A.A.; Omidi, A.; Adetunji, C.O.; Omorefosa, O.O.; Bera, A. Application of Nanotechnology in Agroecosystems: Nanoparticles for Improving Agricultural Production. Rev. Agric. Sci. 2023, 11, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Santás-Miguel, V.; Arias-Estévez, M.; Rodríguez-Seijo, A.; Arenas-Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef]
- Ndaba, B.; Roopnarain, A.; Rama, H.; Maaza, M. Biosynthesized metallic nanoparticles as fertilizers: An emerging precision agriculture strategy. J. Integr. Agric. 2022, 21, 1225–1242. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Vargas-Ortiz, J.R.; Gonzalez, C.; Esquivel, K. Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes 2022, 10, 2282. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, P.; Mishra, V. Recent Advances on Classification, Properties, Synthesis, and Characterization of Nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 83–97. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Hassanien, H.; Darweesh, M. Nanomaterials: Classification and Properties-Part I. J. Nanosci. 2018, 1, 1–11. [Google Scholar]
- García-Ovando, A.E.; Ramírez Piña, J.E.; Esquivel Naranjo, E.U.; Cervantes Chávez, J.A.; Esquivel, K. Biosynthesized nanoparticles and implications by their use in crops: Effects over physiology, action mechanisms, plant stress responses and toxicity. Plant Stress 2022, 6, 109. [Google Scholar] [CrossRef]
- Asha, A.B.; Narain, R. Chapter 15—Nanomaterials properties. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–359. [Google Scholar]
- Karunakaran, G.; Sudha, K.G.; Ali, S.; Cho, E.-B. Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules 2023, 28, 4527. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Rani, N.; Kumari, K.; Sangwan, P.; Barala, P.; Yadav, J.; Vijeta; Rahul; Hooda, V. Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata. Sustainability 2022, 14, 8251. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Gandhi, N.; Shruthi, Y.; Sirisha, G.; Anusha, C.R. Facile and Eco-Friendly Method for Synthesis of Calcium Oxide (CaO) Nanoparticles and its Potential Application in Agriculture. Haya Saudi J. Life Sci. 2021, 6, 89–103. [Google Scholar]
- Sheoran, P.; Goel, S.; Boora, R.; Kumari, S.; Yashveer, S.; Grewal, S. Biogenic synthesis of potassium nanoparticles and their evaluation as a growth promoter in wheat. Plant Gene 2021, 27, 310. [Google Scholar] [CrossRef]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial activity of CaO nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Zhang, S.; Sharifan, H.; Ma, X. Elucidating the Effects of Cerium Oxide Nanoparticles and Zinc Oxide Nanoparticles on Arsenic Uptake and Speciation in Rice (Oryza sativa) in a Hydroponic System. Environ. Sci. Technol. 2018, 52, 10040–10047. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; González-Arias, B.; Rosales, A.; Esquivel, K.; Escamilla-Silva, E.M.; Ortega-Torres, A.E.; Guevara-González, R.G. Elicitation of Bacillus cereus-Amazcala (B.c-A) with SiO2 Nanoparticles Improves Its Role as a Plant Growth-Promoting Bacteria (PGPB) in Chili Pepper Plants. Plants 2022, 11, 3445. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.u.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 2016, 159, 326–334. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Tariverdizadeh, N.; Mohebodini, M.; Chamani, E.; Ebadi, A. Iron and zinc oxide nanoparticles: An efficient elicitor to enhance trigonelline alkaloid production in hairy roots of fenugreek. Ind. Crops Prod. 2021, 162, 113240. [Google Scholar] [CrossRef]
- Yousaf, N.; Ishfaq, M.; Qureshi, H.A.; Saleem, A.; Yang, H.; Sardar, M.F.; Zou, C. Characterization of Root and Foliar-Applied Iron Oxide Nanoparticles (α-Fe2O3, γ-Fe2O3, Fe3O4, and Bulk Fe3O4) in Improving Maize (Zea mays L.) Performance. Nanomaterials 2023, 13, 3036. [Google Scholar] [CrossRef] [PubMed]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M.d.l.L. Impact on Some Soil Physical and Chemical Properties Caused by Metal and Metallic Oxide Engineered Nanoparticles: A Review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dou, F.; Li, C.; Ma, X.; Ma, L.Q. Impacts of metallic nanoparticles and transformed products on soil health. Crit. Rev. Environ. Sci. Technol. 2021, 51, 973–1002. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Syed, A.; Jailani, A.; Elgorban, A.M.; Khan, M.S.; Al-Shwaiman, H.A.; Lee, J. Differential bioaccumulations and ecotoxicological impacts of metal-oxide nanoparticles, bulk materials, and metal-ions in cucumbers grown in sandy clay loam soil. Environ. Pollut. 2021, 289, 117854. [Google Scholar] [CrossRef]
- Peng, C.; Tong, H.; Shen, C.; Sun, L.; Yuan, P.; He, M.; Shi, J. Bioavailability and translocation of metal oxide nanoparticles in the soil-rice plant system. Sci. Total Environ. 2020, 713, 136662. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.Y.; Jurng, J.; Park, Y.K. The synthesis and coating process of TiO2 nanoparticles using CVD process. Powder Technol. 2011, 214, 64–68. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Azar, S.M.A.; Mousa, A.A. 8—Mechanical and physical methods for the metal oxide powders production. In Metal Oxide Powder Technologies; Al-Douri, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–187. [Google Scholar]
- Kalyani, N.T.; Dhoble, S.J. 1—Introduction to nano materials. In Quantum Dots; Thejo Kalyani, N., Dhoble, S.J., Michalska-Domańska, M., Vengadaesvaran, B., Nagabhushana, H., Arof, A.K., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 3–40. [Google Scholar]
- Chen, X.; Gao, C.; Guo, L.; Hu, G.; Luo, Q.; Liu, J.; Nielsen, J.; Chen, J.; Liu, L. DCEO Biotechnology: Tools to Design, Construct, Evaluate, and Optimize the Metabolic Pathway for Biosynthesis of Chemicals. Chem. Rev. 2018, 118, 4–72. [Google Scholar] [CrossRef] [PubMed]
- Bhati, M. Biogenic synthesis of metallic nanoparticles: Principles and applications. Mater. Today Proc. 2021, 81, 882–887. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Aqeel Salim, A.; Bidin, N.; Bakhtiar, H.; Krishna Ghoshal, S.; Al Azawi, M.; Krishnan, G. Optical and structure characterization of cinnamon nanoparticles synthesized by pulse laser ablation in liquid (PLAL). J. Phys. Conf. Ser. 2018, 1027, 012002. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vazquez, M.; Brabazon, D. A Review of Bimetallic and Monometallic Nanoparticle Synthesis via Laser Ablation in Liquid. Crystals 2023, 13, 253. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef]
- Khairani, I.Y.; Mínguez-Vega, G.; Doñate-Buendía, C.; Gökce, B. Green nanoparticle synthesis at scale: A perspective on overcoming the limits of pulsed laser ablation in liquids for high-throughput production. Phys. Chem. Chem. Phys. 2023, 25, 19380–19408. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, S.; Kodam, K.; Dhaygude, M.; Hudlikar, M. Novel route for rapid biosynthesis of lead nanoparticles using aqueous extract of Jatropha curcas L. latex. Mater. Lett. 2011, 65, 3170–3172. [Google Scholar] [CrossRef]
- Laokula, P.; Klinkaewnaronga, J.; Phokha, S.; Seraphin, S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. Optoelectron. Adv. Mater. Rapid Commun. 2008, 2, 101174. [Google Scholar]
- Torres-Limiñana, J.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Cervantes-Chávez, J.A.; Esquivel, K. Green Synthesis via Eucalyptus globulus L. Extract of Ag-TiO2 Catalyst: Antimicrobial Activity Evaluation toward Water Disinfection Process. Nanomaterials 2022, 12, 1944. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Ahamed, M.; Majeed Khan, M.A.; Siddiqui, M.K.J.; Alsalhi, M.S.; Alrokayan, S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low-Dimens. Syst. Nanostructures 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 368, 58–63. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.-L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant-Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 2009, 29, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Sangar, S.; Sharma, S.; Vats, V.K.; Mehta, S.K.; Singh, K. Biosynthesis of silver nanocrystals, their kinetic profile from nucleation to growth and optical sensing of mercuric ions. J. Clean. Prod. 2019, 228, 294–302. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Antunes Filho, S.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- El-Kemary, M.; Zahran, M.; Khalifa, S.A.M.; El-Seedi, H.R. Spectral characterisation of the silver nanoparticles biosynthesised using Ambrosia maritima plant. Micro Nano Lett. 2016, 11, 311–314. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab. J. Chem. 2018, 11, 1097–1106. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid. Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef]
- Mishra, D.; Chitara, M.K.; Negi, S.; Pal Singh, J.; Kumar, R.; Chaturvedi, P. Biosynthesis of Zinc Oxide Nanoparticles via Leaf Extracts of Catharanthus roseus (L.) G. Don and Their Application in Improving Seed Germination Potential and Seedling Vigor of Eleusine coracana (L.) Gaertn. Adv. Agric. 2023, 2023, 7412714. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Cyndrowska, J.; Rutkowski, B.; Hessel, V. Synthesis of Gold Clusters and Nanoparticles Using Cinnamon Extract—A Mechanism and Kinetics Study. Molecules 2024, 29, 1426. [Google Scholar] [CrossRef]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Dehari, D.; Nath, G.; Singh, M. Facile green synthesis of silver nanoparticles derived from the medicinal plant Clerodendrum serratum and its biological activity against Mycobacterium species. Heliyon 2024, 10, e31116. [Google Scholar] [CrossRef] [PubMed]
- Bahattab, O.; Khan, I.; Bawazeer, S.; Rauf, A.; Qureshi, M.N.; Al-Awthan, Y.S.; Muhammad, N.; Khan, A.; Akram, M.; Islam, M.N.; et al. Synthesis and biological activities of alcohol extract of black cumin seeds (Bunium persicum)-based gold nanoparticles and their catalytic applications. Green. Process. Synth. 2021, 10, 440–455. [Google Scholar] [CrossRef]
- Gnanasekaran, R.; Yuvaraj, D.; Reddy, G.K.; Shangar, S.N.; Vijayakumar, V.; Iyyappan, J. Zinc oxide nanoparticles from leaf extract of Eclipta prostrata: Biosynthesis and characterization towards potential agent against film forming bacteria in metal working fluids. Environ. Chem. Ecotoxicol. 2024, 6, 206–215. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Tan, X.; Wang, Z.; Li, Y.; Li, W. Extract of Ginkgo biloba leaves mediated biosynthesis of catalytically active and recyclable silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 31–36. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayaraa, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G.-D. Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater. Res. 2020, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Samari, F.; Salehipoor, H.; Eftekhar, E.; Yousefinejad, S. Low-temperature biosynthesis of silver nanoparticles using mango leaf extract: Catalytic effect, antioxidant properties, anticancer activity and application for colorimetric sensing. New J. Chem. 2018, 42, 15905–15916. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Ngom, I.; Ndiaye, N.M.; Fall, A.; Bakayoko, M.; Ngom, B.D.; Maaza, M. On the Use of Moringa Oleifera Leaves Extract for the Biosynthesis of NiO and ZnO Nanoparticles. MRS Adv. 2020, 5, 1145–1155. [Google Scholar] [CrossRef]
- Selvaraj, R.; Nagendran, V.; Varadavenkatesan, T.; Goveas, L.C.; Vinayagam, R. Stable silver nanoparticles synthesis using Tabebuia aurea leaf extract for efficient water treatment: A sustainable approach to environmental remediation. Chem. Eng. Res. Des. 2024, 208, 456–463. [Google Scholar] [CrossRef]
- Vincent, J.; Lau, K.S.; Evyan, Y.C.-Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312. [Google Scholar] [CrossRef] [PubMed]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Patiño-Ruiz, D.; Sánchez-Botero, L.; Tejeda-Benitez, L.; Hinestroza, J.; Herrera, A. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: Nanotoxicological considerations for potential environmental applications. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100377. [Google Scholar] [CrossRef]
- Huang, Y.; Haw, C.Y.; Zheng, Z.; Kang, J.; Zheng, J.-C.; Wang, H.-Q. Biosynthesis of Zinc Oxide Nanomaterials from Plant Extracts and Future Green Prospects: A Topical Review. Adv. Sustain. Syst. 2021, 5, 2000266. [Google Scholar] [CrossRef]
- Hatipoğlu, A.; Baran, A.; Keskin, C.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Janas, D.; Khalilov, R.; Adican, M.T.; Kandemir, S.İ. Green synthesis of silver nanoparticles based on the Raphanus sativus leaf aqueous extract and their toxicological/microbiological activities. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Raut, R.W.; Mendhulkar, V.D.; Kashid, S.B. Photosensitized synthesis of silver nanoparticles using Withania somnifera leaf powder and silver nitrate. J. Photochem. Photobiol. B Biol. 2014, 132, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.A.; Burmistrov, D.E.; Shumeyko, S.A.; Gudkov, S.V. Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy 2024, 14, 1646. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Fatemi, M.; Mollania, N.; Momeni-Moghaddam, M.; Sadeghifar, F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: Characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J. Biotechnol. 2018, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Kumar, P. Plant Response to Engineered Nanoparticles. Nanomater. Plants Algae Microorg. 2018, 1, 103–118. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; De La Rosa, G.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. XAS Corroboration of the Uptake and Storage of CeO2 Nanoparticles and Assessment of their Differential Toxicity in Four Edible Plant Species. J. Agric. Food Chem. 2010, 58, 3689. [Google Scholar] [CrossRef]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects—Pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, Uptake, and Translocation of Engineered Nanomaterials in Vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. PPB 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Meza, D.Y.; Guevara-González, R.G.; Esquivel, K.; Carbajal-Valenzuela, I.; Avila-Quezada, G.D. Green silver nanoparticles display protection against Clavibacter michiganensis subsp. michiganensis in tomato plants (Solanum lycopersicum L.). Plant Stress 2023, 10, 100256. [Google Scholar] [CrossRef]
- Müller, A.; Behsnilian, D.; Walz, E.; Gräf, V.; Hogekamp, L.; Greiner, R. Effect of culture medium on the extracellular synthesis of silver nanoparticles using Klebsiella pneumoniae, Escherichia coli and Pseudomonas jessinii. Biocatal. Agric. Biotechnol. 2016, 6, 107–115. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. Environ. Sci. Pollut. Res. 2021, 28, 13712–13724. [Google Scholar] [CrossRef]
- Cordoba, A.; Hernández, R.; Viveros-Palma, I.; Mendoza, S.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Esquivel, K. Effect on plant growth parameters and secondary metabolite content of lettuce (Lactuca sativa L.), coriander (Coriandrum sativum), and chili pepper (Capsicum annuum L.) watered with disinfected water by Ag-TiO2 nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 37130–37141. [Google Scholar] [CrossRef]
- Younes, N.A.; Hassan, H.S.; Elkady, M.F.; Hamed, A.M.; Dawood, M.F.A. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 2020, 6, e03188. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.; Seo, S.M.; Kim, D. Physiological effects of zero-valent iron nanoparticles in rhizosphere on edible crop, Medicago sativa (Alfalfa), grown in soil. Ecotoxicology 2019, 28, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Alabdallah, N.; Saeed Alzahrani, H. Impact of ZnO Nanoparticles on Growth of Cowpea and Okra Plants under Salt Stress Conditions. Biosci. Biotechnol. Res. Asia 2020, 17, 329–340. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Abdel-Megeed, A.; El-Settawy, A.A.A.; Böhm, M.; Mansour, M.M.A.; Salem, A.Z.M. Plants-derived bioactives: Novel utilization as antimicrobial, antioxidant and phytoreducing agents for the biosynthesis of metallic nanoparticles. Microb. Pathog. 2021, 158, 105107. [Google Scholar] [CrossRef]
- Wen, Y.; Liao, Y.; Tang, Y.; Zhang, H.; Zhang, J.; Liao, Z. Metabolic Effects of Elicitors on the Biosynthesis of Tropane Alkaloids in Medicinal Plants. Plants 2023, 12, 3050. [Google Scholar] [CrossRef]
- Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Li, X.; Zhang, M.; Zhang, Z.; Lu, T.; Pan, X.; Qian, H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci. Total Environ. 2018, 644, 1070–1079. [Google Scholar] [CrossRef]
- Marano, F.; Hussain, S.; Rodrigues-Lima, F.; Baeza-Squiban, A.; Boland, S. Nanoparticles: Molecular targets and cell signalling. Arch. Toxicol. 2011, 85, 733–741. [Google Scholar] [CrossRef]
- Li, P.; Wang, A.; Du, W.; Mao, L.; Wei, Z.; Wang, S.; Yuan, H.; Ji, R.; Zhao, L. Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level. Sci. Total Environ. 2020, 738, 139795. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics Reveals the “Invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Ribeiro, T.P.; Santos, C.; Pinto, D.C.G.A.; Silva, A.M.S. TiO2 nanoparticles induced sugar impairments and metabolic pathway shift towards amino acid metabolism in wheat. J. Hazard. Mater. 2020, 399, 122982. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ghorbanpour, M.; Kariman, K. Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa belladonna L.). Ind. Crops Prod. 2018, 126, 403–414. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Miras-Moreno, B.; Chehregani Rad, A.; Pii, Y.; Mimmo, T.; Cesco, S.; Lucini, L. Relatively Low Dosages of CeO2 Nanoparticles in the Solid Medium Induce Adjustments in the Secondary Metabolism and Ionomic Balance of Bean (Phaseolus vulgaris L.) Roots and Leaves. J. Agric. Food Chem. 2020, 68, 67–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, G.; Yao, L.; Huang, L.; Wang, J.; Gao, W. Effects of Metal Nanoparticles and Other Preparative Materials in the Environment on Plants: From the Perspective of Improving Secondary Metabolites. J. Agric. Food Chem. 2022, 70, 916–933. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and environmental management through nanotechnology: Eco-friendly nanomaterial synthesis for soil-plant systems, food safety, and sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef]
- Rai, P.K.; Kumar, V.; Lee, S.S.; Raza, N.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef]
- Mura, S.; Greppi, G.; Irudayaraj, J. Latest Developments of Nanotoxicology in Plants; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 125–151. [Google Scholar]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the soil–plant system: A review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Saquib, Q.; Ahamed, M.; Farshori, N.N.; Ahmad, J.; Wahab, R.; Khan, S.T.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A.A.; et al. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloids Surf. B Biointerfaces 2015, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; Fernández van Raap, M.B.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16, 341. [Google Scholar] [CrossRef]
- Kurczyńska, E.; Godel-Jędrychowska, K.; Sala, K.; Milewska-Hendel, A. Nanoparticles—Plant Interaction: What We Know, Where We Are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green. Chem. 2015, 18, 20–52. [Google Scholar] [CrossRef]
- Kaphle, A.; Navya, P.N.; Umapathi, A.; Daima, H.K. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Lett. 2017, 16, 43–58. [Google Scholar] [CrossRef]
- Djibril Sekou, K.; Patel, H. A Review on the interaction between Nanoparticles and Toxic metals in Soil: Meta-analysis of their effects on soil, plants and human health. Soil. Sediment. Contam. Int. J. 2023, 32, 417–447. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Zidan, A.; El-Mageed, A.I.A.A. Preparation methods of different nanomaterials for various potential applications: A review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Lenka, S.K.; Cahill, D.M.; Rookes, J.E. Designer nanoparticles for plant cell culture systems: Mechanisms of elicitation and harnessing of specialized metabolites. BioEssays 2021, 43, 2100081. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, J.; Wang, Y.; Wang, Y. Nano on micro: Tuning microbial metabolisms by nano-based artificial mediators to enhance and expand production of biochemicals. Curr. Opin. Biotechnol. 2020, 64, 161–168. [Google Scholar] [CrossRef]
- Kuska, J.; O’Reilly, E. Engineered biosynthetic pathways and biocatalytic cascades for sustainable synthesis. Curr. Opin. Chem. Biol. 2020, 58, 146–154. [Google Scholar] [CrossRef]
- Matur, M.; Madhyastha, H.; Shruthi, T.S.; Madhyastha, R.; Srinivas, S.P.; Navya, P.N.; Daima, H.K. Engineering bioactive surfaces on nanoparticles and their biological interactions. Sci. Rep. 2020, 10, 19713. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Xie, X.; Zheng, W.; Li, A.; Liu, Y.; Liu, X.; Zhang, R.; Deng, C.; Cheng, J.; et al. Exploring the Biological Effect of Biosynthesized Au–Pd Core–Shell Nanoparticles through an Untargeted Metabolomics Approach. ACS Appl. Mater. Interfaces 2021, 13, 59633–59648. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.-H. Advantage of Nanotechnology-Based Genome Editing System and Its Application in Crop Improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Lv, Z.; Jiang, R.; Chen, J.; Chen, W. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. Plant J. 2020, 104, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Jat, S.K.; Bhattacharya, J.; Sharma, M.K. Nanomaterial based gene delivery: A promising method for plant genome engineering. J. Mater. Chem. B 2020, 8, 4165–4175. [Google Scholar] [CrossRef]
- Wu, K.; Xu, C.; Li, T.; Ma, H.; Gong, J.; Li, X.; Sun, X.; Hu, X. Application of Nanotechnology in Plant Genetic Engineering. Int. J. Mol. Sci. 2023, 24, 14836. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Singh, S.; Chattopadhyay, R.; Kaur, J.; Bari, V.K. Integration of CRISPR/Cas9 with multi-omics technologies to engineer secondary metabolite productions in medicinal plant: Challenges and Prospects. Funct. Integr. Genom. 2024, 24, 207. [Google Scholar] [CrossRef] [PubMed]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Yin, H. Recent advances in chemical modifications of guide RNA, mRNA and donor template for CRISPR-mediated genome editing. Adv. Drug Deliv. Rev. 2021, 168, 246–258. [Google Scholar] [CrossRef]
- Chen, X.; Shukal, S.; Zhang, C. Integrating Enzyme and Metabolic Engineering Tools for Enhanced α-Ionone Production. J. Agric. Food Chem. 2019, 67, 13451–13459. [Google Scholar] [CrossRef]
- Hartline, C.J.; Schmitz, A.C.; Han, Y.; Zhang, F. Dynamic control in metabolic engineering: Theories, tools, and applications. Metab. Eng. 2021, 63, 126–140. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef]
- Iula, G.; Miras-Moreno, B.; Rouphael, Y.; Lucini, L.; Trevisan, M. The Complex Metabolomics Crosstalk Triggered by Four Molecular Elicitors in Tomato. Plants 2022, 11, 678. [Google Scholar] [CrossRef]
- García-Pérez, P.; Miras-Moreno, B.; Lucini, L.; Gallego, P.P. The metabolomics reveals intraspecies variability of bioactive compounds in elicited suspension cell cultures of three Bryophyllum species. Ind. Crops Prod. 2021, 163, 113322. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, J.; Li, Y.; Luo, G.; Li, L.; Yuan, H.; Mur, L.A.J.; Guo, S. Negative effects of the simulated nitrogen deposition on plant phenolic metabolism: A meta-analysis. Sci. Total Environ. 2020, 719, 137442. [Google Scholar] [CrossRef]
- Lynch, J.H.; Huang, X.Q.; Dudareva, N. Silent constraints: The hidden challenges faced in plant metabolic engineering. Curr. Opin. Biotechnol. 2021, 69, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Sudani, W.K.K.; Al-Shammari, R.S.S.; Abed, M.S.; Al-Saedi, J.H.; Mernea, M.; Lungu, I.I.; Dumitrache, F.; Mihailescu, D.F. The Impact of ZnO and Fe2O3 Nanoparticles on Sunflower Seed Germination, Phenolic Content and Antiglycation Potential. Plants 2024, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, P.; Wu, Y.; He, Y.; Munir, S. Environmental effect of agriculture-related manufactured nano-objects on soil microbial communities. Environ. Int. 2023, 173, 107819. [Google Scholar] [CrossRef]
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Understanding the Role of Nanomaterials in Agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 2: Functional Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–288. [Google Scholar] [CrossRef]
- Avila-Quezada, G.D.; Ingle, A.P.; Golińska, P.; Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotechnol. Rev. 2022, 11, 2123–2140. [Google Scholar] [CrossRef]
- kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Shang, Y.; Kamrul Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Mujaddidi, N.; Nisa, S.; Al Ayoubi, S.; Bibi, Y.; Khan, S.; Sabir, M.; Zia, M.; Ahmad, S.; Qayyum, A. Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 2021, 28, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Datkhile, K.D.; Patil, S.R.; Durgawale, P.P.; Patil, M.N.; Jagdale, N.J.; Deshmukh, V.N.; More, A.L. Biogenic Silver Nanoparticles Synthesized Using Mexican Poppy Plant Inhibits Cell Growth in Cancer Cells through Activation of Intrinsic Apoptosis Pathway. Nano Biomed. Eng. 2020, 12, 241–252. [Google Scholar] [CrossRef]
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Anjum, S. Nano-Elicitation as an Effective and Emerging Strategy for In Vitro Production of Industrially Important Flavonoids. Appl. Sci. 2021, 11, 1694. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Ko-mariah; et al. Nano-Enabled Products: Challenges and Opportunities for Sustainable Agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Ahmed, T.; Ijaz, U.; Hameed, A.; Shahid, M.; Azizullah; Li, D.; Song, F. Microbe-oriented nanoparticles as phytomedicines for plant health management: An emerging paradigm to achieve global food security. Crit. Rev. Food Sci. Nutr. 2023, 63, 7489–7509. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Shandilya, N.; Marcoulaki, E.; Barruetabeña, L.; Llopis, I.R.; Noorlander, C.; Jiménez, A.S.; Oudart, Y.; Puelles, R.C.; Pérez-Fernández, M.; Falk, A.; et al. Perspective on a risk-based roadmap towards the implementation of the safe innovation approach for industry. NanoImpact 2020, 20, 100258. [Google Scholar] [CrossRef]
- Marcoulaki, E.; López de Ipiña, J.M.; Vercauteren, S.; Bouillard, J.; Himly, M.; Lynch, I.; Witters, H.; Shandilya, N.; van Duuren-Stuurman, B.; Kunz, V.; et al. Blueprint for a self-sustained European Centre for service provision in safe and sustainable innovation for nanotechnology. NanoImpact 2021, 23, 100337. [Google Scholar] [CrossRef]
- Shandilya, N.; Barreau, M.-S.; Suarez-Merino, B.; Porcari, A.; Pimponi, D.; Jensen, K.A.; Fransman, W.; Franken, R. TRAAC framework to improve regulatory acceptance and wider usability of tools and methods for safe innovation and sustainability of manufactured nanomaterials. NanoImpact 2023, 30, 100461. [Google Scholar] [CrossRef]

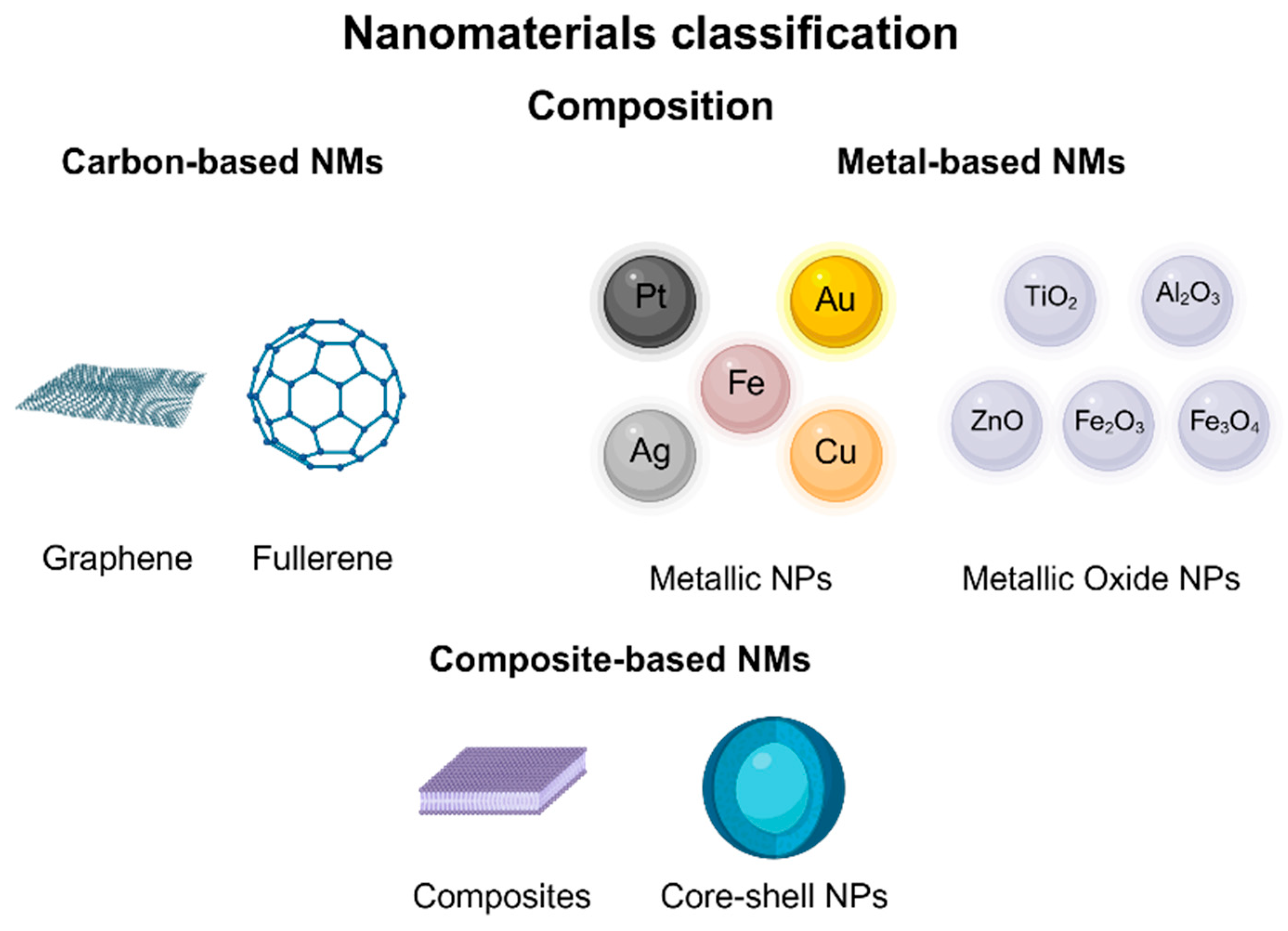


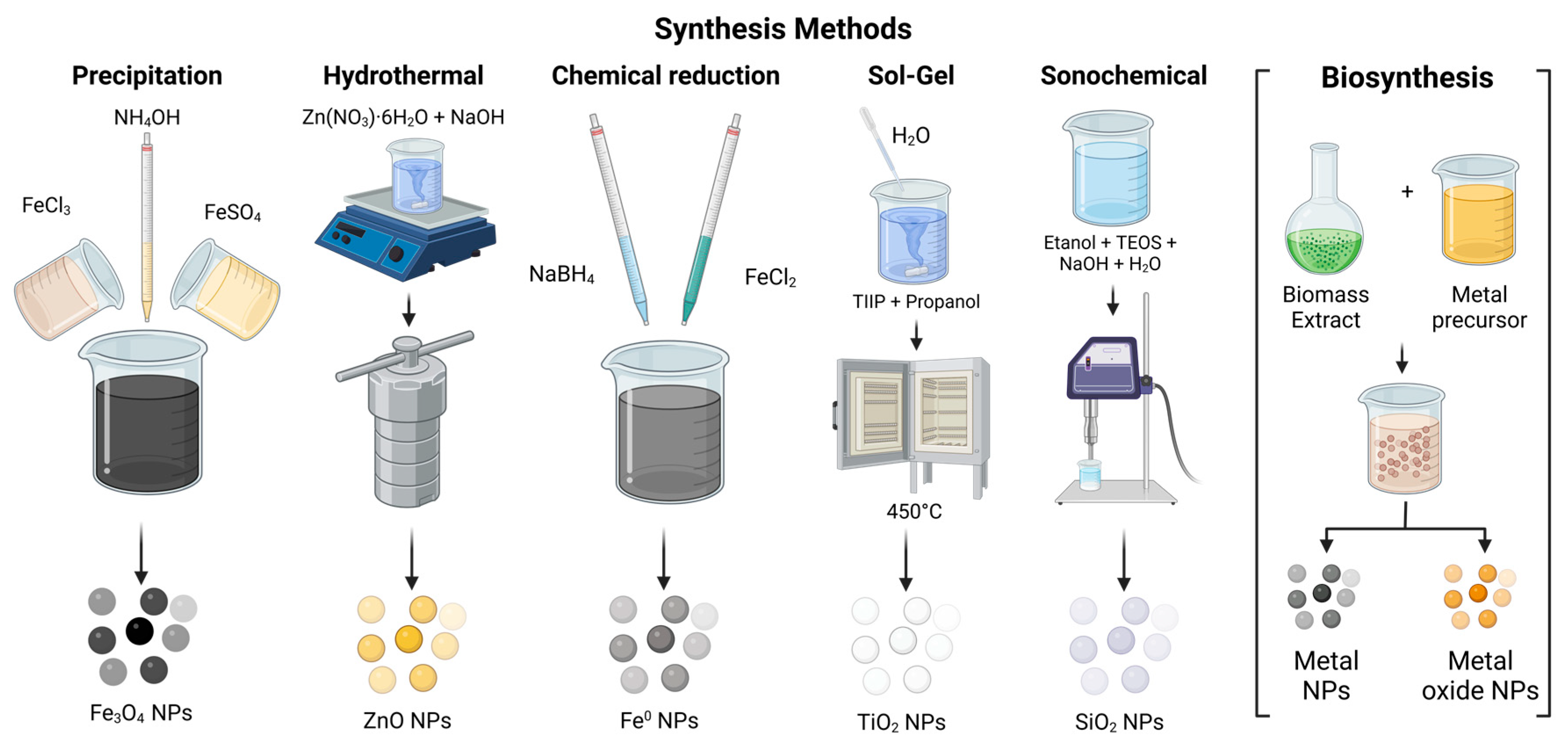
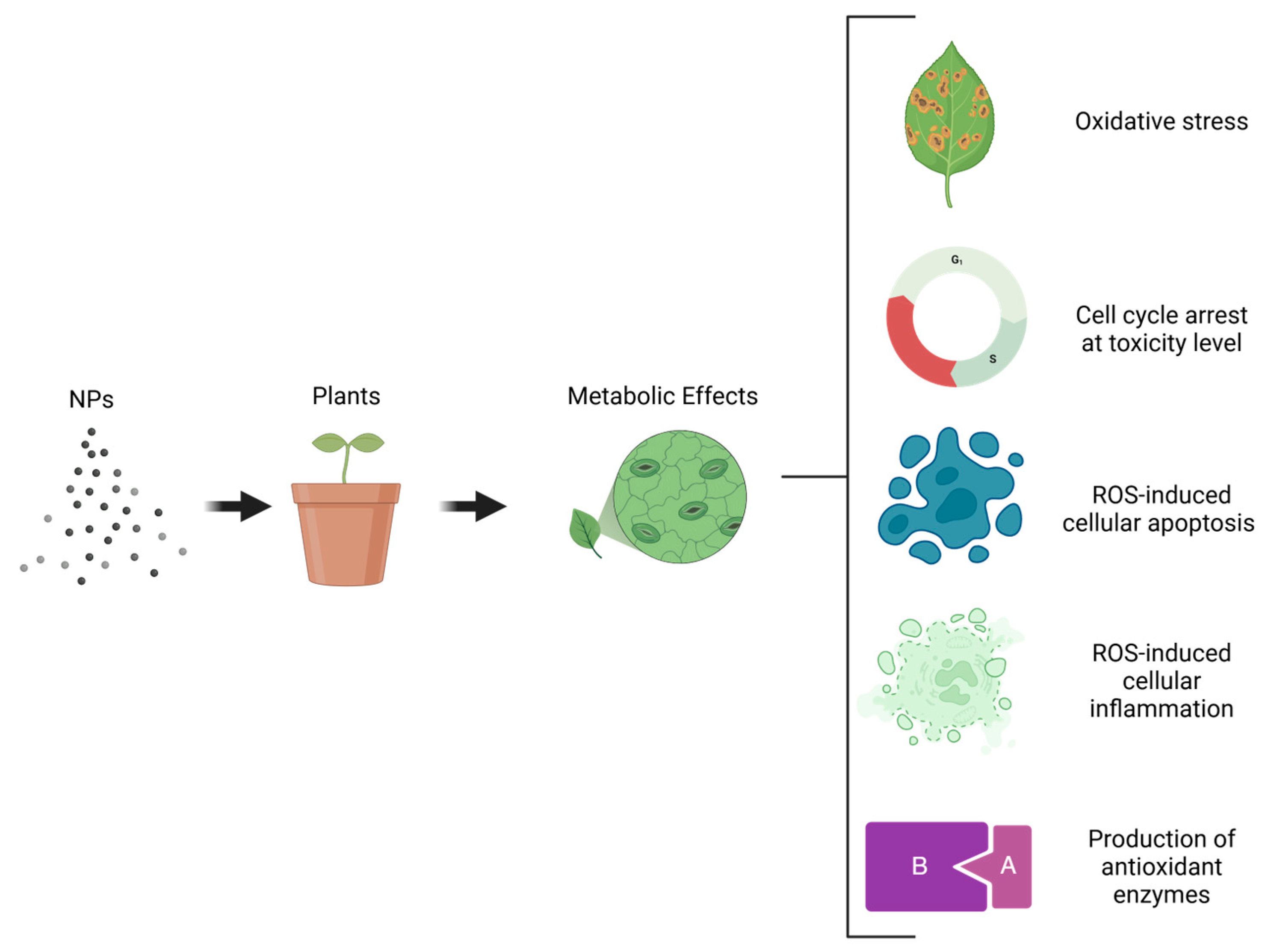
| Factor | Eustressor | Crop | Action | Reference |
|---|---|---|---|---|
| Chemical | Metallic nanoparticles (Ti, Pd, Au, and Ag) | Different crops | The antioxidant defense is activated, the photosynthetic rate is enhanced, plant metabolism is improved, and yield is better. | [66,67,68] |
| Ag NPs (silver nanoparticles), graphene (G), and nanocomposite (Ag NPs/G) | Stevia | Increase total phenolic, flavonoid, chlorophyll, soluble sugar, and protein contents. | [69] | |
| Nanostructured mesoporous silica | Pepper | Hormetic effect on growth, germination, metabolite synthesis, and cross-stress tolerance. | [70] | |
| Silver nanoparticles (Ag NPs) | Tomato | In total, 5 mgL−1 decreases plant height. Increases root length and shoot fresh weight. In total, 20 mgL−1 decreases plant height, root length, and shoot fresh weight. Increases root fresh weight. | [71] | |
| Titanium dioxide nanoparticles (TiO2 NPs) | Wild Fennel | Activation of antioxidant defense and photosynthetic rate, and increases soluble protein, soluble sugar, plant height, root length, and root dry weight. | [72] | |
| Physical | The electric field with Cd-exposure | Honeysuckle | In total, 5 mgL−1 Cd and 2 V cm−1 increase carotenoids, dry weight of root and leaf, and chlorophyll. In total, 5 mgL−1 Cd and 3 V cm−1 decrease the previous parameters. | [50] |
| UV-C | Lettuce | In total, 1.6 kJm−2 increases differentially expressed genes involved in growth and defense. | [44] | |
| UV-B | Broccoli | In total, 1.5 kJm−2 increases glucoraphanin, glucobrassicins, and enzymatic expression. Decreases weight loss and color change in time. In total, 7.2 kJm−2 increases antioxidant activity, weight loss, and color change in time. | [42] | |
| UV-B | Brassicas | Seven consecutive daily doses of 0.5 kJm−2 d−1 (1 h UV-B with acclimation intervals of 23 h) increase flavonoid content. | [43] | |
| UV-B | Thyme | Increases salicylic acid (SA) in seeds. | [73] | |
| UV-B | Rice | Increase flavonoid content and anthocyanins in leaves. | [45] | |
| Gamma-Radiation | Barley | In total, 5, 10, 15, 20, and 100 Gy decrease root length, and 5 and 15 Gy increase shoot and root dry weights, while 100 Gy decreases all factors. | [74] | |
| LED | Sweet pepper | Red and blue. Increases plant height and total leaf number, and stimulates carotenoid and antioxidant levels. | [48] | |
| LED | Saffron | Red (660 nm), blue (450 nm), and green (500–600 nm). Increases flower induction and total flavonoids, flavonols, and flavonol glycosides | [75] | |
| LED | Carnation | White (400–730 nm), blue (460 nm), and red (660 nm). Increases vase-life flower numbers. Decreases carotenoids. | [76] | |
| Magnetic field | Different crops | In total, 0–100 uT, including GMF (geomagnetic fields), Improves germination rate. Increases root and shoot growth, productivity, and increases photosynthetic pigments and the absorption of water and nutrients. | [77,78,79] | |
| Magnetic field | Sunflower Seeds | In total, 100 mT Reduces the mean germination time. Stimulates root length, shoot length, and root–shoot radio. | [80] | |
| Geomagnetic field | Different crops | Regulates gene expression in shoots and roots and suggests involvement in the regulation of reactive oxygen species (ROS) signaling. | [81] | |
| Acoustic emission | Brassicas | In total, 200 Hz changes ion fluxes (Ca2+ and K+) and increases superoxide production. | [82] |
| Biomass Source | Bio-Extract | Nanoparticle | Reference |
|---|---|---|---|
| Bacteria | Rhodococcus species Escherichia coli Sachcharomyces cerevisiae Lactobacillus species Klebsilla pneumonias Enterobacter coacae Bacillus species | Au, Pd, Pt, Ag, Fe3O4, SiO2 | [142,143,152,164,186,195] |
| Fungi | Fusarium oxysporum Aspergillus fumigates Trichoderma reesei Candida albicans Aspergillus terre | Ag, Au, Se | [142,143,152,186,195] |
| Algae | Tetraselmis Kochinensis Klebsormidium flaccidum Lyngbya majuscula Spirulina subsalsa, Fucus vesiculosus Sargassum wightii Chlorella pyrenoidosa Klebsormidium flaccidum Coelastrella sp. Spirulina platensis Chlorococcum humícola Euglena intermedia Uglena gracilis Kappaphycusalvarezii | Au, Ag | [53,142,143,152,162,186,187,190,195,196,197,198,199] |
| Plant Extract | NP Obtained | Size–Shape | Synthesis Conditions | Reference |
|---|---|---|---|---|
| Aloe barbadensis Miller | ZnO | 25–40 nm—spherical | Aloe leaf and aloe gel at 50, 25, 15, 10, 5% concentration 5–6 h at 150 °C | [187] |
| Ambrosia maritime | Ag | 30 nm—spherical | 0.1 mM AgNO3, 1 h at 60 °C | [210] |
| Anogeissus latifolia | Pd | 2–4 nm—spherical | Gum ghati (0.1%–0.5%) and PdCl2 (0.125–1.0 mM), 30 min at 121 °C | [211] |
| Cacumen platycladi | Pt | 2 nm—Spherical | Na2PtCl4 (52.24 mM), 10 min, 30°, 60°, and 90 °C | [212] |
| Catharanthus roseus | ZnO | 43 nm—nonspherical | ZnSO4·7H2O (1 mM); NaOH (2 M), 2 h, 65 °C | [213] |
| Cinnamom zeylanicum | Au | 10–100 nm—spherical | HAuCl4 (0.1 M) and HCl (0.1 M), 24 h, 20–60 °C | [214] |
| Clerodendron serratum L. | Ag | 9–35 nm—spherical | AgNO3 (1 mM), 48 h | [215] |
| Bunium persicum | Au | 25–50 nm—quasi-spherical | HAuCl4 (mM), 4 h | [216] |
| Eclipta prostrata L. | ZnO | <100 nm—rod shaped | ZnSO4 (1 M), 24 h at 200 °C | [217] |
| Eucalyptus globulus L. | Ag Ag-TiO2 TiO2 | 11–14 nm—spherical | AgNO3 (1 mM) 4 h at 40 °C; TTIP (97%) and isopropanol (99%), 30 min at 450 °C | [199] |
| Ginkgo biloba L. | Ag | 20–40 nm—spherical | AgNO3 (2 mM), 60 °C | [218] |
| Jasminum fruticans L. | TiO2 | 31–42 nm | TTIP (0.1 M), 5 h at 110 °C | [219] |
| Lonicera japonica L. | Au | 10–20 nm—spherical, 40 nm—hexagonal | HAuCl4 (0.125, 0.5, 1, 1.5, and 2 mM); 1, 1.5, 2, 2.5, and 3 min; 40, 50, 60, 70, and 80 °C | [220] |
| Magnolia champaca | CuO | 20–40 nm—spherical | Copper acetate (3 mM), 24 h at 37 °C | [221] |
| Mangifera indica L. | Ag | 14–28 nm—quasi-spherical | AgNO3 (1 mM), 80 °C | [222] |
| Morinda citrifolia | TiO2 | 15–19 nm—quasi-spherical | TiCl4 (0.1 M), 8 h, 120 °C | [223] |
| Moringa oleífera | ZnO NiO | Data not available | Zn(NO3)2·6H2O; 2 h at 80 °C Ni(NO3)2·6H2O; 2 h at 80 °C | [224] |
| Tabebuia berteroi | Ag | 48 nm—spherical | AgNO3 (1 mM), 10 min at 80 °C | [225] |
| Tabernaemontana Alternifolia L. | Cu | 10–50 nm—triangular, flaky, and prismatic | 20 min at room temperature | [226] |
| Tie guanyin L. | Fe | 6.6 nm—spherical | FeCl3 (0.05 M), 1 h, 60 °C | [227] |
| Carica papaya L. | α-Fe2O3 | 21.59 nm | FeCl3 (0.1 M), 30 min at room temperature | [228] |
| Cymbopogon citratus | Fe3O4 and γ-Fe2O3 | 2–27 nm—spherical | FeCl3 (0.26 M and 0.52 M), 1 h, 60, and 85 °C | [229] |
| Vitex negundo L. | Ag | 90–120 nm—spherical | AgNO3 (0.1 mM), 2 h | [208] |
| Parthenium hysterophorus L. | ZnO | 16–45 nm—quasi-spherical, radial, and cylindrical | Zn(NO3)2 (1 mM), 24 h | [230] |
| Raphanus sativus var. aegyptiacus | Ag | 9–24 nm—spherical | AgNO3 (15 mM), 10 min at 65 °C | [231] |
| Withania somnifera | Ag | 86 nm | AgNO3 (1 mM), 10 min under sunlight | [232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Chávez, L.A.; Hernández-Ramírez, M.Y.; Feregrino-Pérez, A.A.; Esquivel Escalante, K. Cutting-Edge Strategies to Enhance Bioactive Compound Production in Plants: Potential Value of Integration of Elicitation, Metabolic Engineering, and Green Nanotechnology. Agronomy 2024, 14, 2822. https://doi.org/10.3390/agronomy14122822
Martínez-Chávez LA, Hernández-Ramírez MY, Feregrino-Pérez AA, Esquivel Escalante K. Cutting-Edge Strategies to Enhance Bioactive Compound Production in Plants: Potential Value of Integration of Elicitation, Metabolic Engineering, and Green Nanotechnology. Agronomy. 2024; 14(12):2822. https://doi.org/10.3390/agronomy14122822
Chicago/Turabian StyleMartínez-Chávez, Luis Alejandro, Mariana Y. Hernández-Ramírez, Ana Angélica Feregrino-Pérez, and Karen Esquivel Escalante. 2024. "Cutting-Edge Strategies to Enhance Bioactive Compound Production in Plants: Potential Value of Integration of Elicitation, Metabolic Engineering, and Green Nanotechnology" Agronomy 14, no. 12: 2822. https://doi.org/10.3390/agronomy14122822
APA StyleMartínez-Chávez, L. A., Hernández-Ramírez, M. Y., Feregrino-Pérez, A. A., & Esquivel Escalante, K. (2024). Cutting-Edge Strategies to Enhance Bioactive Compound Production in Plants: Potential Value of Integration of Elicitation, Metabolic Engineering, and Green Nanotechnology. Agronomy, 14(12), 2822. https://doi.org/10.3390/agronomy14122822








