Arthropod Pests, Nematodes, and Microbial Pathogens of Okra (Abelmoschus esculentus) and Their Management—A Review
Abstract
:1. Introduction
2. Okra Animal Pests and Their Distribution
3. Major Okra Pests and Their Management Strategies
3.1. Cotton Aphid (Aphis gossypii Glover (Hemiptera: Aphididae))
3.1.1. Cultural Control
3.1.2. Biological Control
3.1.3. Botanical Control
3.2. Cotton Spotted Bollworm (Earias vittella Fabricius (Lepiodeptera: Nolidae)) and Egyptian bollworm (E. insulana Boisduval)
3.2.1. Chemical Control
3.2.2. Botanical Control
3.2.3. Biological Control
3.2.4. Microbial Control
3.2.5. Host Plant Resistance
3.2.6. Cultural Control
3.3. Cotton Mealybug (Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae))
3.3.1. Chemical Control
3.3.2. Botanical Control
3.3.3. Microbial Control
3.3.4. Biological Control
3.4. Whitefly (Bemisia tabaci Gennadius (Hemiptera: Alyerodidae))
3.4.1. Chemical Control
3.4.2. Botanical Control
3.4.3. Host Plant Resistance
3.5. Cotton Leafhopper (Amrasca biguttula Ishida (Hemiptera: Cicadellidae))
3.5.1. Chemical Control
3.5.2. Cultural Control
3.5.3. Microbial Control
3.5.4. Host Plant Resistance
3.6. Cotton Bollworm (Helicoverpa armigera Hübner (Lepidoptera: Noctuidae))
3.6.1. Chemical Control
3.6.2. Microbial Control
3.6.3. Host Plant Resistance
3.7. Two-Spotted Spider Mite (Tetranychus urticae Koch (Acarida: Tetranychidae))
3.7.1. Chemical Control
3.7.2. Biological Control
3.7.3. Botanical Control
3.8. Root-Knot Nematodes (Meloidogyne spp.)
3.8.1. Botanical Control
3.8.2. Integrated Control
3.8.3. Cultural Control
3.8.4. Microbial Control
3.8.5. Host Plant Resistance
3.9. Reniform Nematode (Rotylenchulus reniformis Linford & Oliveira (Rhabditidae: Hoplolaimidae))
3.9.1. Cultural Control
3.9.2. Botanical Control
3.9.3. Microbial Control
3.9.4. Chemical Control
3.10. Cotton Leaf Roller (Syllepte derogata Fabricius (Lepidoptera: Crambidae))
3.10.1. Chemical Control
3.10.2. Botanical Control
3.10.3. Microbial Control
3.11. Flea Beetles (Nisotra uniformis Jacoby (Coleoptera: Chrysomelidae) and N. sjoestedti Jacoby)
3.11.1. Chemical Control
3.11.2. Botanical Control
4. Minor Okra Pests and Their Management Strategies
4.1. Blister Beetles (Mylabris pustulata Olivier (Coleoptera: Meloidae) and M. phalerata Pallas)
4.1.1. Chemical Control
4.1.2. Microbial Control
4.1.3. Integrated Control
4.2. Okra Petiole Maggot (Melanagromyza hibisci Spencer (Diptera: Agromyzidae))
4.2.1. Biological Control
4.2.2. Integrated Control
4.3. Red Cotton Bug (Dysdercus koenigii Fabricius (Hemiptera: Pyrrcohoridae))
Botanical Control
4.4. Cotton Seed Bug (Oxycarenus hyalinipennis A. Costa (Hemiptera: Oxycarenidae))
Botanical Control
4.5. Cotton Looper (Anomis flava Fabricius (Lepidoptera: Erebidae))
4.5.1. Biological Control
4.5.2. Microbial Control
4.6. Onion Thrips (Thrips tabaci Lindeman (Thysanoptera: Thripidae))
4.6.1. Botanical Control
4.6.2. Cultural Control
4.7. Green Plant Bug (Nezara viridula Linnaeus (Hemiptera: Pentatomidae))
4.7.1. Botanical Control
4.7.2. Chemical Control
4.8. Lesion Nematode (Pratylenchus brachyurus Godfrey (Rhabditida: Pratylenchidae))
Cultural Control
5. Oomycete and Fungal Diseases of Okra, and Their Management Strategies
5.1. Damping-Off
5.1.1. Chemical Control
5.1.2. Microbial Control
5.1.3. Botanical Control
5.1.4. Integrated Control
5.2. Powdery Mildew
5.2.1. Botanical Control
5.2.2. Microbial Control
5.2.3. Chemical Control
5.2.4. Integrated Control
5.2.5. Host Plant Resistance
5.3. Cercospora Leaf Spot (Cercosporiosis)
5.3.1. Microbial Control
5.3.2. Chemical Control
5.3.3. Host Plant Resistance
5.4. Gray Mold
Chemical Control
5.5. Alternaria Leaf Spot
Botanical Control
5.6. Alternaria Pod Blight
5.7. Phyllosticta Leaf Spot
5.8. Wilt Diseases
5.8.1. Fusarium Wilt
Microbial Control
Botanical Control
Host Plant Resistance
5.8.2. Verticillium Wilt
Host Plant Resistance
5.9. Collar Rot
5.9.1. Microbial Control
5.9.2. Host Plant Resistance
5.10. Stem Canker
5.11. Anthracnose
Botanical Control
5.12. Fruit Rot
6. Viral Diseases of Okra and Their Management Strategies
6.1. Okra Yellow Vein Mosaic Disease
6.2. Okra Enation Leaf Curl Disease
6.3. Okra Mosaic Disease
6.4. Management Strategies
6.4.1. Host Plant Resistance
6.4.2. Cultural Control
7. Bacterial Diseases of Okra and Their Management Strategies
7.1. Management Strategies
7.1.1. Host Plant Resistance
7.1.2. Cultural Control
7.1.3. Mechanical Control
7.1.4. Chemical Control
7.1.5. Microbial Control
8. Integrated Management of Okra Pests and Diseases: Perspectives and Gaps
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naveed, A.; Khan, A.A.; Khan, I.A. Generation mean analysis of water stress tolerance in okra (Abelmoschus esculentus L.). Pak. J. Bot. 2009, 41, 195–205. [Google Scholar]
- Tong, P. Okra (Abelmoschus esculentus)–a popular crop and vegetable. UTAR Agric. Sci. J. 2016, 2, 39–42. [Google Scholar]
- Adiaha, M.S. Effect of Okra (Abelmoschus esculentus L. Moench) on human development and its impact on the economy of farmers in Obubra Rainforest Zone of Nigeria. World News Nat. Sci. 2017, 10, 80–85. [Google Scholar]
- Kumar, A.; Kumar, P.; Nadendla, R. A review on: Abelmoschus esculentus (Okra). Int. Res. J. Pharm. Appl. Sci. 2013, 3, 129–132. [Google Scholar]
- Food and Agriculture Organization (Statistics Division). Production: Crops and Livestock Products: World, Production Quantity, Okra, 2022 (from Pick Lists). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 September 2024).
- Tindall, H.D. Vegetables in the Tropics; Macmillan Press Ltd.: London, UK, 1983. [Google Scholar] [CrossRef]
- Charrier, A. Genetic Resources of the Genus Abelmoschus Med. (Okra); IBPGR: Rome, Italy, 1984. [Google Scholar]
- Adebooye, O.; Oputa, C. Effects of galex (r) on growth and fruit nutrient composition of okra (Abelmoschus esculentus (L.) Moench). Ife J. Agric. 1996, 18, 1–9. [Google Scholar]
- Kendall, C.W.; Jenkins, D.J. A dietary portfolio: Maximal reduction of low-density lipoprotein cholesterol with diet. Curr. Atheroscler. Rep. 2004, 6, 492–498. [Google Scholar] [CrossRef]
- Tantawy, I.; Abdalla, R.M.; EL-Ashmony, R.; Galal, A. Effectiveness of Peroxy Acetic Aacid (PAA), Perbicarbonate (PB) and Potassium Silicate (PS) on Okra Growth, Yield and Resistance to Powdery Mildew. J. Plant Prod. 2020, 11, 1417–1425. [Google Scholar] [CrossRef]
- Ardestani, S.T.; Khodaparast, S.A.; Moghaddam, A.A.; Ghanavati, F.; Darsaraei, H. First report of powdery mildew caused by Golovinomyces bolayi on okra (Abelmoschus esculentus). Australas. Plant Dis. Notes 2020, 15, 26. [Google Scholar] [CrossRef]
- Savello, P.A.; Martin, F.W.; Hill, J.M. Nutritional composition of okra seed meal. J. Agric. Food Chem. 1980, 28, 1163–1166. [Google Scholar] [CrossRef]
- Lengsfeld, C.; Titgemeyer, F.; Faller, G.; Hensel, A. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Agric. Food Chem. 2004, 52, 1495–1503. [Google Scholar] [CrossRef]
- Adetuyi, F.; Osagie, A.; Adekunle, A. Effect of postharvest storage techniques on the nutritional properties of benin indigenous okra Abelmoschus esculentus (L) Moench. Pak. J. Nutr. 2008, 7, 652–657. [Google Scholar] [CrossRef]
- Kumar, S.; Dagnoko, S.; Haougui, A.; Ratnadass, A.; Pasternak, N.; Kouame, C. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res. 2010, 5, 3590–3598. [Google Scholar]
- Liu, I.-M.; Liou, S.-S.; Lan, T.-W.; Hsu, F.-L.; Cheng, J.-T. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Medica 2005, 71, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patil, M.; Patil, S.R.; Paschapur, M.S. Evaluation of Abelmoschus esculentus mucilage as suspending agent in paracetamol suspension. Int. J. PharmTech Res. 2009, 1, 658–665. [Google Scholar]
- Çalışır, S.; Özcan, M.; Hacıseferoğulları, H.; Yıldız, M.U. A study on some physico-chemical properties of Turkey okra (Hibiscus esculenta L.) seeds. J. Food Eng. 2005, 68, 73–78. [Google Scholar] [CrossRef]
- Kochlar, S. Tropical crops. A Text Book Econ. Bot. 1986, 21, 263–264. [Google Scholar]
- Jiskani, M. Okra diseases and IPDM. J. Plant Pathol. 2006, 4, 32. [Google Scholar]
- Gulati, R. Incidence of Tetranychus cinnabarinus (Boisd.) Infestation in Different Varieties of Abelmoschus esculentus L. Ann. Plant Prot. Sci. 2004, 12, 45–47. [Google Scholar]
- Ewete, F.K. Insect species and description of damage caused on okra, Abelmoschus esculentus (L.) Moench. East Afr. Agric. For. J. 1978, 44, 152–163. [Google Scholar] [CrossRef]
- Srinivasa, R.; Rajendran, R. Joint action potential of neem with other plant extracts against the leaf hopper Amrasca devastans (Distant) on okra. Pest Manag. Econ. Zool. 2003, 10, 131–136. [Google Scholar]
- Hill, D.S. Agricultural Insect Pests of the Tropics and Their Control; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Thippeswamy, B.; Krishnappa, M.; Chakravarthy, C.; Sathisha, A.; Jyothi, S.; Kumar, K. Pathogenicity and management of brown lesion and leaf spot in okra caused by Macrophomina phaseolina and Alternaria alternata. J. Plant Dis. Sci. 2007, 2, 43–47. [Google Scholar]
- Appiah, A.; Amiteye, S.; Boateng, F.; Amoatey, H. Evaluation of okra (Abelmoschus esculentus L. Moench) cultivars for resistance to okra mosaic virus and okra yellow vein mosaic virus. Australas. Plant Pathol. 2020, 49, 541–550. [Google Scholar] [CrossRef]
- Kon, T.; Rojas, M.R.; Abdourhamane, I.K.; Gilbertson, R.L. Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. J. Gen. Virol. 2009, 90, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Jamir, I.; Mandal, A.K.; Devi, A.P.; Bhattacharjee, T.; Maurya, P.K.; Dutta, S.; Chattopadhyay, A.; Pramanik, K.; Banik, S. Screening of genotypes against viral diseases and assessment of yield loss due to yellow vein mosaic virus in okra grown in the eastern part of India. Indian Phytopathol. 2020, 73, 125–133. [Google Scholar] [CrossRef]
- Mishra, G.P.; Singh, B.; Seth, T.; Singh, A.K.; Halder, J.; Krishnan, N.; Tiwari, S.K.; Singh, P.M. Biotechnological advancements and begomovirus management in okra (Abelmoschus esculentus L.): Status and perspectives. Front. Plant Sci. 2017, 8, 360. [Google Scholar] [CrossRef]
- Young, J. Pathogenicity and identification of the lilac pathogen, Pseudomonas syringae pv. syringae van Hall 1902. Ann. Appl. Biol. 1991, 118, 283–298. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). EPPO Global Database. Available online: https://gd.eppo.int/ (accessed on 11 August 2024).
- Malik, M.U.; Javed, H.; Ayyaz, M. Evaluation of different groundnut Arachis hypogea L. cultivars against termites, Odontotermes obesus (Rambur) in Rawalpindi, Pakistan. Turk. J. Agric.-Food Sci. Technol. 2015, 3, 448–452. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Integrated Pest Management (IPM) Principles. Available online: https://www.epa.gov/safepestcontrol/integrated-pest-management-ipm-principles (accessed on 5 April 2023).
- Ashfaq, M.; Muhammad, N.-u.-A.; Khuram, Z.; Abida, N. The correlation of abiotic factors and physico-morphic charateristics of (Bacillus thuringiensis) Bt transgenic cotton with whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) and jassid, Amrasca devastans (Homoptera: Jassidae) populations. Afr. J. Agric. Res. 2010, 5, 3102–3107. [Google Scholar]
- Henry III, C.W.; Shamsi, S.A.; Warner, I.M. Separation of natural pyrethrum extracts using micellar electrokinetic chromatography. J. Chromatogr. A 1999, 863, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, G.; Shukla, N. Evaluation of different modules for the management of pest complex of okra. Pestology 2009, 33, 31–37. [Google Scholar]
- CABI. CABI Compendium. Available online: https://www.cabidigitallibrary.org/journal/cabicompendium (accessed on 17 August 2024).
- CABI. PlantwisePlus Knowledge Bank. Available online: https://plantwiseplusknowledgebank.org/ (accessed on 18 August 2024).
- Cocuzza, G. Aphis gossypii (cotton aphid). In Pest, Natural Enemy, Invasive Species, Vector of Plant Pest (CABI Compendium); CABI: Wallingford, UK, 2024. [Google Scholar] [CrossRef]
- EPPO Global Database. Phenacoccus solenopsis (Distribution). Available online: https://gd.eppo.int/taxon/PHENSO/distribution (accessed on 21 September 2024).
- EPPO Global Database. Bemisia tabaci (Distribution). Available online: https://gd.eppo.int/taxon/BEMITA/distribution (accessed on 21 September 2024).
- PlantwisePlus Knowledge Bank. Amrasca biguttula biguttula (Indian Cotton Jassid). Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.20857 (accessed on 21 September 2024).
- PlantwisePlus Knowledge Bank. Earias vittella (Spiny Bollworm). Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.20306 (accessed on 21 September 2024).
- EPPO Global Database. Earias insulana (Distribution). Available online: https://gd.eppo.int/taxon/EARIIN/distribution (accessed on 21 September 2024).
- EPPO Global Database. Helicoverpa armigera (Distribution). Available online: https://gd.eppo.int/taxon/HELIAR/distribution (accessed on 21 September 2024).
- PlantwisePlus Knowledge Bank. Haritalodes derogata (Cotton Leaf Roller). Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.52198 (accessed on 21 September 2024).
- Bapfubusa Niyibizi, I.A.; Hanna, R.; Kekeunou, S.; Membang, G.; Fiaboe, K.K.M.; Mahot, H.C.; Fomumbod Abang, A.; Kumar, P.L.; Fotso Kuate, A. Potential of Cameroon-indigenous isolates of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae as microbial control agents of the flea beetle Nisotra uniformis. Biocontrol Sci. Technol. 2023, 33, 226–240. [Google Scholar] [CrossRef]
- Egwuatu, R. Field trials with systemic and contact insecticides for the control of Podagrica uniforma and P. sjostedti (Coleoptera, Chrysomelidae) on okra. Int. J. Pest Manag. 1982, 28, 115–121. [Google Scholar]
- Pitan, O.O.; Olatunde, G. Effects of intercropping tomato (Lycopersicon esculentum) at different times with cowpea (Vigna unguiculata) or okra (Abelmoschus esculentus) on crop damage by major insect pests. J. Agric. Sci. 2006, 144, 361–368. [Google Scholar] [CrossRef]
- Odewole, A.; Adebayo, T.; Dada, A. Efficacy of Plant Based Insecticides in Control of Leaf Defoliators (Podagrica uniformis Jacoby and Nisotra sjostedti Jacoby (Coleoptera: Chrysomelidae) of Okra (Abelmoschus esculentus L. Moench). J. Northeast Agric. Univ. (Engl. Ed.) 2016, 23, 1–7. [Google Scholar] [CrossRef]
- Emosairue, S.; Ukeh, D. Field trial of neem products for the control of okra flea beetles (Podagrica spp.) in south eastern Nigeria. Afr. J. Plant Prot. 1997, 6, 22–26. [Google Scholar]
- Obeng-Ofori, D.; Sackey, J. Field evaluation of non-synthetic insecticides for the management of insect pests of okra Abelmoschus esculentus (L.) Moench in Ghana. SINET Ethiop. J. Sci. 2003, 26, 145–150. [Google Scholar] [CrossRef]
- Bedford, H. The Pests of Cotton in the Anglo-Egyptian Sudan; CABI Digital Library: Wallingford, UK, 1923. [Google Scholar]
- King, H. Clean Cultivation and Its Relation to the Control of Insect Pests; CABI Digital Library: Wallingford, UK, 1918. [Google Scholar]
- Traoré, O. Positive development in integrated pest control for cotton in West Africa. In Proceedings of the 67th Plenary Meeting of the International Cotton Advisory Committee, Ouagadougou, Burkina Faso, 17–21 November 2008. [Google Scholar]
- Eisenback, J.D. Meloidogyne incognita (root-knot nematode). In Pest, Natural Enemy, Invasive Species; CABI: Wallingford, UK, 2020. [Google Scholar] [CrossRef]
- EPPO Global Database. Rotylenchulus reniformis (Distribution). Available online: https://gd.eppo.int/taxon/ROTYRE/distribution (accessed on 21 September 2024).
- CABI. Tetranychus urticae (two-spotted spider mite). In Pest, Natural Enemy, Invasive Species; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Kamble, S.T. Bionomics of Dysdercus koenigii Fabr.(Hemiptera: Pyrrhocoridae). J. N. Y. Entomol. Soc. 1971, 79, 154–157. [Google Scholar]
- Jaleel, W.; Saeed, S.; Naqqash, M.N. Biology and bionomics of Dysdercus koenigii F.(Hemiptera: Pyrrhocoridae) under laboratory conditions. Pak. J. Agric. Sci. 2013, 50, 373–378. [Google Scholar]
- Shah, S.I.A. The cotton stainer (Dysdercus koenigii): An emerging serious threat for cotton crop in Pakistan. Pak. J. Zool. 2014, 46, 329–335. [Google Scholar]
- Smith, T.R.; Brambila, J. A major pest of cotton, Oxycarenus hyalinipennis (Heteroptera: Oxycarenidae) in the Bahamas. Fla. Entomol. 2008, 91, 479–482. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, R.M.; Shad, S.A. Genetics, realized heritability and possible mechanism of chlorfenapyr resistance in Oxycarenus hyalinipennis (Lygaeidae: Hemiptera). Pestic. Biochem. Physiol. 2016, 133, 91–96. [Google Scholar] [CrossRef]
- Bilal, M.; Freed, S.; Ashraf, M.Z.; Rehan, A. Resistance and detoxification enzyme activities to bifenthrin in Oxycarenus hyalinipennis (Hemiptera: Lygaeidae). Crop Prot. 2018, 111, 17–22. [Google Scholar] [CrossRef]
- Shah, Z.U.; Ali, A.; Haq, I.; Hafeez, F. Seasonal history of dusky cotton bug (Oxycarenus hyalinipennis Costa). J. Entomol. Zool. Stud. 2016, 4, 228–233. [Google Scholar]
- Cabral-de-Mello, D.C.; Mora, P.; Rico-Porras, J.M.; Ferretti, A.B.; Palomeque, T.; Lorite, P. The spread of satellite DNAs in euchromatin and insights into the multiple sex chromosome evolution in Hemiptera revealed by repeatome analysis of the bug Oxycarenus hyalinipennis. Insect Mol. Biol. 2023, 32, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, T.W. The Egyptian Cotton Seed Bug (Oxycarenus hyalinipennis, Costa). Its Bionomics, Damage and Suggestions for Remedial Measures. Minist. Agric. Egypt Technol. Sci. Serv. Bull 1923, 35, 107. [Google Scholar]
- Slater, J.A.; Baranowski, R.M. The occurrence of Oxycarenus hyalinipennis (Costa) (Hemiptera: Lygaeidae) in the West Indies and new Lygaeidae records for the Turks and Caicos Islands of Providenciales and North Caicos. Fla. Entomol. 1994, 77, 495–497. [Google Scholar] [CrossRef]
- CABI. Nezara viridula (green stink bug). In Pest, Natural Enemy, Invasive Species, Host Animal; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Srinivasan, K. Seasonality of Eurytoma sp.—A parasitoid on okra petiole maggot, Melanagromyza hibisci Spencer. Int. J. Pest Manag. 1988, 34, 331–332. [Google Scholar]
- Peter, C.; Govindrarajulu, V. A note on the infestation pattern and parasitism of Melanagromyza hibisci on okra. Curr. Sci. 1989, 58, 643–644. [Google Scholar]
- Nair, N.; Giri, U.; Bhattacharjee, T.; Thangjam, B.; Paul, N.; Debnath, M. Biodiversity of insect pest complex infesting okra (Abelmoschus esculentus) in Tripura, NE India. J. Entomol. Zool. Stud. 2017, 5, 1968–1972. [Google Scholar]
- Sharma, R.K.; Singh, S. Host range and abundance of blister beetle [Mylabris pustulata (thunberg)] in sub-mountainous Punjab. Agric. Res. 2018, 55, 696–700. [Google Scholar] [CrossRef]
- EPPO Global Database. Anomis flava (Distribution). Available online: https://gd.eppo.int/taxon/COSPFL/distribution (accessed on 21 September 2024).
- PlantwisePlus Knowledge Bank. Thrips tabaci (Onion Thrips). Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.53746 (accessed on 21 September 2024).
- PlantwisePlus Knowledge Bank. Pratylenchus brachyurus (Root-Lesion Nematode). Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.43894 (accessed on 21 September 2024).
- Ahmed, M.M.M. Studies on the Control of Insect Pests in Vegetables (Okra, Tomato, and Onion) in Sudan with Special Reference to Neem-Preparations; Universitätsbibliothek Giessen: Giessen, Germany, 2000. [Google Scholar]
- Deguine, J.-P.; Vaissayre, M.; Leclant, F. IPM case studies: Cotton. In Aphids as Crop Pests; CABI: Wallingford, UK, 2007; pp. 573–585. [Google Scholar]
- Rohit, S.; Painkra, K.; Painkra, G.; Bhagat, P. Evaluation of new promising pesticides for the management of sucking pests in winter okra crop. J. Entomol. Zool. Stud. 2020, 8, 1176–1180. [Google Scholar]
- Santoshkumar, C.; Kedar, K.K.; Nitin, T. Integrated Pest Management of 12 Important Pests of Okra; Department of Entomology, CCS Haryana Agricultural University: Hisar, India, 2013. [Google Scholar]
- Kedar, S.; Kumaranag, K.; Thodsare, N. Integrated pest management in okra. Pop. Kheti 2013, 2, 112–119. [Google Scholar]
- Nevo, E.; Coll, M. Effect of nitrogen fertilization on Aphis gossypii (Homoptera: Aphididae): Variation in size, color, and reproduction. J. Econ. Entomol. 2001, 94, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Mahr, S.E.R.; Cloyd, R.A.; Mahr, D.L.; Sadof, C.S. Biological control of insects and other pests of greenhouse crops. North Cent. Reg. Publ. 2001, 581, 100. [Google Scholar]
- Flint, M.L.; Dreistadt, S.H. Natural Enemies Handbook: The Illustrated Guide to Biological Pest Control; University of California Press: Berkeley, CA, USA, 1998; Volume 3386. [Google Scholar]
- Sohail, K.; Jan, S.; Usman, A.; Shah, S.F.; Usman, M.; Shah, M.; Mehmood, A. Evaluation of some botanical and chemical insecticides against the insect pests of okra. J. Entomol. Zool. Stud. 2015, 3, 20–24. [Google Scholar]
- Uzair, M.; Khattak, T.N.; Hazir, R.; Daud, M.; Waheed, M.; Azizullah, A. Effects of neem (Azadirachta indica) seed and turmeric (Curcuma longa) rhizome extracts on aphids control, plant growth and yield in okra. J. Appl. Bot. Food Qual. 2018, 91, 194–201. [Google Scholar] [CrossRef]
- Muzemu, S.; Mvumi, B.; Nyirenda, S.; Sileshi, G.; Sola, P.; Chikukura, L.; Kamanula, J.; Belmain, S.; Stevenson, P. Pesticidal effects of indigenous plants extracts against rape aphids and tomato red spider mites. In Proceedings of the African Crop Science Conference Proceedings; African Crop Science Society: Maputo, Mozambique, 2011; pp. 169–171. [Google Scholar]
- Zobayer, N.; Hasan, R. Effects of manually processed Bio-pesticides on crop production and pest managements in okra (Abelmoschus esculentus (L.) Moench). J. Nat. Sci. Res. 2013, 3, 112–118. [Google Scholar]
- Ahmad, T.; Fauzy, Z.M.; Utami, T.; Arbianti, R.; Hermansyah, H. Production of bio-insecticide from extracted Carica papaya using NADES solvent with ultrasound-assisted extraction (UAE). E3S Web Conf. 2018, 67, 03007. [Google Scholar] [CrossRef]
- Murovhi, J.; Phophi, M.M.; Mafongoya, P. Efficacy of plant materials in controlling aphids on Okra (Abelmoschus esculentus L. Moench) in Limpopo Province of South Africa. Agronomy 2020, 10, 1968. [Google Scholar] [CrossRef]
- Akolkar, P.; Deore, B.; Patil, C.; Saindane, Y. Bio-efficacy of newer insecticides against okra shoot and fruit borer, Earias vittella Fabricius. Int. J. Chem. Stud. 2021, 9, 3220–3223. [Google Scholar] [CrossRef]
- Rahman, M.; Uddin, M.; Haque, M.; Rahman, M. Comparative efficacy of different tactics for the Management of Okra Shoot and Fruit Borer, Earias vittella (Fab.) under Field condition. Int. J. Appl. Sci. Biotechnol. 2016, 4, 74–78. [Google Scholar] [CrossRef]
- Rahman, M.M.; Uddin, M.M.; Shahjahan, M. Management of okra shoot and fruit borer, Earias vittella (fabricius) using chemical and botanical insecticides for different okra varieties. Int. Res. J. Appl. Life Sci. 2013, 2, 1–9. [Google Scholar]
- Mohammad, A.; Alam, S.; Miah, M.; Amin, M.; Saif, H. Bio-rational management packages of jassid and shoot and fruit borer of okra. Bangladesh J. Agric. Res. 2018, 43, 323–332. [Google Scholar] [CrossRef]
- Sivakumar, R.; Nachiappan, R.; Selvanarayanan, V. Field evaluation of profenofos (Curacron) against selected pests of okra. Pestology 2003, 27, 7–11. [Google Scholar]
- Ishaaya, I.; Kontsedalov, S.; Mazirov, D.; Horowitz, A.R. Biorational agents: Mechanism and importance in IPM and IRM programs for controlling agricultural pests. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 363–374. [Google Scholar] [PubMed]
- Padwal, K.G.; Kumar, A. Efficacy of plant products and combinations with cypermethrin in management of Earias vittella of Okra. Ann. Plant Prot. Sci. 2014, 22, 73–75. [Google Scholar]
- Gautam, H.; Singh, N.; Rai, A. Effect of some plant extract and an insecticide on the incidence of Earias vittella in okra. Indian J. Agric. Res. 2015, 49, 175–179. [Google Scholar] [CrossRef]
- Nalini, C.; Kumar, A. Population dynamics and comparative efficacy of certain chemicals and biopesticides against okra ainst okra shoot and fruit borer (Earias vitella). Population 2016, 11, 1589–1592. [Google Scholar]
- Reddy, G.N.; Jalali, S. Identification of most suitable species of Trichogramma for the management of Earias vittella (Fabricius) on okra. Mysore J. Agric. Sci. 2015, 49, 329–331. [Google Scholar]
- Patel, N.; Raghunandan, B.; Patel, N.; Sivakumar, G. Bio-efficacy of different biocontrol agents against shoot and fruit borer, Earias vittella (Fabricius) (Lepidoptera: Noctuidae) in okra. J. Biol. Control 2021, 35, 196–204. [Google Scholar] [CrossRef]
- Mahapatro, G.K.; Gupta, G.P.; Kumar, P.A. Bioassay of insecticidal Bacillus thuringiensis Cry IA delta-endotoxins against the spotted bollworm, Earias vittella Fabricius. Int. J. Trop. Insect Sci. 2001, 21, 225–228. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Gulzar, A.; Tariq, M.; Rasool, B.; Khan, D.; Ullah, S.; Asad, M.J. Resistance, cross-resistance and stability of resistance to Bacillus thuringiensis kurstaki in Earias vittella (Fabricius) (Lepidoptera: Noctuidae). Biol. Control 2022, 175, 105058. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Gulzar, A.; Abbas, N.; Tariq, M.; Ali, I.; Hafez, A.M. Realized Heritability, Risk Assessment, and Inheritance Pattern in Earias vittella (Lepidoptera: Noctuidae) Resistant to Dipel (Bacillus thuringiensis kurstaki). Toxins 2022, 14, 686. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Gulzar, A.; Tariq, M.; Asad, M.J. Field evolved resistance in Earias vittella (Lepidoptera: Noctuidae) from Punjab, Pakistan against commercial formulations of Bacillus thuringiensis kurstaki. J. Econ. Entomol. 2021, 114, 2204–2213. [Google Scholar] [CrossRef]
- Ibargutxi, M.a.A.; Estela, A.; Ferré, J.; Caballero, P. Use of Bacillus thuringiensis toxins for control of the cotton pest Earias insulana (Boisd.) (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 2006, 72, 437–442. [Google Scholar] [CrossRef]
- Malik, K.; Jabeen, F.; Talpur, M.M.A.; Andleeb, S.; Farooq, A. Pesticidal activity of Pakistani Bacillus thuringiensis isolates against Helicoverpa armigera (Hubner) and Earias vittella (Lepidoptera: Noctuidae). IOSR J. Pharm. Biol. Sci. 2013, 4, 9–12. [Google Scholar] [CrossRef]
- Afzal, M.; Mukhtar, M.K.; Tahir, H.M.; Babar, M.H.; Sherawat, S.M. Screening of Different Okra Genotypes Against Fruit Borer (Earias spp.) (Lepidoptera: Noctuidae) on Okra Crop. Pak. J. Zool. 2015, 47, 1631–1635. [Google Scholar]
- Raut, P.; Mehendale, S.; Deogharkar, D.; Chavan, S. Effect of Border Crops on Infestation of Okra Shoot and Fruit Borer (Earias spp.). Trends Biosci. 2015, 8, 4378–4380. [Google Scholar]
- Williams, D.J.; Granara de Willink, M.C. Mealybugs of Central and South America; CABI: Wallingford, UK, 1992. [Google Scholar]
- Kulkarni, S.; Adsule, P. Soil drenching of two formulations of imidacloprid for the management of mealybug on grape in Maharashtra, India. Pestology 2010, 34, 46–48. [Google Scholar]
- Mandal, D.; Bhowmik, P.; Chatterjee, M. Cotton mealy bug (Phenacoccus solenopsis Tinsley, Hemiptera: Pseudococcidae) in West Bengal—Host range and infestation. Indian J. Entomol. 2014, 76, 32–36. [Google Scholar]
- Nagrare, V.; Kumar, R.; Amutha, M.; Dharajothi, B.; Kranthi, S.; Vennila, S.; Deshmukh, A.; Bisane, K.; Kranthi, K. A record of host plants of mealybug, Phenacoccus solenopsis Tinsley for devising ecofriendly management strategies. J. Entomol. Res. 2012, 36, 327–344. [Google Scholar]
- Younas, H.; Razaq, M.; Farooq, M.O.; Saeed, R. Host plants of Phenacoccus solenopsis (Tinsley) affect parasitism of Aenasius bambawalei (Hayat). Phytoparasitica 2022, 50, 669–681. [Google Scholar] [CrossRef]
- Rai, A.; Halder, J.; Kodandaram, M. Emerging insect pest problems in vegetable crops and their management in India: An appraisal. Pest Manag. Hortic. Ecosyst. 2014, 20, 113–122. [Google Scholar]
- Culik, M.P.; Gullan, P.J. A new pest of tomato and other records of mealybugs (Hemiptera: Pseudococcidae) from Espirito Santo, Brazil. Zootaxa 2016, 964, 1–8. [Google Scholar] [CrossRef]
- Franco, J.C.; Zada, A.; Mendel, Z. Novel approaches for the management of mealybug pests. In Biorational Control Arthropod Pests; Springer: Dordrecht, The Netherlands, 2009; pp. 233–278. [Google Scholar] [CrossRef]
- Arif, M.I.; Muhammad Rafiq, M.R.; Abdul Ghaffar, A.G. Host plants of cotton mealybug (Phenacoccus solenopsis): A new menace to cotton agroecosystem of Punjab, Pakistan. Int. J. Agric. Biol. 2009, 11, 163–167. [Google Scholar]
- Fand, B.B.; Suroshe, S.S. The invasive mealybug Phenacoccus solenopsis Tinsley, a threat to tropical and subtropical agricultural and horticultural production systems–a review. Crop Prot. 2015, 69, 34–43. [Google Scholar] [CrossRef]
- Zia, A.; Haseeb, M. Seasonal incidence of cotton mealybug, Phenacoccus solenopsis (Tinsley) on okra, Abelmoschus esculentus (L.) and comparative efficacy of insecticides on the mortality. J. Entomol. Zool. Stud. 2019, 7, 421–425. [Google Scholar]
- Ansari, H.; Haseeb, M. Efficacy of combination insecticide and biopesticide against Phenacoccus solenopsis in laboratory condition on Okra. J. Entomol. Zool. Stud. 2019, 7, 1185–1189. [Google Scholar] [CrossRef]
- Haris, H. Efficiency of Some Insecticides Alone and Mixed with Mineral Oil KZ on Cotton Mealybugs, Phenacoccus solenopsis (Tinsley) Hemiptera: Pseudococcidae. J. Plant Prot. Pathol. 2021, 12, 43–46. [Google Scholar] [CrossRef]
- Bala, K.; Arivudainambi, S.; Aravinthraju, K. Efficacy of certain botanicals against cotton mealybug Phenacoccus solenopsis L.(Pseudococcidae: Hemiptera) on Okra. J. Pharmacogn. Phytochem. 2019, 8, 1586–1591. [Google Scholar]
- Khanzada, A.M.; Khanzada, M.A.; Syed, R.N.; Lodhi, A.M. Comparative effectiveness of entomopathogenic fungi against okra mealybug Phenacoccus solenopsis. Pak. J. Bot. 2021, 53, 287–292. [Google Scholar] [CrossRef]
- Halder, J.; Khushwaha, D.; Rai, A.; Nagendran, K.; Singh, B. Host plant mediated susceptibility of Phenacoccus solenopsis (Tinsley) to Lecanicillium lecanii (Zimmermann) Zare and Gams, neem oil and their combination. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 241–247. [Google Scholar] [CrossRef]
- Suroshe, S.S.; Gautam, R.; Fand, B.B. Natural enemy complex associated with the mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) infesting different host plants in India. J. Biol. Control 2013, 27, 204–210. [Google Scholar]
- Ibrahim, S.S. Study on cotton host plants of mealybug Phenacoccus solenopsis (Tinsley) and efficiency release the predator Chrysoperla carnea (Stephens) for its controlling on cotton plants in Egypt. J. Plant Prot. Pathol. 2018, 9, 247–252. [Google Scholar] [CrossRef]
- Mohamed, G.S. Studies on population dynamic, biology of the cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) and its natural enemies as a new insect on okra plant, (Abelmoschus esculentus (L.) Moench) at Qena Governorate, Egypt. Egypt. Acad. J. Biol. Sci. Entomol. 2021, 14, 1–16. [Google Scholar] [CrossRef]
- Dalton, R. Whitefly infestations: The Christmas invasion. Nature 2006, 443, 898–901. [Google Scholar] [CrossRef]
- Liu, S.-S.; De Barro, P.; Xu, J.; Luan, J.-B.; Zang, L.-S.; Ruan, Y.-M.; Wan, F.-H. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Nath, P.; Saikia, A. Assessment of yield loss due to yellow vein mosaic of Bhendi (Abelmoschus esculentus (L.) Moench) in Assam. J. Agric. Sci. Soc. North East India 1993, 6, 87–88. [Google Scholar]
- Chaudhary, H.; Dadheech, L. Incidence of insects attacking okra and the avoidable losses caused by them. Ann. Arid Zone 1989, 28, 305–307. [Google Scholar]
- Atwal, A.; Singh, B. Pest Population and Assessment of Crop Losses; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989. [Google Scholar]
- Sheikh, M.; Safiuddin; Khan, Z.; Mahmood, I. Effect of bhendi yellow vein mosaic virus on yield components of okra plants. J. Plant Pathol. 2013, 95, 391–393. [Google Scholar]
- Nawaz, A.; Habib, A.; Dildar, G.M.; Sajid, F.; Muhammad, A.; Khan, M.A.; Muhammad, Q.; Muhammad, A.; Khan, K.A. Resistance Assessment of Different Cultivars of Okra (Abelmoschus esculentus) Against Whitefly (Bemisia tabaci). Gesunde Pflanz. 2020, 72, 361–369. [Google Scholar] [CrossRef]
- Nadeem, M.; Ayyaz, M.; Begum, H.A. Comparative efficacy of neem oil and lambdacyhalothrin against whitefly (Bemesia tabaci) and jassid (Amrasca devastans Dist.) in okra field. Russ. Agric. Sci. 2015, 41, 138–145. [Google Scholar] [CrossRef]
- Mohanty, K.K.; Chakraborty, D.; Roy, S. Antifeedant activity of oil fraction of seed of some leguminous plants against Diacrisia obliqua. Indian J. Agric. Sci. 1988, 58, 579–580. [Google Scholar]
- Atawodi, S.E.; Atawodi, J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009, 8, 601–620. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Choi, M.-Y.; Paik, C.-H.; Seo, H.-Y.; Kalaivani, K. Toxicity and physiological effects of neem pesticides applied to rice on the Nilaparvata lugens Stål, the brown planthopper. Ecotoxicol. Environ. Saf. 2009, 72, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Rao, J. Botanical Pesticides in Agriculture; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Schmutterer, H.; Singh, R. List of insect pests susceptible to neem products. In The Neem Tree: Azadirachta Indica A. Juss and Other Meliaceae Plants; VCH: New York, NY, USA, 1995; Chapter 3; pp. 326–365. [Google Scholar] [CrossRef]
- Mudathir, M.; Basedow, T. Field experiments on the effects of neem products on pests and yields of okra (Abelmoschus esculentus), tomato (Lycopersicum esculentum), and onion (Allium cepa) in the Sudan. Mitteilungen Der Dtsch. Ges. Für Allg. Und Angew. Entomol. 2004, 14, 407–410. [Google Scholar]
- Jambhulkar, P.; Singh, V.; Babu, S.R.; Yadav, R. Insecticides and Bioproducts against Whitefly Population and Incidence of Yellow vein mosaic virus in Okra. Indian J. Plant Prot. 2013, 41, 253–256. [Google Scholar]
- Pun, K.; Sabitha, D.; Jeyarajan, R. Management of okra yellow vein mosaic virus disease and its whitefly vector. Indian J. Virol. 2005, 16, 32–35. [Google Scholar]
- Oladimeji, A.; Kannike, M.A. Comparative studies on the efficacy of neem, basil leaf extracts and synthetic insecticide, lambda-cyhalothrin, against Podagrica spp. on okra. Afr. J. Microbiol. Res. 2010, 4, 33–37. [Google Scholar]
- Sridharan, S.; Shekhar, K.C.; Ramakrishnan, N. Bioefficacy, phytotoxicity, and biosafety of mineral oil on management of whitefly in okra. Int. J. Veg. Sci. 2015, 21, 28–35. [Google Scholar] [CrossRef]
- Manju, K.; Lakshmi, K.V.; Babu, B.S.; Anitha, K. Morphological and biochemical basis of resistance in okra to whitefly, Bemisia tabaci and okra yellow vein mosaic virus (OYVMV). J. Entomol. Zool. Stud. 2021, 9, 1719–1728. [Google Scholar]
- Niruba, D.; Chandrasekaran, M.; Gailce Leo Justin, C.; Rajanbabu, V.; Satya, V.K. Screening and Identification of Resistant Sources of okra, Abelmoschus esculentus L. accessions against Whitefly, Bemisia tabaci Gennadius. Biol. Forum—Int. J. 2022, 14, 378–385. [Google Scholar]
- Rajpal, S.; Joshi, A. Pests of okra (Abelmoschus esculentus Moench.) in Paonta Valley, Himachal Pradesh. Insect Environ. 2003, 9, 173–174. [Google Scholar]
- Bindra, O.; Mahal, M. Varietal resistance in eggplant (brinjal) (Solanum melongena) to the cotton jassid (Amrasca biguttula biguttula). Phytoparasitica 1981, 9, 119–131. [Google Scholar] [CrossRef]
- Jayarao, B.; Khadar, B.; Naik, L.K.; Vinaykumar, M. Assessment of biology and morphometric characteristics of different stages of leafhopper, Amrasca biguttula biguttula (Ishida) on okra. Bioscan 2015, 10, 671–674. [Google Scholar]
- Sharma, G.; Sharma, P. Biology and development of cotton leafhopper, Amrasca biguttula biguttula (Ishida) on different genotypes of okra (Abelmoschus esculentus (L.) Moench). Crop Res. 1997, 14, 487–492. [Google Scholar]
- Rawat, R.; Sahu, H. Estimation of losses in growth and yield of okra due to Empoasca devastans Distant and Earias species (India). Indian J. Entomol. 1973, 35, 252–254. [Google Scholar]
- Susheelkumar, M.; Rajashekharappa, K.; Maradi, R.; Hegde, J.; Gangaprasad, S. Bio-efficacy of Insecticides against okra leafhopper, Amrasca biguttula biguttula (Ishida). Int. J. Curr. Microbiol. App Sci. 2020, 9, 460–465. [Google Scholar] [CrossRef]
- Thapa, R.; Bista, K.; Bhatta, M.; Bhandari, S.; Acharya, S.R.; Sapkota, B. Comparative performance and economic efficiency of different pesticides against okra jassids (Amrasca biguttula biguttula): Their impact on okra yield and growth attributes. J. Entomol. Zool. Stud. 2019, 7, 525–531. [Google Scholar]
- Sullivan, P. Intercropping Principles and Production Practices. Available online: https://www.iatp.org/documents/intercropping-principles-and-production-practices-0 (accessed on 5 June 2023).
- Degri, M.; Samaila, A. Impact of intercropping tomato and maize on the infestation of tomato fruit borer [Helicoverpa armigera (Hubner)]. J. Agric. Crop Res. 2014, 2, 160–164. [Google Scholar]
- Reddy, P. Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Sharma, A.; Neupane, K.R.; Regmi, R.; Neupane, R.C. Effect of intercropping on the incidence of jassid (Amrasca biguttula biguttula Ish.) and whitefly (Bemisia tabaci Guen.) in okra (Abelmoschus esculentus L. Moench). J. Agric. Nat. Resour. 2018, 1, 179–188. [Google Scholar] [CrossRef]
- Dahal, B.R.; Rijal, S.; Poudel, N.; Gautam, B.; Neupane, R.B. Influence of different bio-pesticides and mulching in management of Okra Jassids Amrasca biguttula biguttula (Hemiptera: Cicadellidae) in Chitwan district of Nepal. Cogent Food Agric. 2020, 6, 1829271. [Google Scholar] [CrossRef]
- Akramuzzaman, M.; Uddin, M.; Islam, K. Biorational management of okra jassid (Amrasca devastans). Progress. Agric. 2018, 29, 205–212. [Google Scholar] [CrossRef]
- Sandhi, R.K.; Sidhu, S.K.; Sharma, A.; Chawla, N.; Pathak, M. Morphological and biochemical basis of resistance in okra to cotton jassid, Amrasca biguttula biguttula (Ishida). Phytoparasitica 2017, 45, 381–394. [Google Scholar] [CrossRef]
- Chandio, M.A.; Kubar, M.I.; Butt, N.A.; Magsi, F.H.; Mangi, S.; Lashari, K.H.; Channa, N.A.; Roonjha, M.A. Varietal resistance of okra against cotton jassid, Amrasca biguttula biguttula (Ishida). J. Entomol. Zool. Stud. 2017, 5, 1647–1650. [Google Scholar]
- Mohankumar, S.; Karthikeyan, G.; Durairaj, C.; Ramakrishnan, S.; Preetha, B.; Sambathkumar, S. Integrated Pest Management of Okra in India. In Integrated Pest Management of Tropical Vegetable Crops; Springer: Dordrecht, The Netherlands, 2016; pp. 167–177. [Google Scholar] [CrossRef]
- Jallow, M.F.; Matsumura, M.; Suzuki, Y. Oviposition preference and reproductive performance of Japanese Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2001, 36, 419–426. [Google Scholar] [CrossRef]
- Aziz, M.A.; Hasan, M.; Ali, A. Impact of abiotic factors on incidence of fruit and shoot infestation of spotted bollworms Earias spp. on okra (Abelmoschus esculentus L.). Pak. J. Zool. 2011, 43, 863–868. [Google Scholar]
- Salim, M. Diversity: Role in integrated pest management. Sci. Technol. Dev. 1999, 18, 26–31. [Google Scholar]
- Latheef, M.; Ortiz, J. Seasonal abundance and oviposition response of the corn earworm, Heliothis zea (Lepidoptera: Noctuidae), on okra in Virginia. Can. Entomol. 1983, 115, 1539–1541. [Google Scholar] [CrossRef]
- Chowdary, L.R.; Bheemanna, M.; Kumar, L.R. Bioefficacy of rynaxypyr (Coragen) 20 SC against fruit borer Helicoverpa armigera (Hubner) in okra. Int. J. Plant Prot. 2010, 3, 379–381. [Google Scholar]
- Ghosh, A.; Chatterjee, M.; Roy, A. Bio-efficacy of spinosad against tomato fruit borer (Helicoverpa armigera Hub.) (Lepidoptera: Noctuidae) and its natural enemies. J. Hortic. For. 2010, 2, 108–111. [Google Scholar]
- Govindan, K.; Gunasekaran, K.; Kuttalam, S.; Aiswariya, K. Bio-Efficacy of Emamectin Benzoate 5 Sg Against Helicoverpa Armigera (Hübner) in Cotton. Ann. Plant Prot. Sci. 2011, 19, 364–368. [Google Scholar]
- Javed, M.; Majeed, M.Z.; Sufyan, M.; Ali, S.; Afzal, M. Field efficacy of selected synthetic and botanical insecticides against lepidopterous borers, Earias vittella and Helicoverpa armigera (Lepidoptera: Noctuidae), on okra (Abelmoschus esculentus (L.) Moench). Pak. J. Zool. 2018, 50, 2019–2028. [Google Scholar] [CrossRef]
- Subbiredy, K.; Patel, H.; Patel, N.; Bharpoda, T. Utilization of plant extract for managing fruit borers in okra [Abelmoschus esculentus (L.) Moench]. Int. J. Curr. Mcrobiol. App. Sci. 2018, 7, 2786–2793. [Google Scholar] [CrossRef]
- Karim, S.; Zafar, A.U.; Nasir, I.A.; Riazuddin, S. Field efficacy of CAMB Bacillus thuringiensis biopesticide to control Helicoverpa armigera (Hubner) and Earias vitella (Fabricius) in okra crop. Pak. J. Biol. Sci. 2000, 3, 1296–1298. [Google Scholar] [CrossRef]
- Kassi, A.K.; Javed, H.; Mukhtar, T. Screening of okra cultivars for resistance against Helicoverpa armigera. Pak. J. Zool. 2018, 50, 91–95. [Google Scholar] [CrossRef]
- Kavitha, J.; Ramaraju, K.; Baskaran, V.; Kumar, P.P. Bioecology and management of spider mites and broad mites occurring on Jatropha curcas L. in Tamil Nadu, India. Syst. Appl. Acarol. 2007, 12, 109–115. [Google Scholar] [CrossRef]
- Siddhapara, M.; Virani, V. Biology of two spotted red spider mite Tetranychus urticae koch (Acari: Tetranychidae) on Okra. Indian J. Entomol. 2018, 80, 90–94. [Google Scholar] [CrossRef]
- Lall, B.; Dutta, C. On the biology of the red spider mite, Tetranychus telarius L. Sci. Cult. 1959, 25, 204–205. [Google Scholar]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Chiasson, H.; Bostanian, N.; Vincent, C. Acaricidal properties of a Chenopodium-based botanical. J. Econ. Entomol. 2004, 97, 1373–1377. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Dermauw, W.; Van De Veire, M.; Tirry, L. Systemic use of spinosad to control the two-spotted spider mite (Acari: Tetranychidae) on tomatoes grown in rockwool. Exp. Appl. Acarol. 2005, 37, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, N. Avermectin, a new group of pesticides. Plant. Chron. 1993, 315–316. [Google Scholar]
- Ghosh, S.K. Incidence of red spider mite (Tetranychus urticae Koch) on okra (Abelmoschus esculentus (L.) Moench) and their sustainable management. Curr. Biot. 2013, 7, 40–50. [Google Scholar]
- Jeyarani, S.; Singh, R.J.; Ramaraju, K. Efficacy of predators against the two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). J. Biol. Control 2012, 26, 279–282. [Google Scholar]
- Rai, A.; Malaviya, M.; Desai, H.; Patel, J. Impact of some weather factors and natural enemies on the incidence of Tetranychus ludeni, and role of botanicals in its control and safety to the predatory mites. J. Acaro 1999, 14, 105–108. [Google Scholar]
- Kaur, P.; Bhullar, M.; Kaur, R. Predatory potential of an anthocorid predator Blaptostethus pallescens (Poppius) against two spotted spider mite Tetranychus urticae Koch on brinjal. In Proceedings of the Agrochemicals Protecting Crops, Health and Natural Environment 2nd International Conference, New Delhi, India, 15–18 February 2012. [Google Scholar]
- Kaur, R.; Sharma, S.; Shera, P.; Sangha, K. Evaluation of anthocorid predator, Blaptostethus pallescens Poppius against spider mite, Tetranychus urticae Koch on Okra under insect net cage condition. J. Biol. Control 2019, 33, 236–241. [Google Scholar] [CrossRef]
- Singh, A.K.; Koul, K.; Shankar, U.; Singh, S.; Mondal, A.; Singh, M. Seasonal incidence and management of red spider mite, Tetranychus urticae Koch on Okra, Abelmoschus esculentus (L.) Moench. J. Entomol. Zool. Stud. 2018, 6, 650–656. [Google Scholar]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI Publishing: Wallingford, UK, 2005; pp. 319–392. [Google Scholar]
- Kayani, M.Z.; Mukhtar, T. Reproductivity of Meloidogyne incognita on Fifteen Cucumber Cultivars. Pak. J. Zool. 2018, 50, 1717–1722. [Google Scholar] [CrossRef]
- Sasser, J.; Carter, C. Overview of the international Meloidogyne project 1975–1984. Biol. Control 1985, 6, 19–24. [Google Scholar]
- Bhatti, D.; Jain, R.K. Estimation of loss in okra, tomato and brinjal yield due to Meloidogyne incognita. Indian J. Nematol. 1977, 7, 37–41. [Google Scholar]
- Shendge, A.; Mhase, N.; Landge, S.; Kadu, R. Assessment of yield losses due to root-knot nematode, Meloidogyne incognita infesting okra [Abelmoschus esculentus (L.) Moench]. Int. J. Plant Prot. 2010, 3, 325–326. [Google Scholar]
- Veronika, K.; Khan, M. Biomanagement of root-knot nematode (Meloidogyne incognita) infecting okra in West Bengal. India Indian J. Nematol. 2015, 45, 178–183. [Google Scholar]
- Begum, N.; Haque, M.; Mukhtar, T.; Naqvi, S.; Wang, J. Status of bacterial wilt caused by Ralstonia solanacearum in Pakistan. Pak. J. Phytopathol. 2012, 24, 11–20. [Google Scholar]
- Taylor, C. Meloidogyne interrelationships with microorganisms. Biol. Control 1979, 375–397. [Google Scholar]
- Williamson, V.M.; Hussey, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Cork, A.; Boomathi, N.; Pandi, R.; Velavan, S.; Dhakshnamoorthy, G. Cold aqueous extracts of African marigold, Tagetes erecta for control tomato root knot nematode, Meloidogyne incognita. Crop Prot. 2006, 25, 1210–1213. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.W. Single and interactive effects of root-knot nematode and coal-smoke on okra. New Phytol. 1994, 126, 337–342. [Google Scholar] [CrossRef]
- Prajapati, V.; Singh, P.; Deshmukh, A. First report of root knot (Meloidogyne incognita) on okra (Abelmoschus esculentus (L) moench) in dang district of Gujarat. Int. J. Econ. Plants 2018, 5, 154–156. [Google Scholar] [CrossRef]
- Olabiyi, T.; Ayeni, B. Assessment of Azadirachta indica and Cleome viscosa liquid-formulations as bio-nematicides in the management of nematode pests of okra. Afr. J. Agric. Res. 2016, 11, 467–471. [Google Scholar] [CrossRef]
- Oluwatoyin, E.; Bello, O.; Dada, A. Investigation of aqueous extracts of leaves of Calotropis procera as a natural nematicide against root knot nematode infection on Abelmoschus esculentus L. Moench’s yield. Int. J. Plant Anim. Environ. Sci. 2013, 3, 175–177. [Google Scholar]
- Ahmad, F.; Rather, M.A.; Siddiqui, M.A. Nematicidal activity of leaf extracts from Lantana camara L. against Meloidogyne incognita (Kofoid and White) Chitwood and its use to manage roots infection of Solanum melongena L. Braz. Arch. Biol. Technol. 2010, 53, 543–548. [Google Scholar] [CrossRef]
- Adediran, J.; Adegbite, A.; Akinlosotu, T.; Agbaje, G.; Taiwo, L.; Owolade, O.; Oluwatosin, G. Evaluation of fallow and cover crops for nematode suppression in three agroecologies of south western Nigeria. Afr. J. Biotechnol. 2005, 4, 1034–1039. [Google Scholar]
- Wami, A. Management of root-knoot nematode, Meloidogyne incognita, on okra and lentil by soil amendment with oil cakes and leaves of different plants. Nematol. Mediterr. 2006, 34, 83–87. [Google Scholar]
- Oyedunmade, E. Laboratory and field toxicities of the African marigold (Targetes erecta) to root-knot nematodes. Plant Sci. 2004, 4, 115–121. [Google Scholar]
- Izuogu, N.; Yakubu, L.; Abolusoro, S.; Nwabia, I. Efficacy of aqueous leaf extracts of Negro coffee (Cassia occidentalis) and lemon grass (Cymbopogon citratus) in the management of nematode pests of okra (Abelmoschus esculentus L. Moench). Sci. Technol. Arts Res. J. 2015, 4, 67–70. [Google Scholar] [CrossRef]
- de Jesus Silva, F.; Ribeiro, R.C.F.; Xavier, A.A.; Neto, J.A.S.; da Silva, C.M.; Mizobutsi, E.H. Management of Meloidogyne javanica in okra using compost of pequi fruit waste. J. Agric. Sci. 2018, 10, 258. [Google Scholar]
- Oyedunmade, E.; Izuogu, N. Efficacy of Aqueous Extract of Lemon Grass (Andropogon citratus L.) against Root-Knot Nematode Pests of Okra.(Abelmoschus esculentus (L.) Moench). Agrosearch 2011, 11, 31–38. [Google Scholar] [CrossRef]
- Fabiyi, O.A. Evaluation of plant materials as root-knot nematode (Meloidogyne incognita) suppressant in okra (Abelmuscous esculentus). Agric. Conspec. Sci. 2021, 86, 51–56. [Google Scholar]
- Abdul-hameed, M.H.; Abood, J.N. Effect of fungal filtrates of Fusarium oxysporum and Aspergillus flavus on Meloidogyne javanica in Okra plant under greenhouse conditions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012100. [Google Scholar]
- Silva, E.H.C.; Soares, R.S.; Diniz, G.M.; Franco, C.A.; Marin, M.V.; Candido, W.S.; Braz, L.T.; Soares, P.L.M. Grafting as a management tool to control Meloidogyne incognita in okra: Identifying rootstocks candidates. Sci. Hortic. 2019, 246, 354–359. [Google Scholar] [CrossRef]
- Mukhtar, T.; Arooj, M.; Ashfaq, M.; Gulzar, A. Resistance evaluation and host status of selected green gram germplasm against Meloidogyne incognita. Crop Prot. 2017, 92, 198–202. [Google Scholar] [CrossRef]
- Hussain, M.; Mukhtar, T.; Kayani, M. Assessment of the damage caused by Meloidogyne incognita on okra (Abelmoschus esculentus). JAPS J. Anim. Plant Sci. 2011, 21, 857–861. [Google Scholar]
- Afzal, M.U.; Khan, S.A.; Salehon, N.; Naz, M.; Khan, N.A. Development of Meloidogyne incognita on selected okra cultivars. Plant Prot. 2019, 3, 85–90. [Google Scholar] [CrossRef]
- Mukhtar, T.; Hussain, M.A.; Kayani, M.Z.; Aslam, M.N. Evaluation of resistance to root-knot nematode (Meloidogyne incognita) in okra cultivars. Crop Prot. 2014, 56, 25–30. [Google Scholar] [CrossRef]
- Luc, M.; Sikora, R.A.; Bridge, J. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Robinson, A.; Inserra, R.; Caswell-Chen, E.; Vovlas, N.; Troccoli, A. Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica 1997, 27, 127–180. [Google Scholar]
- Koenning, S.R.; Wrather, J.A.; Kirkpatrick, T.L.; Walker, N.R.; Starr, J.L.; Mueller, J.D. Plant-parasitic nematodes attacking cotton in the United States: Old and emerging production challenges. Plant Dis. 2004, 88, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Chandra, B.; Khan, M.R. Dynamics of soil nematodes in vegetable based crop sequences in West Bengal, India. J. Plant Prot. Res. 2011, 51, 7–13. [Google Scholar] [CrossRef]
- Jagadeeswaran, R.; Singh, R. Management of reniform nematode, Rotylenchulus reniformis on okra hybrid by organic amendments. Indian J. Nematol. 2011, 41, 9–13. [Google Scholar]
- Jumder, V.M.; Chawla, G.; Singh, J. Effect of urea coated with neem formulations on root-knot and reniform nematodes in Okra. Indian J. Nematol. 2004, 34, 60–63. [Google Scholar]
- Devi, N.; Sharma, M.; Bhargava, S. Biotic Potential of Paeciliomyces lilacinus, Pochonia chlamydosporia and Trichoderma viride Against Rotylenchulus reniformis on Okra. Indian J. Nematol. 2012, 42, 80–82. [Google Scholar]
- Jayakumar, J.; Rajendran, G.; Ramakrishnan, S. Management of reniform nematode, Rotylenchulus reniformis on okra through Streptomyces avermitilis. Indian J. Nematol. 2005, 35, 59–62. [Google Scholar]
- Sontakke, P.P.; Radhika, N.; Pattapu, S. Biological attributes of cotton leaf roller on okra. Ann. Plant Soil Res. 2015, 17, 219–220. [Google Scholar]
- Mariselvi, S.; Manimegalai, K. Biochemical studies of cotton pest Sylepta derogata Fab. by Econeem, Acorus calamus and Piper longum extracts. Int. J. Sci. Res. Publ. 2016, 6, 388–401. [Google Scholar]
- Atulukwu, S. Management of Leaf Roller Sylepta Derogata Fab. (Lepidoptera: Pyralidae) on Some Okra Varieties in Samaru and Kadawa, Nigeria; Ahmadu Bello University: Zaria, Nigeria, 2021. [Google Scholar]
- Jalgaonkar, V.; Patil, R.; Naik, K.; Mule, R.; Pawar, S. Field management of okra leaf roller, Sylepta derogata (Fabricius). Health Environ. Res. 2011, 35, 47–49. [Google Scholar]
- Misra, H.; Dash, D.; Mahapatra, D. Efficacy of Some Insecticides Against Okra Fruit Borer, Earias spp. and leafroller, Sylepta derogata Fab. Ann. Plant Prot. Sci. 2002, 10, 51–54. [Google Scholar]
- Kamaraj, C.; Rahuman, A.A.; Bagavan, A. Screening for antifeedant and larvicidal activity of plant extracts against Helicoverpa armigera (Hübner), Sylepta derogata (F.) and Anopheles stephensi (Liston). Parasitol. Res. 2008, 103, 1361–1368. [Google Scholar] [CrossRef]
- Anaso, C. Cost-benefits of spraying sole and intercropped okra with neem seed extracts and deltamethrin in the Nigerian Sudan Savanna. ASSET—Ser. Agric. Environ. 2003, 3, 171–177. [Google Scholar]
- Adhikary, S. Results of field trials to control common insect pests of okra, Hibiscus esculentus L., in Togo by application of crude methanolic extracts of leaves and seed kernels of the neem tree, Azadirachta indica A. Juss. Z. Für Angew. Entomol. 1984, 98, 327–331. [Google Scholar] [CrossRef]
- Ajibola Taylor, T. Evaluation of Dipel for control of lepidopterous pests of okra. J. Econ. Entomol. 1974, 67, 690–691. [Google Scholar] [CrossRef]
- Cobbinah, J.; Osei-Owusu, K. Effects of neem seed extracts on insect pests of eggplant, okra and cowpea. Int. J. Trop. Insect Sci. 1988, 9, 601–607. [Google Scholar] [CrossRef]
- Anaso, C.; Lale, N. Spraying intervals and cost-benefit of using aqueous neem kernel extract and deltamethrin against some foliage and fruit pests of okra in Sudan savanna of Nigeria. J. Sustain. Agric. Environ. 2002, 4, 122–128. [Google Scholar]
- Fasunwon, B.; Banjo, A. Seasonal population fluctuations of Podagrica Species on okra plant (Abelmoschus esculentus). Res. J. Agric. Biol. Sci. 2010, 6, 283–288. [Google Scholar]
- Vanlommel, S.; Duchateau, L.; Coosemans, J. The effect of okra mosaic virus and beetle damage on yield of four okra cultivars. Afr. Crop Sci. J. 1996, 4, 71–77. [Google Scholar]
- Echereobia, C.; Okerere, C.; Emeaso, K. Determination of repellence potentials of some aqueous plant extracts against okra flea beetles Podagrica uniforma. J. Biopestic. 2010, 3, 505. [Google Scholar] [CrossRef]
- Onunkun, O. Evaluation of aqueous extracts of five plants in the control of flea beetles on okra (Abelmoschus esculentus (L.) Moench). J. Biopestic. 2012, 5, 62–67. [Google Scholar]
- Mobolade, A.J.; Ejemen, I.J.; Rufus, J.A.; Festus, E.A. Control of flea beetles Podagrica spp.(Coleoptera: Chrysomelidae) infestation on okra (Abelmoschus esculentus (L.) Moench) using Piper guineense seed extracts. Arch. Phytopathol. Plant Prot. 2014, 47, 2332–2339. [Google Scholar] [CrossRef]
- Ojo, J.A.; Olunloyo, A.A.; Ibitoye, O. Evaluation of botanical insecticides against flea beetles Podagrica sjostedti and Podagrica uniforma of okra. Int. J. Adv. Res. 2014, 2, 236–244. [Google Scholar]
- Adesina, J.; Idoko, J. Field evaluation of insecticidal activity of Chenopodium ambrosiodes and Spondias mombin crude extracts for the control of okra flea beetles Podagrica uniforma Jacq.(Coleoptera: Chysomelidae). Res. J. Agric. Sci. 2013, 4, 37–39. [Google Scholar]
- Dhingra, S.; Sarup, P. Detection of resistance in the blister beetle, Mylabris pustulata Thunb. to various insecticides evaluated during the last quarter century. J. Entomol. Res. 1992, 16, 231–235. [Google Scholar]
- Shende, S.; Thakare, A.; Wadaskar, R. Dose mortality responses of blister beetles against some insecticides. Bioscan 2013, 8, 1061–1064. [Google Scholar]
- Anandita, P.K.S.; Jayaram, C. Efficacy of Botanicals and Organic Products against Blister Beetles, Mylabris spp. Infesting Okra. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2912–2916. [Google Scholar]
- Taggar, G.K.; Khanna, V.; Malhotra, A.; Gupta, M. Field efficacy and molecular characterization of native Bacillus thuringiensis isolates against blister beetle, Mylabris pustulata (Thunberg) (Coleoptera: Meloidae) in pigeonpea. J. Pure Appl. Microbiol. 2015, 9, 2405–2410. [Google Scholar]
- Singh, S.; Yadav, G.S.; Das, A.; Das, B.; Devi, H.L.; Raghuraman, M.; Kumar, A. Bioefficacy, environmental safety and synergistic impacts of biorational formulations against whitefly, leafhopper and blister beetle in organic okra ecosystem. J. Agric. Sci. 2021, 159, 373–384. [Google Scholar] [CrossRef]
- Kumar, N.K.; Srinivasan, K. Studies on the seasonal abundance and control of okra stemfly, Melanagromyza hibisci Spencer (Diptera: Agromyzidae) in India. Int. J. Trop. Insect Sci. 1985, 6, 533–536. [Google Scholar] [CrossRef]
- Moorthy, P.K.; Kumar, N.K. Evaluation of neem products for management of insect pests of okra. J. Eco-Friendly Agric. 2012, 7, 51–55. [Google Scholar]
- Ahmad, I.; Mohammad, A. Biology and immature systematics of red cotton stainer Dysdercus koenigii (Fabr).(Hemiptera: Pyrrohocoridae) with a note on their phylogenetic value. Bull. Zool. 1983, 1, 1–9. [Google Scholar]
- Ashfaq, S.; Khan, I.; Saeed, M.; Saljoqi, A.; Manzoor, F.; Sohail, K.; Habib, K.; Sadozai, A. Population dynamics of insect pests of cotton and their natural enemies. Sarhad J. Agric. 2011, 27, 251–253. [Google Scholar]
- Claver, M.A.; Yadav, S. Biocontrol efficiency of Rhynocoris fuscipes (Hemiptera: Reduviidea) against Dysdercus koenigii (Hemiptera: Pyrrhocoridae), a severe pest of Abelmuschus esculentus. J. Entomol. Zool. Stud. 2024, 12, 162–165. [Google Scholar] [CrossRef]
- Naqqash, M.N.; Saeed, S.; Jaleel, W.; Zaka, S.M.; Saeed, Q. Effect of host plants on life history traits of Dysdercus koenigii (Hemiptera: Pyrrhocoridae). J. Biodivers. Environ. Sci. 2014, 4, 187–194. [Google Scholar]
- Shahzad, A.; Khan, M.A.; Ashfaq, M.; Nazir, J.; Ahmed, M.Z.; Mustafa, F.-u.; Baloch, R. Comparative effect of botanical extracts and synthetic pesticides against Dysdercus cingulatus F. and Amrasca biguttula I. in Okra. J. Entomol. Zool. Stud. 2019, 7, 1291–1295. [Google Scholar]
- Shukla, S.; Dubey, A.; Chandel, B. Application of toxicological compatibility of sweet flag and nirgundi extract against cotton strainer, Dysdercus koenigii Fabr.(Hemiptera: Pyrrhocoridae) on okra, Abelmoschus esculentus. J. Entomol. Zool. Stud. 2022, 10, 39–44. [Google Scholar] [CrossRef]
- Akram, M.; Asi, M.R.; Mehfooz-ul-Haq, M.A.; Saleem, M.S. Bioefficacy of organophosphates, pyrethroids and new chemistry insecticides against a field population of dusky cotton bug, Oxycarenus spp.(Hemiptera: Oxycarenidae) in Bt cotton ecosystem. Pak. J. Life Soc. Sci. 2013, 11, 48–52. [Google Scholar]
- Srinivas, M.; Patil, B. Quantitative and qualitative loss caused by dusky cotton bug, Oxyacarenus laetus Kirby on cotton. Karnataka J. Agric. Sci. 2004, 17, 487–490. [Google Scholar]
- Nurulain, S.; Tabassum, R.; Naqvi, S. Toxicity of neem fraction and malathion (57% EC) against dusky cotton bug (Oxycarenus lugubris Mostch.). Pak. J. Entomol. 1989, 4, 13–14. [Google Scholar]
- Abbas, M.; Hafeez, F.; Farooq, M.; Ali, A. Dusky cotton bug Oxycarenus spp. (Hemiptera: Lygaeidae): Hibernating sites and management by using plant extracts under laboratory conditions. Pol. J. Entomol. 2015, 84, 127–136. [Google Scholar] [CrossRef]
- Li, S.; Rahmann, H. Baumwoll-Schädlingsmanagement in China. I: Schädlingsspektrum/Cotton pest management in China. I: The cotton pests. Z. Für Pflanzenkrankh. Und Pflanzenschutz/J. Plant Dis. Prot. 1997, 104, 611–621. [Google Scholar]
- Kalshoven, L.G.E.; Van der Laan, P. Pests of crops in Indonesia. In De Plagen van de Cultuurgewassen in Indonesie; Uitgeverij van Hoeve: Gravenhage, The Netherlands, 1981. [Google Scholar]
- Sidhu, A.; Dhawan, A. Development of Anomis flava on different cotton varieties and its control. Indian J. Plant Prot. 1979, 7, 189–196. [Google Scholar]
- Li, L.; Wang, R.; Waterhouse, D.F. The Distribution and Importance of Arthropod Pests and Weeds of Agriculture and Forestry Plantations in Southern China; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 1997.
- Ferino, M.; Calora, F.; Magallona, E. Population dynamics and economic threshold level of the cotton semi-looper, Anomis flava flava (Fabr.) (Noctuidae, Lepidoptera). Philipp. Entomol. 1982, 5, 401–446. [Google Scholar]
- Wang, F.; Zhang, S.; Hou, S. Inoculative release of Trichogramma dendrolimi in vegetable gardens to regulate populations of cotton pests. Colloq. L’inra 1988, 43, 613–619. [Google Scholar]
- Xie, M. Observations on the parasitic wasps of Anomis flava Fabricius in the fields of Hibiscus cannabinus. Nat. Enemies Insects (Kunchong Tiandi) 1984, 6, 68–70. [Google Scholar]
- Steffan, J. Brachymeria (Hym. Chalcididae) parasites de Anomis flava f. à Madagascar. Entomophaga 1958, 3, 275–280. [Google Scholar] [CrossRef]
- Yin, Y.; Chang, J.; Pei, M.; Qiu, Y.; Sun, G.; Sun, T.; Sun, T.; Wang, Y.; Liu, W. Study on a granulosis virus in the larvae of Anomis flava (Fabricius). Nat. Enemies Insects 1991, 13, 180–185. [Google Scholar]
- Khan, M. Cotton Semilooper, Anomis flava (Fb.) in Hyderabad State. Indian J. Entomol. 1956, 18, 461–462. [Google Scholar]
- Ananthakrishnan, T. Bionomics of thrips. Annu. Rev. Entomol. 1993, 38, 71–92. [Google Scholar] [CrossRef]
- Iqbal, J.; Ali, H.; Hassan, M.W.; Jamil, M. Evaluation of indigenous plant extracts against sucking insect pests of okra crop. Pak. Entomol. 2015, 37, 39–44. [Google Scholar]
- Khaliq, A.; Afzal, M.; Khan, A.A.; Raza, A.M.; Kamran, M.; Tahir, H.M.; Aqeel, M.A.; Ullah, M.I. Management of Thrips tabaci (Thysanoptera: Thripidae) through agronomic practices in onion field plots. Pak. J. Zool. 2016, 48, 1675–1680. [Google Scholar]
- Devi, M.S.; Roy, K. Comparable study on different coloured sticky traps for catching of onion thrips, Thrips tabaci Lindeman. J. Entomol. Zool. Stud. 2017, 5, 669–671. [Google Scholar]
- Kumar, A.; Lal, R.; Kumar, A.; Wagan, M.A. Thrips and Whitefly Trapping With Three Colors Sticky Trap on Okra Crop. Int. J. Innov. Res. Sci. Eng. Stud. 2023, 3, 16–18. [Google Scholar]
- Pramudi, M.I.; Febrianti, E.; Rosa, H.O. Pengaruh Ekstrak Patikan Kebo (Euphorbhia hirta Linn) Terhadap Serangan Hama Daun Sawi. J. Prot. Tanam. Trop. 2022, 5, 407–413. [Google Scholar] [CrossRef]
- Azhari, A.A.; Sayuthi, M.; Hasnah, H. Patogenisitas Cendawan Metarhizium anisopliae (Metsch) dalam mengendalikan Kepik Hijau (Nezara viridula L.) pada Stadia Perkembangan yang Berbeda di Laboratorium. J. Ilm. Mhs. Pertan. 2019, 4, 178–187. [Google Scholar] [CrossRef]
- Abudulai, M.; Shepard, B.; Mitchell, P. Antifeedant and toxic effects of a neem (Azadirachta indica A. Juss)-based formulation Neemix® against Nezara viridula (L.) (Hemiptera: Pentatomidae). J. Entomol. Sci. 2003, 38, 398–408. [Google Scholar] [CrossRef]
- Reddy, P.P. Nematode Diseases of Crops and Their Management; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Lordello, L.G.E.; Zamith, A.P.L.; Boock, O. Novo nematódeo parasito da batatinha. Bragantia 1954, 13, 141–149. [Google Scholar] [CrossRef]
- Danso, Y.; Abugri, B. Plant Parasitic Nematodes Associated with Okra in the Forest Savanna Transition and Semi-Deciduous Forest Agro-Ecologies of Ghana. Agric. Food Sci. J. Ghana 2021, 14, 1483–1492. [Google Scholar] [CrossRef]
- Adekunle, O. Population dynamics of Meloidogyne incognita and three other phytonematodes on okra cultivars planted in alleys of Leucaena leucocephala and Gliricidia sepium. Australas. Plant Pathol. 2009, 38, 211–215. [Google Scholar] [CrossRef]
- Waller, J.; Bigger, M.; Hillocks, R. Nematodes. In Coffee Pests, Diseases and Their Management; CABI: Wallingford, UK, 2007; pp. 258–276. [Google Scholar]
- Subarjah, C.; Himawan, T.; Puspitarini, R.D. Effects of compost on nematode Pratylenchus sp.(Tylenchida: Pratylenchidae) population in patchouli. J. Trop. Life Sci. 2016, 6, 101–106. [Google Scholar] [CrossRef]
- Jukte, S.; Badgujar, S.; Suryawanshi, A.; Utpal, D.; Kuldhar, D. Symptomatology, isolation, identification and pathogenicity test of damping off disease in okra. Int. J. Plant Prot. 2016, 9, 358–361. [Google Scholar] [CrossRef]
- Dahivelkar, P.; Atre, G.; Gawande, P.; Mate, G. Management of powdery mildew of okra caused by Erysiphe cichoracearum. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3183–3189. [Google Scholar] [CrossRef]
- Bolie, H.; Ndongo, B.; Ngatsi, P.Z.; Kuate, W.N.T.; Dida, S.L.L.; Essogue Etame, A.; Essomé, C.S.; Tonfack, L.B. Antifungal activity of Annona muricata seed extracts against Cercospora malayensis, causal agent of cercospora leaf spot disease of okra (Abelmoschus esculentus L.). Int. J. Pathog. Res. 2021, 6, 12–24. [Google Scholar] [CrossRef]
- Afroz, T.; Aktaruzzaman, M.; Kim, B.-S. First Report of Gray Mold on Okra Caused by Botrytis cinerea in Korea. Plant Dis. 2019, 103, 1038. [Google Scholar] [CrossRef]
- Cho, J.; Moon, B. The occurrence of strawberry black leaf spot caused by Alternaria alternata (Fr.) Keissler in Korea. Korean J. Plant Prot. 1980, 19, 221–227. [Google Scholar]
- Werner, M. Necrotic leaf spot of apple caused by fungi of the genus Alternaria. Ochr. Rosl. 1987, 31, 6–7. [Google Scholar]
- Canihos, Y.; Peever, T.; Timmer, L. Temperature, leaf wetness, and isolate effects on infection of Minneola tangelo leaves by Alternaria sp. Plant Dis. 1999, 83, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Gappa-Adachi, R. Studies on the taxonomy and ecology of pathogenic microorganisms related to the generation of an integrated pest management model for important horticultural crop diseases. J. Gen. Plant Pathol. 2018, 84, 435–436. [Google Scholar] [CrossRef]
- Awasthi, L. Recent Advances in the Diagnosis and Management of Plant Diseases; Springer Nature: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Beckman, C.H. The Nature of Wilt Diseases of Plants; APS Press: St. Paul, MN, USA, 1987. [Google Scholar]
- Hao, J.J.; Yang, M.E.; Davis, R.M. Effect of soil inoculum density of Fusarium oxysporum f. sp. vasinfectum race 4 on disease development in cotton. Plant Dis. 2009, 93, 1324–1328. [Google Scholar] [CrossRef]
- Schnathorst, W.; Mathre, D. Host range and differentiation of a severe form of Verticillium albo-atrum in cotton. Phytopathology 1966, 56, 1155–1161. [Google Scholar]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Pearson, C.; Schwenk, F.; Crowe, F.; Kelley, K. Colonization of soybean roots by Macrophomina phaseolina. Plant Dis. 1984, 68, 1086–1088. [Google Scholar] [CrossRef]
- Fugro, P. A new disease of okra (Abelmoschus esculentus L.) in India. J. Mycol. Plant Pathol. 1999, 29, 264. [Google Scholar]
- Amadi, J.; Nnamani, C.; Ozokonkwo, O.; Eze, C. Survey of the incidence and severity of okra (Abelmoschus esculentus L. Moench) Fruit rot in Awka South lga, Anambra state, Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1114–1121. [Google Scholar]
- Shi, Y.-X.; Zhang, X.-H.; Zhao, Q.; Li, B.-J. First report of Colletotrichum gloeosporioides causing anthracnose on okra in China. Plant Dis. 2019, 103, 1023. [Google Scholar] [CrossRef]
- Balogun, O.; Babatola, J. Effect of plant age and injury on the pathogenicity of Choanephora cucurbitarum in okra Abelmoschus esculentus Moench. Agrosearch 1999, 5, 62–69. [Google Scholar]
- Park, J.H.; Cho, S.E.; Choi, I.Y.; Shin, H.D. First Report of Choanephora Rot of Okra Caused by Choanephora cucurbitarum in Korea. J. Phytopathol. 2015, 163, 503–506. [Google Scholar] [CrossRef]
- Henz, G.P.; Lopes, C.A.; Reis, A. A novel postharvest rot of okra pods caused by Rhizoctonia solani in Brazil. Fitopatol. Bras. 2007, 32, 237–240. [Google Scholar] [CrossRef]
- Matny, O.N. First report of damping-off of okra caused by Phytophthora nicotianae in Iraq. Plant Dis. 2013, 97, 558. [Google Scholar] [CrossRef]
- Ghoneem, K.M.; El-Wakil, D.A.; Ahmed, M.I.; Kamel, H.M.; Rashad, E.M.; Al-Askar, A.A.; Elsherbiny, E.A.; Ibrahim, A.A. Biodiversity of Rhizoctonia solani in Phaseolus vulgaris Seeds in East Delta of Egypt. Agronomy 2023, 13, 1317. [Google Scholar] [CrossRef]
- Gaur, S.; Chauhan, S. Seasonal diversity of Pythium at Yamuna River Agra, India. J. Mycol. Pl. Pathol. 2007, 37, 37–39. [Google Scholar]
- Jukte, S.R.; Badgujar, S. In vitro Efficacy of Fungicides Against Pythium aphanidermatum Causing Damping off in Okra. Adv. Life Sci. 2016, 5, 10309–10312. [Google Scholar]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.-N. Revisiting sustainability of fungicide seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Patel, J.V.; Singh, A.; Kaur, K.; Bhandhari, D.; Banyal, D. Biological Control of Damping Off of Okra caused by Rhizoctonia solani. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2277–2292. [Google Scholar] [CrossRef]
- Abdelzaher, H.M.; Imam, M.; Shoulkamy, M.; Gherbawy, Y. Biological control of Pythium damping-off of bush okra using rhizosphere strains of Pseudomonas fluorescens. Mycobiology 2004, 32, 139–147. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Hashem, A.H.; Al-Askar, A.A.; AbdElgawad, H.; Attia, M.S. Efficient Role of Endophytic Aspergillus terreus in Biocontrol of Rhizoctonia solani Causing Damping-off Disease of Phaseolus vulgaris and Vicia faba. Microorganisms 2023, 11, 1487. [Google Scholar] [CrossRef]
- Jukte, S.R.; Badgujar, S.; Suryawanshi, A. In vitro Efficacy of Plant Extracts and Bioagents Against Pythium aphanidermatum Causing Damping off in Okra. Adv. Life Sci. 2016, 5, 10336–10338. [Google Scholar]
- Al-Lashi, N.B.; Mahmood, A.A. Biological Control of Damping–off of Okra by the Biopesticides Pseudomones fluorescens and Bacillus subtilis. Rafidain J. Sci. 2014, 25, 23–39. [Google Scholar] [CrossRef]
- Jalal, A.M.; Sulaiman, E.D.; Al Lashi, N.B. The Effect of Water Extract of Ocimum basilicum and the Fungal Biocontrol Agent Trichoderma harzianum on Fungi causing Damping-off of Okra. Rafidain J. Sci. 2009, 20, 12–27. [Google Scholar] [CrossRef]
- Jabur, S.G. ُEfficiency test of both bacterial isolation Bacillus subtilis and water extraction of dry bulb Allium sativum and chemical fungicide Ridomil–MZ-50 in control of damping–off of Okra caused by fungus Pythium aphanidermatum. Kufa J. Agric. Sci. 2009, 1, 51–60. [Google Scholar]
- Ale-Agha, N.; Boyle, H.; Braun, U.; Butin, H.; Jage, H.; Kummer, V.; Shin, H.-D. Taxonomy, host range and distribution of some powdery mildew fungi (Erysiphales). Schlechtendalia 2008, 17, 39–54. [Google Scholar]
- Gangwar, S.; Qadri, S.; Maji, M.; Kumar, P.P.; Saratchandra, B. Evaluation of fresh plant extracts for the control of mulberry powdery mildew. Indian J. Seric. 2000, 39, 76–78. [Google Scholar]
- Paulitz, T.C.; Bélanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [CrossRef]
- Naik, K.; Nagaraja, A. Chemical control of powdery mildew of okra. Indian J. Plant Prot. 2000, 28, 41–42. [Google Scholar]
- Jadav, A.; Kumar, B.M.; Kadvani, D.; Patel, T. Efficacy of various fungicides for the management of okra powdery mildew caused by Erysiphe cichoracearum DC. Int. J. Chem. Stud. 2019, 7, 4293–4295. [Google Scholar]
- Ashfaq, M.; Atiq, M.; Sahi, S.T.; Younas, M.; Ghafoor, K.; Shafiq, M.; Sajid, M.; Bashir, M.R.; Latif, S.; Mubeen, I. Management of powdery mildew of okra through plant extracts and chemicals under field conditions. Pak. J. Phytopathol. 2015, 27, 163–168. [Google Scholar]
- Zaher, E.A.; Abada, K.; Zyton, M.A. Management of Okra Powdery Mildew. J. Plant Prod. 2008, 33, 4945–4956. [Google Scholar] [CrossRef]
- Alexis, Z.P.; Pierre, E.O.; Pierre-François, D. Performance of Trichoderma harzianum and Bacillus amyloliquefaciens bioformulation on germination and expression of bioprotective molecules against Cercosporiosis in Abelmoschus esculentus in the field. J. Adv. Biol. Biotechnol. 2023, 26, 14–29. [Google Scholar] [CrossRef]
- Shrawan, K.; Dabbas, M.; Priti, T. Evaluation chemicals against cercospora leaf spot of okra. Int. J. Plant Prot. 2015, 8, 384–388. [Google Scholar] [CrossRef]
- Armand, N.M.; Martin, B.J.; Célestin, D.; Mireille, T.F.; BILLE, N.H.; Ntsefong, G.N.; AKOA, A. Assessment of the sensitivity of some varieties of okra [Abelmoschus esculentus (Moench)] to cercosporiose of the leaves caused by Cercospora malayensis and Pseudocercospora abelmoschii. World 2013, 1, 1–11. [Google Scholar]
- Diao, Y.; Larsen, M.M.; Kamvar, Z.N.; Zhang, C.; Li, S.; Wang, W.; Lin, D.; Peng, Q.; Knaus, B.J.; Foster, Z.S. Genetic differentiation and clonal expansion of Chinese Botrytis cinerea populations from tomato and other crops in China. Phytopathology 2020, 110, 428–439. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Li, D.; Reymick, O.O.; Tan, X.; Tao, N. Isolation and control of Botrytis cinerea in postharvest green pepper fruit. Sci. Hortic. 2022, 302, 111159. [Google Scholar] [CrossRef]
- Pansambal, S.; Raut, R.; Mahajan, P. Bio-efficacy of different fungicides against Alternaria leaf spot of okra caused by Alternaria chlamydospora. Trends Biosci. 2015, 8, 5583–5587. [Google Scholar]
- Feng, W.; Zheng, X. Essential oils to control Alternaria alternata in vitro and in vivo. Food Control 2007, 18, 1126–1130. [Google Scholar] [CrossRef]
- Devi, N.S.; Basu, A. Antifungal activity of some botanical oils against Alternaria solani, causing leaf blight of potato. Indian J. Plant Prot. 2013, 41, 167–171. [Google Scholar]
- Bhattarai, B.; Jha, S.K. Antifungal effects of some plant essential oils against Alternaria alternata (Fr.) kiessl. and Aspergillus niger van Tiegh. from grapes. Biol. Forum Int. J. 2016, 8, 259–263. [Google Scholar]
- Naik, U.; Fugro, P.; Kadam, J.; Jadhav, D. Exploration of fungicides, bio-agents and botanicals against leaf blight of okra incited by Alternaria chlamydospora. J. Plant Dis. Sci. 2010, 5, 37–40. [Google Scholar]
- Ziedan, E. First report of Alternaria pod blight of okra in Egypt. J. Agric. Technol. 2012, 8, 2239–2243. [Google Scholar]
- Okwu, D.E.; Awurum, A.N.; Okoronkwo, J.I. Phytochemical composition and in vitro antifungal activity screening of extracts from citrus plants against Fusarium oxysporum of okra plant (Hibiscus esculentus). Summa Phytopathol. 2007, 30, 145–148. [Google Scholar]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.; Crous, P.W. Epitypification of Fusarium oxysporum–clearing the taxonomic chaos. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Burgess, L. General Ecology of the Fusaria; Pennsylvania State Univ. Press: University Park, PA, USA, 1981. [Google Scholar]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef]
- Sain, S.K.; Pandey, A.K. Evaluation of some Trichoderma harzianum isolates for the management of soilborne diseases of brinjal and okra. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 905–914. [Google Scholar] [CrossRef]
- Shah, N.; Syed, R.N.; Khanzada, M.A.; Rajput, N.A.; Lodhi, A.M. Non-chemical management of okra wilt caused by Fusarium oxysporum. Int. J. Agric. Appl. Sci. 2015, 7, 86–94. [Google Scholar]
- Gowda, B.H.; Ramesh, P.; Saifulla, M. In vitro and field screening of okra cultivars against Fusarium wilt disease. Pest Manag. Hortic. Ecosyst. 2020, 26, 129–133. [Google Scholar] [CrossRef]
- McLeod, J.M.; Witcher, W.; Epps, W.; Robbins, M. Resistance of Okra Plant Introductions to Root Knot Nematode and Fusarium Wilt. HortScience 1983, 18, 249–250. [Google Scholar] [CrossRef]
- Aguiar, F.M.; Michereff, S.J.; Boiteux, L.S.; Reis, A. Search for sources of resistance to Fusarium wilt (Fusarium oxysporum f. sp. vasinfectum) in okra germplasm. Crop Breed. Appl. Biotechnol. 2013, 13, 33–40. [Google Scholar] [CrossRef]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Farag, M.M.; Arafa, R.A.; Abou-Zeid, M.A.; Alwutayd, K.M.; Abd El Moneim, D.; Ghebrial, E.W. First Appearance of Verticillium tricorpus Causing Verticillium Wilt in tested Okra varieties. Res. Sq. 2023; preprints. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef]
- Tok, F.M.; Dervis, S.; Yetisir, H. Vegetative Compatibility and Virulence Diversity of Verticillium dahliae from Okra (Abelmoschus esculentus) Plantations in Turkey and Evaluation of Okra Landraces for Resistance to V. dahliae. Phyton 2020, 89, 303. [Google Scholar] [CrossRef]
- Jana, T.; Singh, N.; Koundal, K.; Sharma, T. Genetic differentiation of charcoal rot pathogen, Macrophomina phaseolina, into specific groups using URP-PCR. Can. J. Microbiol. 2005, 51, 159–164. [Google Scholar] [CrossRef]
- Prabhu, H.V.; Adiver, S.; Bhat, R.; Narayana, Y.; Jahagirdar, S.; Parameshwarappa, K. Genetic variability in Macrophomina phaseolina (Tassi.) Goid., causal agent of charcoal rot of sorghum. Karnataka J. Agric. Sci. 2012, 25, 72–76. [Google Scholar]
- Pun, K.; Doraiswamy, S.; Valluvaparidasan, V. Studies on seed-borne nature of Macrophomina phaseolina in okra. Plant Dis. Res. 1998, 13, 162–164. [Google Scholar]
- Reuveni, R.; Nachmias, A.; Krikun, J. The role of seedborne inoculum on the development of Macrophomina phaseolina on melon. Plant Dis. 1983, 67, 280–281. [Google Scholar] [CrossRef]
- Gulya, T.; Krupinsky, J.; Draper, M.; Charlet, L. First report of charcoal rot (Macrophomina phaseolina) on sunflower in North and South Dakota. Plant Dis. 2002, 86, 923. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Iqbal, R.; Hannan, A.; Sahi, S.T. Antagonism of selected fungal species against Macrophomina phaseolina (Tassi) goid, causing charcoal rot of Mungbean. Pak. J. Bot. 2022, 54, 1129–1138. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J. Plant Prot. Res. 2016, 56, 21–31. [Google Scholar] [CrossRef]
- Aravind, T.; Brahmbhatt, A. Management of root and collar rot (Macrophomina phaseolina (Tassi) Goid.) of Okra (Abelmoschus esculentus (L.) Moench) through bioagents, oil cakes and fungicides. J. Pharmacogn. Phytochem. 2018, 7, 631–635. [Google Scholar]
- Aravind, T.; Brahmbhatt, A. Estimation of biochemical factors responsible for resistance to root and collar rot (Macrophomina phaseolina (Tassi) Goid.) in okra. J. Appl. Nat. Sci. 2018, 10, 1266–1270. [Google Scholar] [CrossRef]
- Fugro, P.; Jadhav, N. Stem canker of okra in Konkan region of Maharashtra. J. Mycol. Plant Pathol. (India) 2003, 33, 288–289. [Google Scholar]
- Batista, I.C.A.; Boari, A.d.J.; Kauffmann, C.M.; Nechet, K.d.L. Colletotrichum plurivorum causes anthracnose on okra in Brazil. J. Plant Pathol. 2020, 102, 1331. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Li, Y.; Liu, X.; Liu, L.; Yan, J.; Hu, X.; Qin, W. Thymol Edible Coating Controls Postharvest Anthracnose by Regulating the Synthesis Pathway of Okra Lignin. Foods 2023, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Ngonadi, E.; Mohammed, Z.; Onwughalu, J. Hort communication Efficacy of plant extracts on control of okra anthracnose (Colletotrichum gloeosporiodes) in the Sudan Savannah ecology of Nigeria. Niger. J. Plant Prot. 2019, 33, 105–113. [Google Scholar]
- Esuruoso, O.; Ogundiran, S.; Chheda, H.; Fatokun, D. Seedborne fungi and some fungal diseases of okra in Nigeria. Plant Dis. Report. 1975, 59, 660–663. [Google Scholar]
- Hussein, E.; Ziedan, E. First report of pod blight of okra caused by Choanephora cucurbitarum in Egypt. J. Agric. Technol. 2013, 9, 135–140. [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV). Current ICTV Taxonomy Release. Available online: https://ictv.global/taxonomy (accessed on 21 August 2024).
- Briddon, R.W.; Patil, B.L.; Bagewadi, B.; Nawaz-ul-Rehman, M.S.; Fauquet, C.M. Distinct evolutionary histories of the DNA-A and DNA-B components of bipartite begomoviruses. BMC Evol. Biol. 2010, 10, 97. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Reddy, C.L.; Jalali, S.; Reddy, M.K. Association of tomato leaf curl New Delhi virus DNA-B with bhendi yellow vein mosaic virus in okra showing yellow vein mosaic disease symptoms. Acta Virol. 2015, 59, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.; Singh, S. Effect of yellow-vein mosaic virus infection on growth and yield of okra crop. Indian Phytopathol. 1974, 27, 294–297. [Google Scholar]
- Givord, L.; Boer, L.D. Insect transmission of okra mosaic virus in the Ivory Coast. Ann. Appl. Biol. 1980, 94, 235–241. [Google Scholar] [CrossRef]
- Hernandez-Zepeda, C.; Isakeit, T.; Scott Jr, A.; Brown, J. First report of Okra yellow mosaic Mexico virus in okra in the United States. Plant Dis. 2010, 94, 924. [Google Scholar] [CrossRef]
- Singh, S. Assessment of losses in okra due to enation leaf curl virus. Indian J. Virol. 1996, 12, 51–53. [Google Scholar]
- Sattar, M.N. Diversity and Interactions of Begomoviruses and Their Associated DNA-Satellites; Department of Plant Biology and Forest Genetics, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2012. [Google Scholar]
- Akhtar, S.; Khan, A.J.; Singh, A.S.; Briddon, R.W. Identification of a disease complex involving a novel monopartite begomovirus with beta-and alphasatellites associated with okra leaf curl disease in Oman. Arch. Virol. 2014, 159, 1199–1205. [Google Scholar] [CrossRef]
- Hoffmann, L.V.; Inoue-Nagata, A.K.; Vaz, L.N.A.; Barroso, P.A.V.; Faria, J.C.d. Identification of sida micrantha mosaic virus as the causal agent of common mosaic in cotton in Goiás. Summa Phytopathol. 2022, 47, 222–224. [Google Scholar] [CrossRef]
- Tahir, M.; Haider, M.S.; Briddon, R.W. Complete nucleotide sequences of a distinct bipartite begomovirus, bitter gourd yellow vein virus, infecting Momordica charantia. Arch. Virol. 2010, 155, 1901–1905. [Google Scholar] [CrossRef]
- Sergius, U.O.; Esther, D.U. Screening of Abelmoschus esculentus and Abelmoschus callei cultivars for resistance against okra leaf curl and okra mosaic viral diseases, under field conditions in South Eastern Nigeria. Afr. J. Biotechnol. 2014, 13, 4419–4429. [Google Scholar] [CrossRef]
- Costa, A. Anthocyanosis, a virus disease of Cotton in Brazil. Phytopathol. Z. 1956, 28, 167–186. [Google Scholar]
- Wintermantel, W.M.; Wisler, G.C. Vector specificity, host range, and genetic diversity of Tomato chlorosis virus. Plant Dis. 2006, 90, 814–819. [Google Scholar] [CrossRef]
- Adkins, S. Tomato spotted wilt virus—Positive steps towards negative success. Mol. Plant Pathol. 2000, 1, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, A.; Singh, S.; Roy, J.; Lalit, A.; Parmar, D.; Sharma, N.; Tuli, R. First report of Radish leaf curl virus infecting okra in India. New Dis. Rep. 2012, 7, 13–24. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Lakshminarayana Reddy, C.; Swarnalatha, P.; Devaraju; Jalali, S.; Reddy, M.K. Molecular evidence for association of Cotton leaf curl Alabad virus with yellow vein mosaic disease of okra in North India. Arch. Phytopathol. Plant Prot. 2012, 45, 2095–2113. [Google Scholar] [CrossRef]
- Kumar, R.; Esakky, R.; Palicherla, S.R. The emergence of okra enation leaf curl virus—An important begomovirus, infecting okra in several states across India. Arch. Phytopathol. Plant Prot. 2019, 52, 234–238. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Reddy, C.L.; Jalali, S.; Briddon, R.W.; Reddy, M.K. Molecular identification and biological characterisation of a begomovirus associated with okra enation leaf curl disease in India. Eur. J. Plant Pathol. 2015, 141, 217–235. [Google Scholar] [CrossRef]
- Saeed, F.; Sattar, M.N.; Hameed, U.; Ilyas, M.; Haider, M.S.; Hamza, M.; Mansoor, S.; Amin, I. Infectivity of okra enation leaf curl virus and the role of its V2 protein in pathogenicity. Virus Res. 2018, 255, 90–94. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Lakshminarayana Reddy, C.; Swaranalatha, P.; Jalali, S.; Briddon, R.W.; Reddy, M.K. Diversity and phylogeography of begomovirus-associated beta satellites of okra in India. Virol. J. 2011, 8, 555. [Google Scholar] [CrossRef]
- Chandran, S.; Packialakshmi, R.; Subhalakshmi, K.; Prakash, C.; Poovannan, K.; Nixon Prabu, A.; Gopal, P.; Usha, R. First report of an alphasatellite associated with Okra enation leaf curl virus. Virus Genes 2013, 46, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Rishishwar, R.; Khan, Z.A.; Dasgupta, I. Agrobacterium-mediated co-inoculation of okra plants with cloned okra enation leaf curl virus DNA and bhendi yellow vein mosaic beta-satellite DNA furthers Koch’s postulates for enation leaf curl disease. J. Virol. Methods 2022, 300, 114413. [Google Scholar] [CrossRef]
- Pullaiah, N.; Reddy, T.; Moses, G.; Reddy, B.; Reddy, D. Inheritance of resistance to yellow vein mosaic virus in okra (Abelmoschus esculentus (L.) Moench). Indian J. Genet. Plant Breed. 1998, 58, 349–352. [Google Scholar]
- Lana, A.O.; Taylor, T.A. The insect transmission of an isolate of okra mosaic virus occurring in Nigeria. Ann. Appl. Biol. 1976, 82, 361–364. [Google Scholar] [CrossRef]
- Atiri, G. The occurrence and importance of okra mosaic virus in Nigerian weeds. Ann. Appl. Biol. 1984, 104, 261–265. [Google Scholar] [CrossRef]
- Wasala, S.; Senevirathne, S.I.; Senanayake, J.B.; Navoditha, A. Genetic analysis of Okra Yellow Vein Mosaic Virus disease resistance in wild relative of okra Abelmoschus angulosus Wall. ex Wight & Arn. Plant Genet. Resour. 2019, 17, 346–351. [Google Scholar] [CrossRef]
- Singh, G.; Pathak, M.; Sharma, A. Identification of novel sources of yellow vein mosaic disease resistance in okra (Abelmoschus esculentus L. Moench). Euphytica 2023, 219, 111. [Google Scholar] [CrossRef]
- Rashid, M.; Yasmin, L.; Kibria, M.; Mollik, A.; Hossain, S.M. Screening of okra germplasm for resistance to yellow vein mosaic virus under field conditions. Pak. J. Plant Pathol. 2002, 1, 61–62. [Google Scholar] [CrossRef]
- Yadav, Y.; Maurya, P.K.; Devi, A.P.; Jamir, I.; Bhattacharjee, T.; Banerjee, S.; Dutta, S.; Debnath, D.; Mandal, A.K.; Dutta, S. Enation leaf curl virus (ELCV): A real threat in major okra production belts of India: A review. J. Pharmacogn. Phytochem. 2018, 7, 3795–3802. [Google Scholar]
- Kumar, S.D. Enumerations on seed-borne and post-harvest microflora associated with okra [Abelmoschus esculentus (L.) Moench] and their management. GSC Biol. Pharm. Sci. 2019, 8, 119–130. [Google Scholar]
- Goss, O.M. Hibiscus leaf spot. J. Dep. Agric. West. Aust. 1962, 3, 7–9. [Google Scholar]
- Kimura, O.; Ribeiro, R.D.L.; Robbs, C. Fruit rot and leaf blight of H. esculentum caused by Pseudomonas syringae. Arq. Univ. Fed. Rural Rio Jan. 1982, 5, 105–110. [Google Scholar]
- Stăncescu, C.; Zurini, I. Bacterial disease of okra, a new disease identified in Romania. Analele Instilutului Cercet. Pentru Prot. Plantelor 1987, 20, 43–50. [Google Scholar]
- Brown, C.; McCarter, S. A leaf spot and blight of Abelmoschus moschatus caused by a pathovar of Pseudomonas syringae. Plant Dis. 1991, 75, 424–429. [Google Scholar] [CrossRef]
- Scortichini, M.; Marchesi, U.; Dettori, M.; Rossi, M. Genetic diversity, presence of the syrB gene, host preference and virulence of Pseudomonas syringae pv. syringae strains from woody and herbaceous host plants. Plant Pathol. 2003, 52, 277–286. [Google Scholar] [CrossRef]
- Ranković, T.; Nikolić, I.; Berić, T.; Popović, T.; Lozo, J.; Medić, O.; Stanković, S. Genome analysis of two Pseudomonas syringae pv. aptata strains with different virulence capacity isolated from sugar beet: Features of successful pathogenicity in the phyllosphere microbiome. Microbiol. Spectr. 2023, 11, e03598-22. [Google Scholar] [CrossRef] [PubMed]
- Addy, S. Hypersensitive reactions in cotton and okra by Xanthomonas oryzae (Uyeda & Ishiyama) Dowson. Curr. Sci. 1974, 43, 526–527. [Google Scholar]
- Phookan, A.; Addy, S. The effect of extracellular polysaccharide from Xanthomonas campestris pv. oryzae on some plant cuttings. J. Res. Assam Agric. Univ. 1983, 4, 74–76. [Google Scholar]
- Jain, S.; Saxena, A.; Saxena, S. Two new post harvest diseases of fruits from India [dry rot of Pyrus communis caused by Fusarium solani (Mart.) Sacc. and dry rot of Abelmoschus esculantum caused by Aspergillus flavus Link ex Fries]. Curr. Sci. 1982, 51, 1071. [Google Scholar]
- Delannoy, E.; Lyon, B.; Marmey, P.; Jalloul, A.; Daniel, J.; Montillet, J.; Essenberg, M.; Nicole, M. Resistance of cotton towards Xanthomonas campestris pv. malvacearum. Annu. Rev. Phytopathol. 2005, 43, 63–82. [Google Scholar] [CrossRef]
- Van Loon, L. Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 1997, 103, 753–765. [Google Scholar] [CrossRef]
- Vrancken, K.; Holtappels, M.; Schoofs, H.; Deckers, T.; Valcke, R. Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: State of the art. Microbiology 2013, 159, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J. Implementing biopesticides as part of an integrated pest management (IPM) programme. In Biopesticides for Sustainable Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 21–44. [Google Scholar]
- European Commission. Integrated Pest Management (IPM). Available online: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/integrated-pest-management-ipm_en (accessed on 22 June 2024).
- Food and Agriculture Organization of the United Nations. Integrated Pest Management. Available online: https://www.fao.org/pest-and-pesticide-management/ipm/integrated-pest-management/en/ (accessed on 26 June 2024).
- Singh, P.; Chauhan, V.; Tiwari, B.K.; Chauhan, S.S.; Simon, S.; Bilal, S.; Abidi, A. An overview on okra (Abelmoschus esculentus) and it’s importance as a nutritive vegetable in the world. Int. J. Pharm. Biol. Sci. 2014, 4, 227–233. [Google Scholar]
- Parsa, S.; Morse, S.; Bonifacio, A.; Chancellor, T.C.; Condori, B.; Crespo-Pérez, V.; Hobbs, S.L.; Kroschel, J.; Ba, M.N.; Rebaudo, F. Obstacles to integrated pest management adoption in developing countries. Proc. Natl. Acad. Sci. USA 2014, 111, 3889–3894. [Google Scholar] [CrossRef]
- Vikaspedia. Integrated Pest Management Strategies for Okra. Available online: https://agriculture.vikaspedia.in/viewcontent/agriculture/crop-production/integrated-pest-managment/ipm-for-vegetables/integrated-pest-management-strategies-for-okra?lgn=en (accessed on 22 September 2024).
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Mohapatra, S.; Padhi, J.; Singh, S. Enhancing yield and economic benefits through sustainable pest management in Okra cultivation. Sci. Rep. 2024, 14, 22220. [Google Scholar] [CrossRef]
- Sushil, S.N.; Singh, J.P.; Kapoor, K.S.; Chakraborty, A. Integrated Pest Management (IPM) in Okra (Abelmoschus esculentus) for Export Purpose; Directorate of Plant Protection, Quarantine & Storage, Ministry of Agriculture & Farmers Welfare, Government of India: New Delhi, India.
- Bhalodia, M.; Devi, Y.K. Assessment of various integrated pest management modules to manage the incidence of okra shoot and fruit borer for adaptation to climate change: A review. Int. J. Entomol. Res. 2022, 7, 98–103. [Google Scholar]
- Ghuge, D.; Gosalwad, S.; Patil, S. Effect of weather factors on the incidence of major insect pest on okra. J. Entomol. Zool. Stud. 2020, 8, 1474–1479. [Google Scholar]
- Onyeneke, R.U.; Agyarko, F.F.; Onyeneke, C.J.; Osuji, E.E.; Ibeneme, P.A.; Esfahani, I.J. How does climate change affect tomato and okra production? evidence from Nigeria. Plants 2023, 12, 3477. [Google Scholar] [CrossRef]
- Olabiyi, T.I.; Afolabi, E.A.; Adewole, E.I.; Omomowo, I.O. Impact assessment of climate change on crop diseases incidence and severity in Nigeria. In Climate Change Adaptation in Africa: Fostering Resilience and Capacity to Adapt; Springer: Cham, Switzerland, 2017; pp. 235–244. [Google Scholar] [CrossRef]
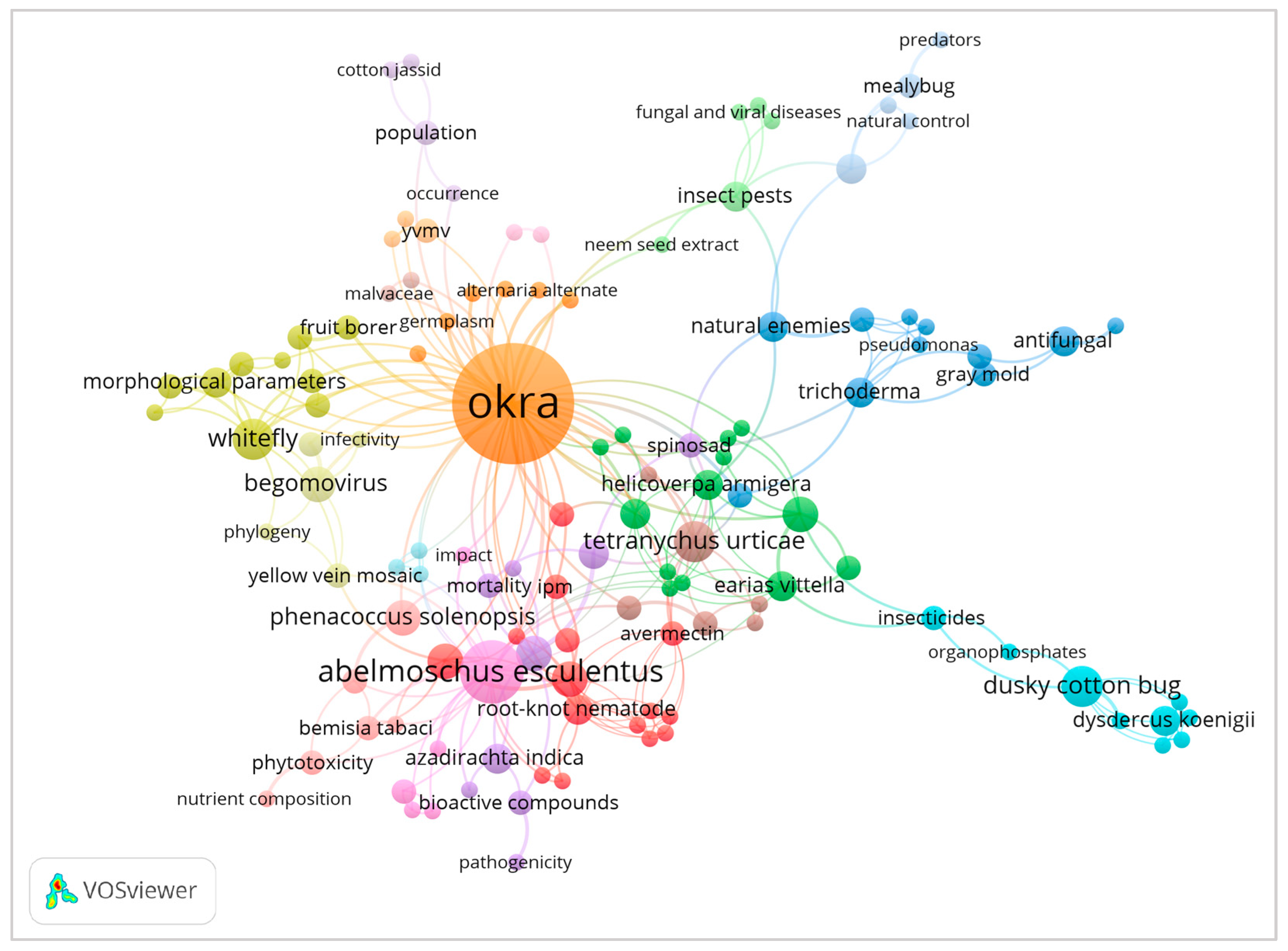

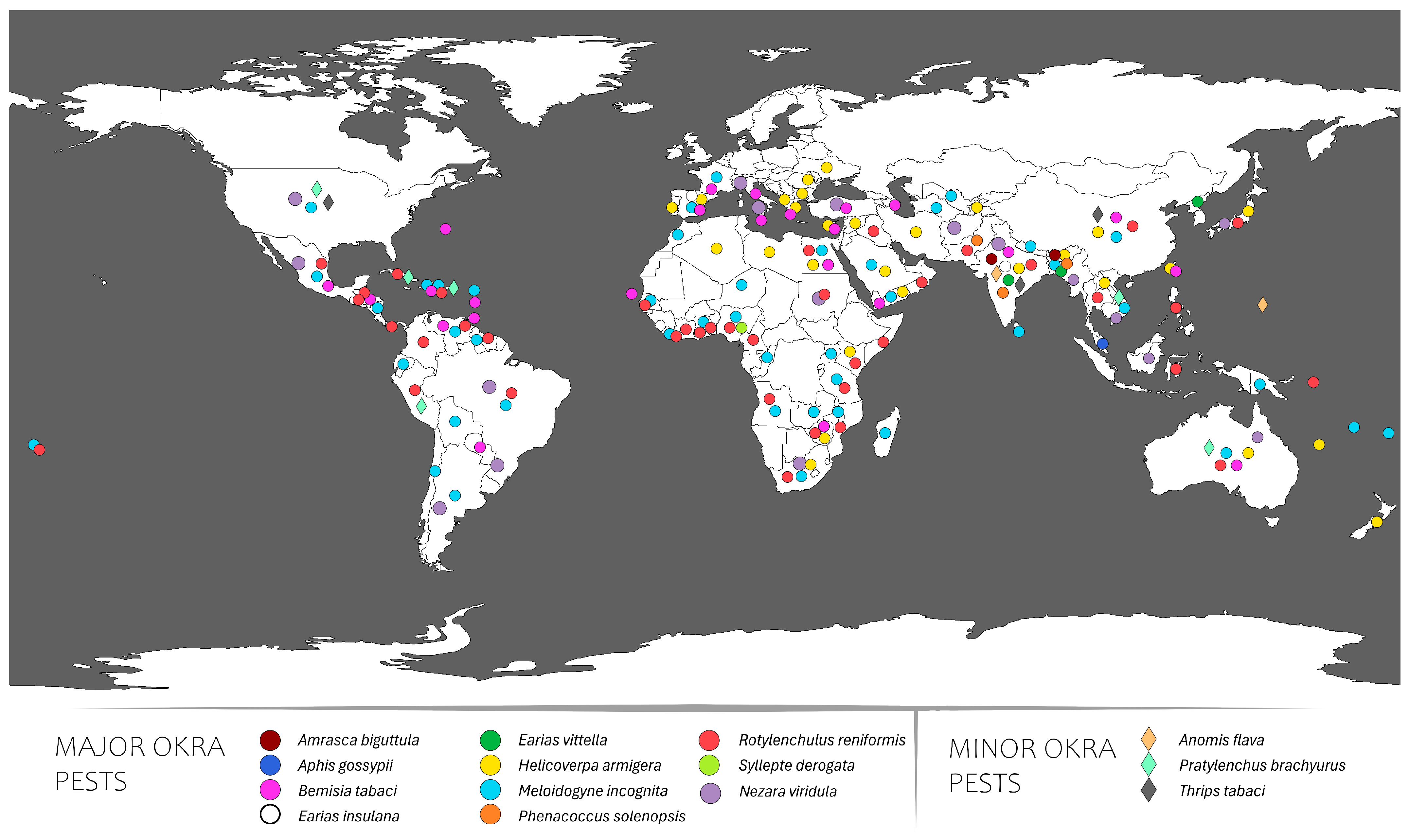
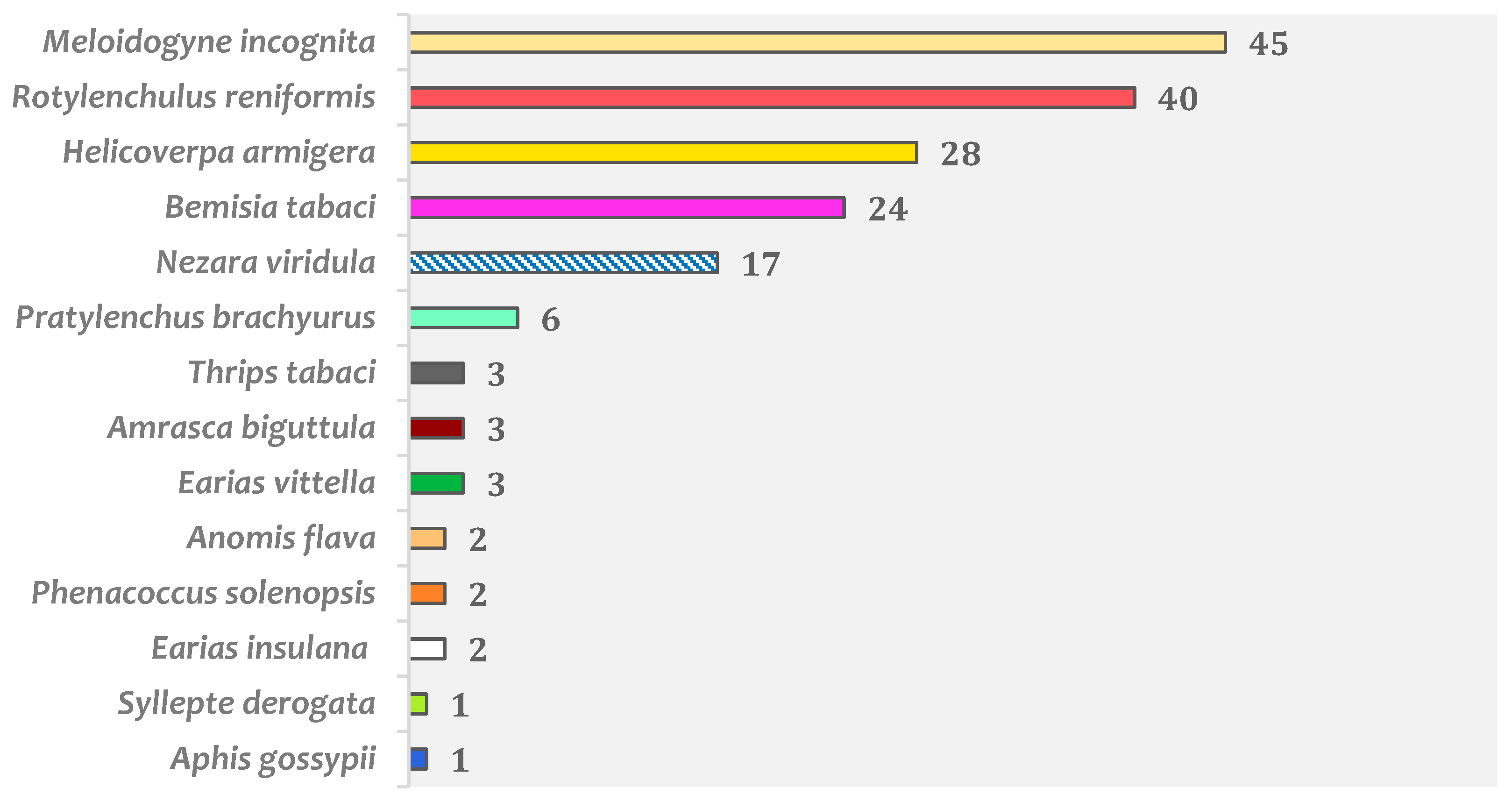
| Taxonomical Group | Common Name | Scientific Name | Global Distribution | Countries Reporting Pest as Widespread | |
|---|---|---|---|---|---|
| Major pests | Hemiptera | Cotton aphid | Aphis gossypii Glover 1877 | Present in most countries across North and South America, Europe, Africa, Asia, and Australia [39] | Singapore [39] |
| Hemiptera | Cotton mealybug | Phenacoccus solenopsis Tinsley 1898 | Present in all countries of North and South America, as well as in many countries across Africa, Asia, and Australia [40] | China, India (due to the difficulty of identifying the species, the pest has been reported to be likely more widespread than the countries listed) [40] | |
| Hemiptera | Whitefly | Bemisia tabaci Gennadius 1889 | Present in all countries of North and South America, as well as in most countries across Africa, Europe, Asia, and Australia [41] | Cape Verde, Egypt, Sudan, Zimbabwe, Belize, Bermuda, Haiti, Martinique (France), Mexico, Paraguay, Trinidad and Tobago, Venezuela, China, India, Taiwan, Yemen, Azerbaijan, Cyprus, Greece, Italy, Malta, Spain, Turkey, Australia [41] | |
| Hemiptera | Cotton leafhopper | Amrasca biguttula Ishida 1912 | Present in East Asia and Southeast Asia, with some regions of Africa [42] | India, Pakistan, Bangladesh [42] | |
| Lepidoptera | Cotton spotted bollworm | Earias vittella Fabricius 1794 | Present in East Asia, Southeast Asia, parts of the Middle East, and Australia, with some presence in Southern Europe, specifically in Spain [43] | India, Bangladesh, North Korea [43] | |
| Lepidoptera | Egyptian bollworm | Earias insulana Boisduval 1833 | Present in Eastern Europe, East Asia, Southeast Asia, parts of the Middle East, Australia, and Africa [44] | India, Spain [44] | |
| Lepidoptera | Cotton bollworm | Helicoverpa armigera Hübner 1805 | Present across most countries of South America, Africa, Europe, Asia, and Australia. It is notably absent from North America, northern countries of South America, and Mongolia [45] | Algeria, Egypt, Kenya, Libya, South Africa, Zimbabwe, Bangladesh, China, India, Iran, Japan, Laos, Saudi Arabia, Syria, Taiwan, Tajikistan, Yemen, Albania, Bulgaria, Cyprus, Greece, Portugal, Romania, Spain, Ukraine, Australia, New Caledonia (France), New Zealand [45] | |
| Lepidoptera | Cotton leaf roller | Syllepte derogata Fabricius 1775 | Present in many African countries, East Asia, Southeast Asia, and Australia. It is notably absent from Europe and the Americas [46] | Nigeria [46] | |
| Coleoptera | Flea beetles | Nisotra uniformis Jacoby 1906 & N. sjoestedti Jacoby 1903 | Reported presence in Sub-Saharan Africa [47], with N. uniformis and N. stotjedii recorded in Nigeria [48,49,50,51] and N. uniformis also documented in Ghana [52], Sudan [53,54], and Burkina Faso [55] | N/A | |
| Rhabditida | Root-knot nematodes | Meloidogyne spp. | M. incognita is present in all countries of the Americas, Oceania, and most countries of Europe, Asia, and Africa, excluding Norway, Sweden, Finland, Ireland, Chad, South Sudan, Eq. Guinea, Rwanda, Burundi, Namibia, Cambodia, North Korea, and Laos [56] | In the case of M. incognita: Chile, Argentina, Brazil, Bolivia, Guyana, Venezuela, Ecuador, Guadeloupe, Dominican Republic, Cuba, Honduras, Mexico, USA, Australia, Fiji, Vanuatu, Samoa, Papua New Guinea, Vietnam, China, Bangladesh, Sri Lanka, Nepal, Uzbekistan, Turkmenistan, Yemen, Saudi Arabia, Egypt, Libya, France, Spain, Morocco, Senegal, Niger, Nigeria, Ghana, Liberia, Congo, Uganda, Tanzania, Malawi, Zambia, Angola, Madagascar, South Africa [56] | |
| Rhabditida | Reniform nematodes | Rotylenchulus reniformis Linford & Oliveira 1940 | Present in parts of North America, Central America, South America, Sub-Saharan Africa, East Asia, Southeast Asia, and Australia. In Europe, its presence has only been recorded in Austria [57] | Mexico, Cuba, Belize, Brazil, Suriname, Venezuela, Colombia, Peru, Panama, Honduras, Dominican Republic, Gambia, Liberia, Côte d’Ivoire, Ghana, Togo, Nigeria, Cameroon, Angola, South Africa, Mozambique, Zimbabwe, Tanzania, Kenya, Somalia, Sudan, Egypt, Oman, Iraq, Pakistan, India, China, Japan, Thailand, Philippines, Indonesia, Australia, Solomon Islands, Fiji [57] | |
| Acari | Two spotted spider mite | Tetranychus urticae Koch 1836 | Recorded in most countries in Europe, Asia, Africa, Australasia, Pacific and Caribbean islands, and North, Central, and South America [58] | N/A | |
| Minor pests | Hemiptera | Red cotton bug | Dysdercus koenigii Fabricius 1775 | India [59] and Pakistan [60,61] | N/A |
| Hemiptera | Cotton seed bug | Oxycarenus hyalinipennis A. Costa 1843 | Has a worldwide distribution, including a first report in the Bahamas [62], as well as reports in Pakistan [63,64], India [65], Spain [66], Egypt [67], and the UK [68] | N/A | |
| Hemiptera | Green plant bug | Nezara viridula Linnaeus 1758 | Widely distributed, especially in the southern USA, Central and South America, the Mediterranean, the Middle East, Africa, Malaysia, the Philippines, Indonesia, Japan, and the Pacific Islands [69] | USA, Mexico, Brazil, Uruguay, Argentina, South Africa, Sudan, Italy, Sardinia (Italy) Turkey, Afghanistan, India, Myanmar, Bangladesh, Vietnam, Indonesia, Japan, Australia [69] | |
| Diptera | Okra stem fly | Melanagromyza hibisci Spencer 1961 (Diptera) | Present in India [70,71] | N/A | |
| Coleoptera | Blister beetles | Mylabris pustulata Olivier 1795 & M. phalerata Pallas 1781 (Coleoptera) | Widely distributed in India, with many records on okra [51,72,73] | N/A | |
| Lepidoptera | Cotton looper | Anomis flava Fabricius 1775 | Present in many countries of Africa, Oceania, South and Southeast Asia. It is notably absent in Europe and the Americas [74] | India, Northern Mariana Islands [74] | |
| Thysanoptera | Onion thrips | Thrips tabaci Lindeman 1889 | Distributed across North America and present in most countries of South America, Oceania, Asia, and many countries in Africa. Present throughout Europe, except for Belarus [75] | China, India, USA [75] | |
| Rhabditida | Lesion nematode | Pratylenchus brachyurus Godfrey 1929 | Distributed across Oceania and North America and is present in most countries of South America, Asia, and many countries in Africa. It is found throughout Europe except for Bulgaria and Italy [76] | Australia, Vietnam, Peru, Cuba, Puerto Rico (USA), USA [76] |
| Disease | Causative Fungi | Symptoms | Refs. |
|---|---|---|---|
| Damping-off | Pythium aphanidermatum (Edson) Fitzpatrick Rhizoctonia solani Kühn Fusarium solani (von Martius) Saccardo) Macrophomina phaseolina (Tassi) Goidanich Phytophthora nicotianae Breda de Haan | Water-soaked lesions at the stem collar region. Browning and shrinkage of the stem tissue and seedling collapse. | [284]. |
| Powdery mildew | Golovinomyces cichoracearum (de Candolle) Heluta | White to grayish-white powdery fungal growth on leaves, stems, and occasionally flowers or fruits. Leaf deformation, curling, yellowing, and stunted plant growth. | [285]. |
| Cercospora leaf spot | Cercospora malayensis Stevens & Solheim C. abelmoschi (Ellis & Everhart) Deighton | Irregular brown or black spots on mature leaves, developing into reddish-brown with yellow margins, expanding and reducing photosynthesis. | [15,286]. |
| Gray mold | Botrytis cinerea Persoon | Grayish, web-like fungal growth on leaves, stems, and fruits. Light brown lesions on thin leaves, concentric brown rings on thicker leaves. Wilting in severe cases. | [287]. |
| Alternaria leaf spot | Alternaria alternata (Fries) Keissler A. chlamydospora Mouchacca | Small, light brown-concentric dark brown lesions on the leaves. Necrosis, wilting, and plant death under severe infections. | [288,289,290]. |
| Alternaria pod blight | Alternaria alternata (Fries) Keissler | Wet rot on young okra pods, development of lesions, leading to decay. | [291]. |
| Phyllosticta leaf spot | Phyllosticta hibiscini Ellis & Everh. | Large leaf lesions with a grayish center, progressing to form shot holes. Presence of black pycnidia on both leaf surfaces. | [292]. |
| Fusarium wilt | Fusarium oxysporum f. sp. vasinfectum sensu lato (Atkinson) Snyder & Hansen | Leaf discoloration and wilting, stunted growth, vascular damage, and stem deterioration, leading to wilting and death. | [293,294]. |
| Verticillium wilt | Verticillium dahliae Klebahn V. tricorpus Isaac | Initial symptoms include yellowing followed by wilting and drying, V-shaped chlorosis of leaflets, and yellow-to-red-brown lesions near the leaf tip. Severe infections lead to defoliation, shoot dieback, and plant death. | [295,296]. |
| Collar rot | Macrophomina phaseolina (Tassi) Goidanich | Damping-off, seedling blight, collar rot, stem rot, and root rot in mature plants. Hollow stem formation, pre-emergence and post-emergence damping-off. | [297]. |
| Stem canker | Fusarium chlamydosporum sensu lato Wollenweber & Reinking | Dark circular to oblong lesions on the stems, floral buds, and pods of young plants. Severe infections lead to seedling death. | [298]. |
| Anthracnose | Colletotrichum plurivorum Damm, Alizadeh & Toy. Sato C. gloeosporioides (Penzig) Penzig & Saccardo | Yellow-brown, necrotic, sunken lesions on the leaves. | [299,300]. |
| Fruit rot | Choanephora cucurbitarum (Berkeley & Ravenel) Thaxter Rhizoctonia solani Kühn Fusarium solani (von Martius) Saccardo Phytophthora palmivora E.J. Butler Rhizopus stolonifera (Ehrenberg) Vuillemin Fusarium oxysporum Schlechtendal Aspergillus flavus Link | Brown-black water-soaked lesions on fruits, progressing rapidly and causing fruit decay and drop. | [301,302,303]. |
| Disease | Causative Agent | Synonyms | Symptoms | Refs. |
|---|---|---|---|---|
| Okra yellow vein mosaic disease (YVMV) | Begomovirus abelmoschusflavi | Bhendi yellow vein India virus, bhendi yellow vein mosaic begomovirus, bhendi yellow vein mosaic virus, okra yellow vein mosaic agent, okra yellow vein mosaic virus | Vein yellowing, followed by yellowing of the entire leaf. Reduced fruit size, pale yellow or whitish fruits, and deformation. Stunted plant growth. | [367,368]. |
| Bhendi yellow vein haryana virus (BYVHV) | Begomovirus abelmoschusharyanaense | |||
| Okra yellow mosaic Mexico virus (OkYMMV) | Begomovirus abelsmoschusmexicoense | Irregular yellow patches of leaves, distinctive yellow borders on leaf edges, and chlorosis of newly developing leaves. | [369]. | |
| Okra enation leaf curl disease (OELCV) | Begomovirus abelsmoschusenation | Okra enation leaf curl virus | Upward curling of leaves with thickened texture, with pin-shaped enations on the underside of leaves. Stunted and distorted plants., with deformed and discolored fruits. | [370]. |
| Okra leaf curl disease (OLCV) | Okra leaf curl virus (OLCV) | Okra leaf curl begomovirus | ||
| Okra leaf curl Cameroon virus (OLCuCMV) | Begomovirus gossypigeziraense | Cotton leaf curl Gezira begomovirus, cotton leaf curl Gezira virus, hollyhock leaf crumple virus, okra leaf curl Cameroon virus | Severe leaf curling with margins curling upward. Leaf distortion leading to crinkled and leathery textures. Development of yellow-green mosaics or chlorotic patterns. Stunted plant growth with reduced leaf and plant size, with the production of small, deformed fruits. | [371]. |
| Okra leaf curl Oman virus (OLCOV) | Begomovirus abelsmoschusomanense | Leaf curling, inter-veinal yellowing, vein thickening, and reduced leaf size. | [372]. | |
| Okra yellow crinkle virus (OkYCV) | Begomovirus abelsmoschusretorridi | Okra yellow crinkle virus | Vein thickening in affected leaves, curling, distortion, and yellow crinkling of leaf tissues. Stunted plant growth and reduced vigor. | [371]. |
| Cotton leaf curl disease (CLCuD) | Begomovirus gossypialabadense | Cotton leaf curl Alabad virus | Leaf curling, vein swelling or thickening, interveinal chlorosis, plant stunting, leaf crumpling and deformation, and reduced flowering and fruit production. | [371]. |
| Cotton leaf curl Multan virus (CLCuMuV) | Begomovirus gossypimultanense | Cotton leaf curl Multan begomovirus, cotton leaf curl Rajasthan begomovirus, cotton leaf curl Rajasthan virus | Curled and crumpled leaves, thickened and distorted veins, yellow or greenish mosaic patterns on leaves, stunted plant growth with premature defoliation. | [371]. |
| Sida micrantha mosaic virus (SiMMV) | Begomovirus sidamicranthae | Chlorotic spots distributed in a mosaic pattern, interspersed with green streaks. Random alternation of chlorotic and green areas, not confined to veins. Severe stunting in young plants, and localized symptoms in single branches of mature plants. | [373]. | |
| Bitter gourd yellow vein virus (BGYVV) | Begomovirus solanumdelhiense | Tomato leaf curl New Delhi begomovirus (ToLCNDV), tomato leaf curl New Delhi virus | Yellowing along the leaf veins, with yellow blotches on the leaf surface. | [374]. |
| Okra mosaic disease (OkMV) | Tymovirus abelmoschi | Okra mosaic tymovirus | Yellowish mottling of plants, stunting and reduced yield, smaller leaves, fruit discoloration, distortion, and abortion. | [375]. |
| Cotton anthocyanosis virus (CAV) | Cotton anthocyanosis virus (CAV) | Excessive purple or reddish pigmentation in lower and medium leaves. | [376]. | |
| Tomato chlorosis virus (ToCV) | Crinivirus tomatichlorosis | Tomato chlorosis closterovirus, tomato chlorosis crinivirus | Interveinal chlorosis with green veins, brittleness of leaves, necrotic flecking or bronzing on leaves. | [377]. |
| Tomato spotted wilt virus (TSWV) * | Orthotospovirus tomatomaculae | Tomato spotted wilt orthotospovirus, tomato spotted wilt tospovirus | Chlorosis of plant tissues, necrosis of leaves, stems, and fruits. Stunting of plants, and death in severe cases. | [378]. |
| Radish leaf curl virus (RALCV) | Radish leaf curl virus (RALCV) | Leaf curling, stunted growth, absence of fruit production. | [379]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ounis, S.; Turóczi, G.; Kiss, J. Arthropod Pests, Nematodes, and Microbial Pathogens of Okra (Abelmoschus esculentus) and Their Management—A Review. Agronomy 2024, 14, 2841. https://doi.org/10.3390/agronomy14122841
Ounis S, Turóczi G, Kiss J. Arthropod Pests, Nematodes, and Microbial Pathogens of Okra (Abelmoschus esculentus) and Their Management—A Review. Agronomy. 2024; 14(12):2841. https://doi.org/10.3390/agronomy14122841
Chicago/Turabian StyleOunis, Samara, György Turóczi, and József Kiss. 2024. "Arthropod Pests, Nematodes, and Microbial Pathogens of Okra (Abelmoschus esculentus) and Their Management—A Review" Agronomy 14, no. 12: 2841. https://doi.org/10.3390/agronomy14122841
APA StyleOunis, S., Turóczi, G., & Kiss, J. (2024). Arthropod Pests, Nematodes, and Microbial Pathogens of Okra (Abelmoschus esculentus) and Their Management—A Review. Agronomy, 14(12), 2841. https://doi.org/10.3390/agronomy14122841






