The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Location
2.2. Chemicals and Reagents
2.3. Laboratory Equipment
2.4. Preparation of Aqueous Extracts
2.5. Preparation of the Ethanolic Extracts
2.6. Determination of Moisture and Ash Content
2.7. Determination of Sugar Content
2.8. Determination of Vitamin C Content
2.9. Determination of Antioxidant Activity (DPPH)
2.10. Determination of Total Polyphenol Content
2.11. Determination of Total Tannin Content
2.12. Determination of Total Flavonoid Content
2.13. Determination of Total Anthocyanin Content
2.14. Determination of Total Alkaloid Content
2.15. Determination of Carotenoid Content (Lycopene and β-Carotene)
2.16. ATR-FTIR Analysis
2.17. Statistical Analysis
3. Results and Discussion
3.1. Determination of Moisture, Ash, Sugars, Vitamin C, and DPPH
3.2. Determination of Phenolic Compounds
3.3. Determination of Alkaloids and Carotenes (Lycopene and β-Carotene)
3.4. Pearson Correlation Matrix Between Biological Compounds and Genotypes
3.5. ATR-FTIR Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Natić, M.; Pavlović, A.; Bosco, F.L.; Stanisavljević, N.; Zagorac, D.D.; Akšić, M.F.; Papetti, A. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Ritika Bora, B.; Ismail, B.B.; Garba, U.; Mishra, S.; Jha, A.K.; Naik, B.; Kumar, V.; Rather, M.A.; Preet, M.S.; Rustagi, S.; et al. Himalayan fruit and circular economy: Nutraceutical potential, traditional uses, challenges and opportunities. Food Prod. Process. Nutr. 2024, 6, 71. [Google Scholar] [CrossRef]
- Dimitriadis, K.M.; Karavergou, S.; Tsiftsoglou, O.S.; Karapatzak, E.; Paschalidis, K.; Hadjipavlou-Litina, D.; Charalambous, D.; Krigas, N.; Lazari, D. Nutritional value, major chemical compounds, and biological activities of Petromarula pinnata (Campanulaceae)—A unique nutraceutical wild edible green of Crete (Greece). Horticulturae 2024, 10, 689. [Google Scholar] [CrossRef]
- Dos Santos, O.V.; do Rosário, R.C.; Teixeira-Costa, B.E. Sources of carotenoids in amazonian fruits. Molecules 2024, 29, 2190. [Google Scholar] [CrossRef] [PubMed]
- Stabnikova, O.; Stabnikov, V.; Paredes-López, O. Fruits of wild-grown shrubs for health nutrition. Plant Foods Hum. Nutr. 2024, 79, 20–37. [Google Scholar] [CrossRef]
- Khan, M.N.; Ullah, B.; Kaplan, A.; Wahab, S.; Ali, B.; Al Obaid, S.; Ansari, M.J. An in-depth investigation of the nutraceutical value and medicinal perspectives of wild medicinal plants in Ojhor Valley, Hindukush Range, Chitral, Pakistan. Genet. Resour. Crop Evol. 2024. [Google Scholar] [CrossRef]
- Fantasma, F.; Samukha, V.; Saviano, G.; Chini, M.G.; Iorizzi, M.; Caprari, C. Nutraceutical aspects of selected wild edible plants of the Italian Central Apennines. Nutraceuticals 2024, 4, 190–231. [Google Scholar] [CrossRef]
- Khelfi, S.; Zerizer, S.; Bensouici, C.; Tebibel, S.; Kabouche, Z. The antioxidant activity and the protective effect of Rosa canina L. fruit against intestinal inflammation induced by hyperhomocysteinemia in mice. Pharm. Chem. J. 2024, 57, 1778–1788. [Google Scholar] [CrossRef]
- Sallustio, V.; Rossi, M.; Marto, J.; Coelho, T.; Chinnici, F.; Mandrone, M.; Chiocchio, I.; Cappadone, C.; Luppi, B.; Bigucci, F.; et al. Green extraction of Rosa canina L. and Prunus spinosa L. by NaDES and their encapsulation in chitosan nanoparticles for cosmetic industry. Ind. Crops Prod. 2024, 218, 119042. [Google Scholar] [CrossRef]
- Jariani, P.; Shahnejat-Bushehri, A.A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Molecular and phytochemical characteristics of flower color and scent compounds in dog rose (Rosa canina L.). Molecules 2024, 29, 3145. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Špirović-Trifunović, B.; Pećinar, I.; de Oliveira, L.F.C.; Krstić, Đ.; Mihajlović, D.; Akšić, M.F.; Simal-Gandara, J. Fatty acids in seed oil of wild and cultivated rosehip (Rosa canina L.) from different locations in Serbia. Ind. Crops Prod. 2023, 191, 115797. [Google Scholar] [CrossRef]
- Jariani, P.; Shahnejat-Bushehri, A.A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Characterization of key genes in anthocyanin and flavonoid biosynthesis during floral development in Rosa canina L. Int. J. Biol. Macromol. 2024, 276, 133937. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical profile and antioxidant and antimicrobial activity of Rosa canina L. dried fruit commercially available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef] [PubMed]
- Bhiri, F.; Kallel, F.; Bouallegue, A.; Abidi, S.; Bensidhom, G.; Ellouz Chaabouni, S.; Trabelsi, A.B.H. Hydro-distilled wastes from Rosa canina: A new renewable bioresource for the extraction and characterization of cellulosic microfibers. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1–13. [Google Scholar] [CrossRef]
- Ashrafi, A.; Ahari, H.; Asadi, G.; Nafchi, A.M. Enhancement of the quality and preservation of frozen burgers by active coating containing Rosa canina L. extract nanoemulsions. Food Chem. X 2024, 23, 101749. [Google Scholar] [CrossRef]
- Stoenescu, A.M.; Cosmulescu, S.N. Variability of some Rosa canina L. genotypes from southern area of Oltenia. Sci. Pap. Ser. B Hortic. 2021, 65, 250–255. [Google Scholar]
- Gunaydin, S.; Alibas, I. The influence of short, medium, and long duration common dehydration methods on total protein, nutrients, vitamins, and carotenoids of rosehip fruit (Rosa canina L.). J. Food Compos. Anal. 2023, 124, 105631. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical origin influence on some honey physicochemical characteristics and antioxidant properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef]
- Hera, O.; Sturzeanu, M.; Vîjan, L.E.; Tudor, V.; Teodorescu, R. Biochemical evaluation of some fruit characteristics of blueberry progenies obtained from ‘Simultan × Duke’. ACS Omega 2023, 8, 18603–18616. [Google Scholar] [CrossRef]
- Mazilu, I.E.; Vîjan, L.E.; Cosmulescu, S. The influence of harvest moment and cultivar on variability of some chemical constituents and antiradical activity of dehydrated chokeberry pomace. Horticulturae 2022, 8, 544. [Google Scholar] [CrossRef]
- Giosanu, D.; Bărbuceanu, M.; Anghel, M.; Vîjan, L. The determination of the content of phenolic compounds from different Romanian wines using Folin-Ciocîlteu method. Curr. Trends Nat. Sci 2018, 7, 155–159. [Google Scholar]
- Giura, S.; Botu, M.; Vulpe, M.; Vîjan, L.E.; Mitrea, R. Evolution of the polyphenols, flavonoids, and tannins content in walnut leaves and green walnut husk during growing season. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1264–1271. [Google Scholar] [CrossRef]
- Anggraini, T.; Wilma, S.; Syukri, D.; Azima, F. Total phenolic, anthocyanin, catechins, DPPH radical scavenging activity, and toxicity of Lepisanthes alata (Blume) Leenh. Int. J. Food Sci. 2019, 9703176. [Google Scholar] [CrossRef] [PubMed]
- Badea, A.M.; Vijan, L.E. Otravă sau medicament? Alcaloizi în Aristolochia clemantitis L., Chelidonium majus L., Delphinius consolida L. și Digitalis purpurea L. Bul. Științ. Ser. Chim. Fiz. 2023, 1, 58–65. [Google Scholar]
- Cosmulescu, S.; Vijan, L.; Mazilu, I.C.; Badea, G. Bioactive compounds in the residue obtained from fruits of some cultivars of Lonicera caerulea. Horticulturae 2024, 10, 211. [Google Scholar] [CrossRef]
- Munteanu, A.L.; Vijan, L.E.; Topală, C.M.; Mitrea, R. Influence of the phytosanitary status, cultivar, and harvest time on the phenolic, chlorophyll, and alkaloid content of Rosa sp. leaves. Horticulturae 2023, 9, 1169. [Google Scholar] [CrossRef]
- Andronie, L.; Holonec, L.; Ioana, P.O.P.; Truta, A.M.; Odagiu, A.; Sălăgean, T.; Sobolu, R.; Coroian, A.; Balta, I.; Șuba, E.E. Antioxidant capacity of several Romanian forest fruits (Rosa canina L., Prunus spinosa L., Vaccium vitis-idaea L. and Cornus mas L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1178–1184. [Google Scholar] [CrossRef]
- Vasilj, V.; Brekalo, H.; Petrović, D.; Šaravanja, P.; Batinić, K. Chemical composition and mineral content of fresh and dried fruits of the wild rosehip (Rosa canina L.) population. J. Cent. Eur. Agric. 2024, 25, 179–193. [Google Scholar] [CrossRef]
- Pashaei, M.; Hassanpour, H. Phenolic, amino acids, and fatty acids profiles and the nutritional properties in the fresh and dried fruits of black rosehip (Rosa pimpinellifolia L). Sci. Rep. 2024, 14, 19665. [Google Scholar] [CrossRef]
- Grdiša, M.; Šic Žlabur, J.; Varga, F.; Bosilj, P.; Klepo, T.; Šatović, Z. Phytochemical diversity of Rosa canina L. populations in Croatia. Maced. Pharm. Bull. 2022, 68 (Suppl. S2), 57–58. [Google Scholar] [CrossRef]
- Michalec, K.; Wasik, R.; Gach, M.B. The content of vitamin C in dog rose fruit Rosa canina L. depending on the method and duration of storage. Sylwan 2023, 167, 110–119. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea extracts and assessment of their antioxidant activity in human endothelial cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Kayahan, S.; Ozdemir, Y.; Gulbag, F. Functional compounds and antioxidant activity of Rosa species grown in Turkey. Erwerbs-Obstbau 2023, 65, 1079–1086. [Google Scholar] [CrossRef]
- Nađpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative study of biological activiti and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R.; Ercisli, S.; Karatas, N.; Ekiert, H.; Elansary, H.O.; Szopa, A. Morphological and biochemical diversity in fruits of unsprayed Rosa canina and Rosa dumalis ecotypes found in different agroecological conditions. Sustainability 2021, 13, 8060. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, polyphenols, and ascorbic acid in organic rosehips (Rosa spp.) cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Goztepe, B.; Kayacan, S.; Bozkurt, F.; Tomas, M.; Sagdic, O.; Karasu, S. Drying kinetics, total bioactive compounds, antioxidant activity, phenolic profile, lycopene and β-carotene content and color quality of Rosehip dehydrated by different methods. LWT 2022, 153, 112476. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Urbštaitė, R.; Liaudanskas, M.; Obelevičius, K.; Janulis, V. Phenolic content and antioxidant activity in fruit of the genus Rosa L. Antioxidants 2022, 11, 912. [Google Scholar] [CrossRef]
- Ramos, P.M.; Gil, J.M.; Ramos Sánchez, C.R.; Gracia, L.M.N.; Navarro, S.H.; Gil, F.J.M. Vibrational and thermal characterization of seeds, pulp, leaves and seed oil of Rosa rubiginosa. Boletín Soc. Argent. Botánica 2016, 51, 429–439. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Dovbeshko, G.I.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FTIR spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–346. [Google Scholar] [CrossRef] [PubMed]
- Dovbeshko, G.I.; Chegel, V.I.; Gridina, N.Y.; Repnytska, O.P.; Shirshov, Y.M.; Tryndiak, V.P.; Todor, I.M.; Solyanik, G.I. Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study. Biopolymers 2002, 67, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
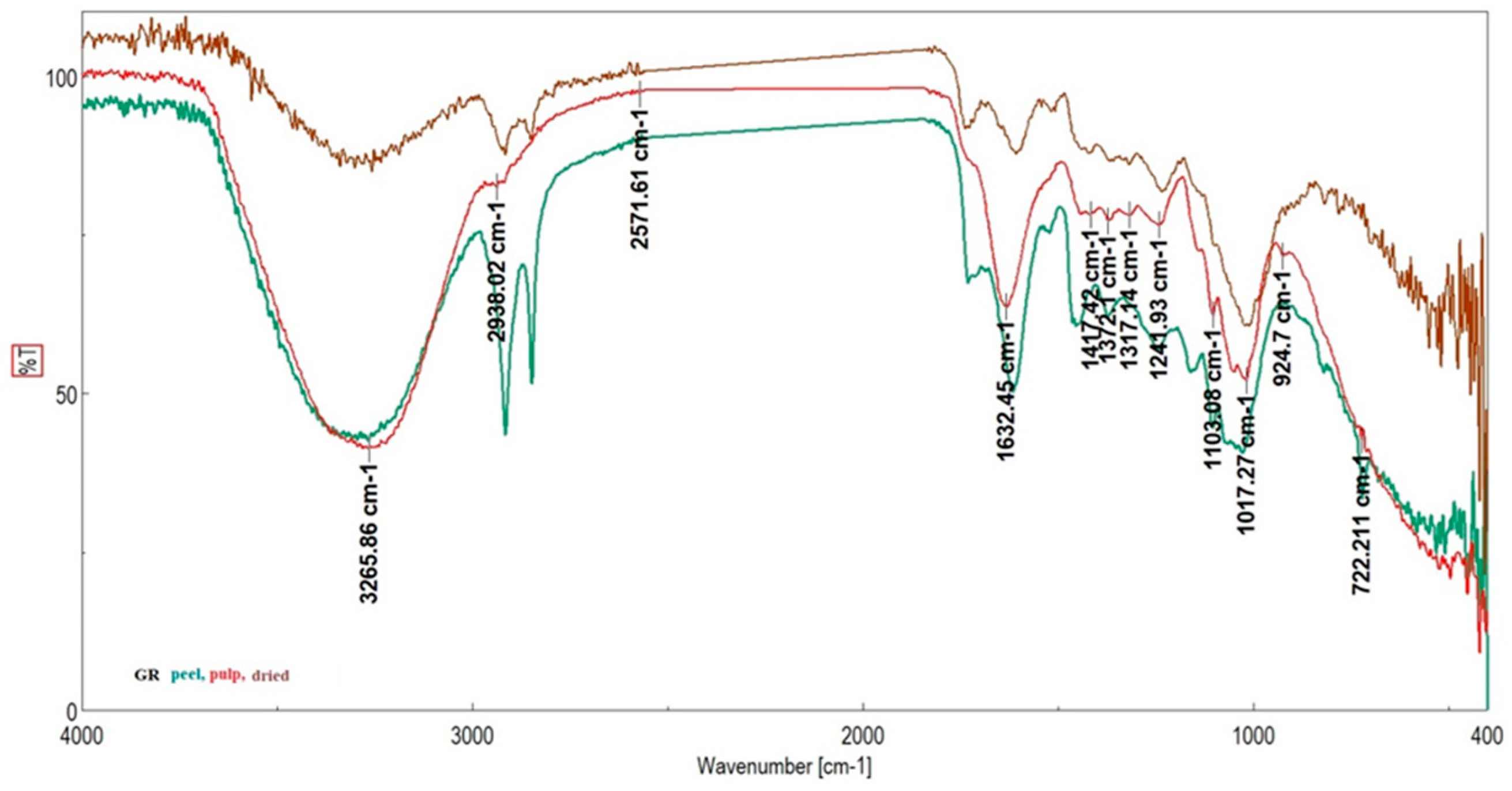
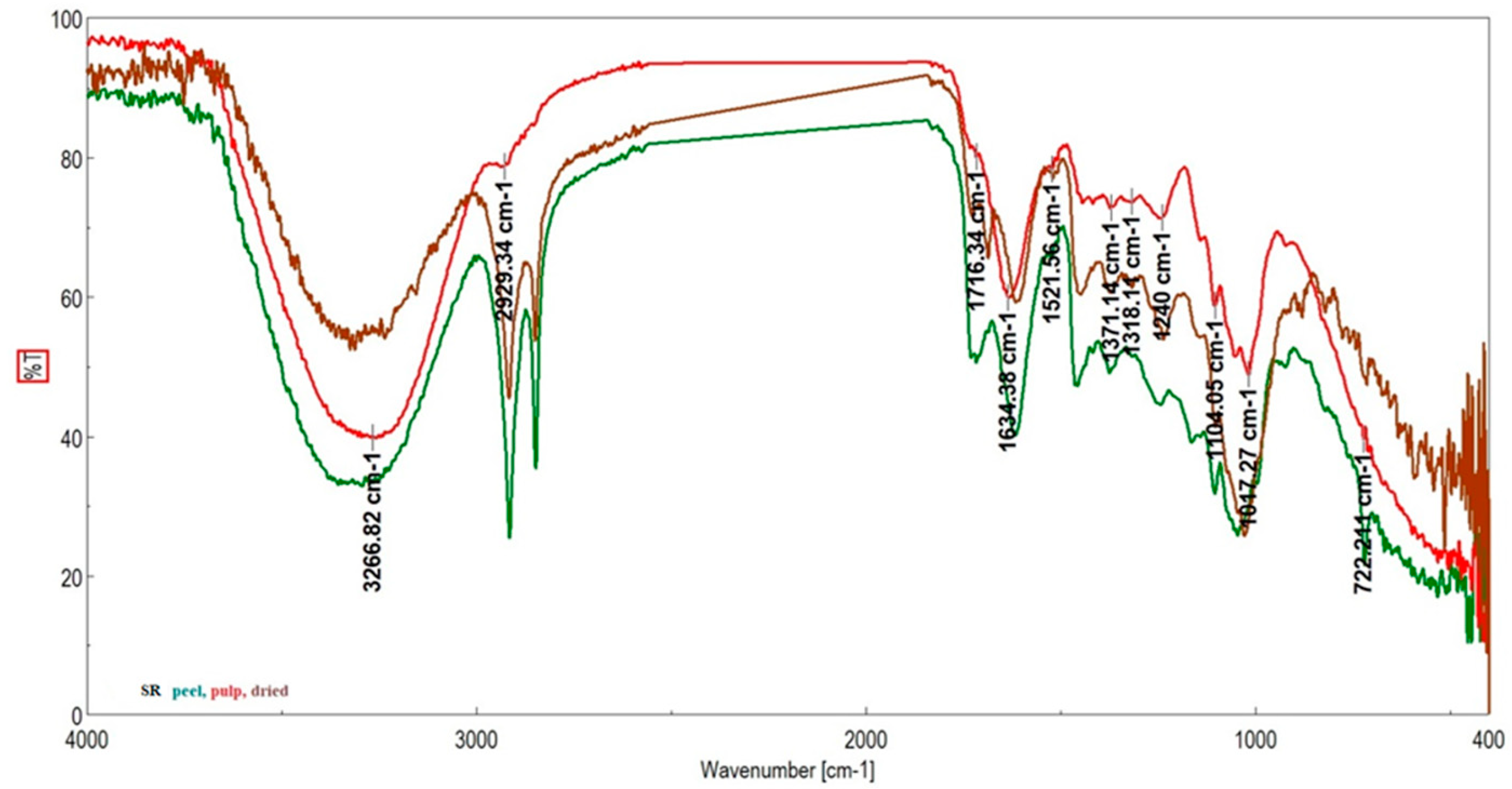
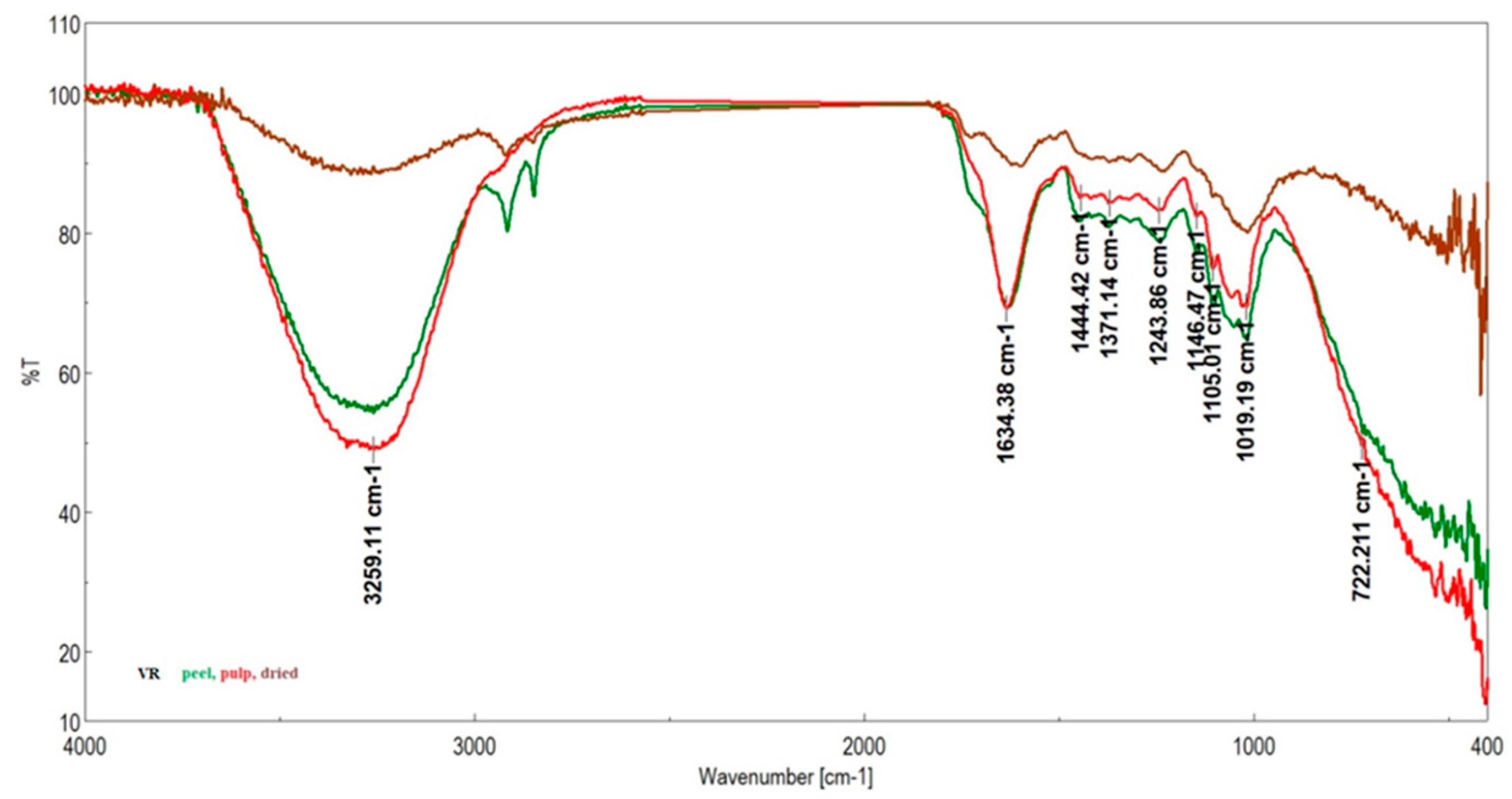
| Genotype | Moisture Content (%) | Ash (%) | TSC (gGluE/100 g) | Vitamin C (g/100 g) | DPPH (%) |
|---|---|---|---|---|---|
| GR1 | 34.89 ± 0.03 e | 2.43 ± 0.02 f | 49.10 ± 1.35 c | 1.74 ± 0.02 e | 95.10 ± 0.29 bc |
| GR2 | 38.71 ± 0.04 c | 2.33 ± 0.01 g | 47.74 ± 0.54 d | 2.10 ± 0.01 b | 94.78 ± 0.16 c |
| GR3 | 23.38 ± 0.07 h | 2.65 ± 0.02 c | 36.67 ± 1.32 f | 2.10 ± 0.01 b | 90.88 ± 0.12 e |
| SR1 | 32.73 ± 0.08 f | 2.51 ± 0.01 e | 57.22 ± 0.33 a | 2.23 ± 0.01 a | 91.84 ± 0.38 c |
| SR2 | 30.35 ± 0.22 g | 2.88 ± 0.01 a | 40.04 ± 0.39 e | 2.08 ± 0.01 b | 96.94 ± 0.26 a |
| SR3 | 41.15 ± 0.11 b | 2.33 ± 0.02 g | 26.24 ± 0.09 h | 2.01 ± 0.02 c | 90.26 ± 0.56 e |
| VR1 | 36.34 ± 0.16 d | 2.82 ± 0.02 b | 31.51 ± 0.33 g | 1.83 ± 0.02 d | 95.78 ± 0.26 b |
| VR2 | 48.34 ± 0.08 a | 2.44 ± 0.02 f | 30.68 ± 0.55 g | 1.68 ± 0.02 f | 89.21 ± 0.58 f |
| VR3 | 36.36 ± 0.29 d | 2.56 ± 0.02 d | 53.10 ± 0.46 b | 1.99 ± 0.01 c | 82.90 ± 0.77 g |
| Mean | 35.81 ± 6.70 | 2.55 ± 0.19 | 41.37 ± 10.51 | 1.97 ± 0.18 | 91.97 ± 4.19 |
| Genotype | TPC (mg GAE/100 g) | TTC (mg GAE/100 g) | TFC (mg CE/100 g) | TAC (mg C3GE/100 g) |
|---|---|---|---|---|
| GR1 | 1884.67 ± 95.30 a | 847.33 ± 28.75 abc | 122.67 ± 18.56 c | 20.63 ± 0.02 a |
| GR2 | 1179.67 ± 37.81 d | 639.67 ± 26.76 e | 82.67 ± 10.02 e | 17.53 ± 0.03 h |
| GR3 | 956.67 ± 22.28 f | 359.33 ± 13.32 f | 66.33 ± 7.51 e | 19.16 ± 0.02 b |
| SR1 | 1264.67 ± 45.24 c | 827.67 ± 31.34 bcd | 163.33 ± 24.03 b | 18.45 ± 0.03 f |
| SR2 | 1445.67 ± 26.08 b | 892.67 ± 33.01 a | 141.33 ± 21.03 bc | 19.05 ± 0.03 c |
| SR3 | 989.67 ± 20.50 f | 808.33 ± 34.15 cd | 114.00 ± 16.52 cd | 17.92 ± 0.03 g |
| VR1 | 1068.00 ± 33.65 e | 780.67 ± 29.14 d | 129.00 ± 19.52 bc | 17.47 ± 0.03 h |
| VR2 | 1029.00 ± 19.92 ef | 873.67 ± 34.59 ab | 112.33 ± 16.04 cd | 18.72 ± 0.07 e |
| VR3 | 1082.33 ± 27.21 e | 848.67 ± 36.30 abc | 202.33 ± 31.56 a | 18.83 ± 0.13 d |
| Mean | 1211.15 ± 285.72 | 764.22 ± 164.23 | 126.00 ± 42.33 | 18.64 ± 0.94 |
| Genotype | Alkaloids (mg CSAE/100 g) | Lycopene (mg/100 g) | β-Carotene (mg/100 g) |
|---|---|---|---|
| GR1 | 67.07 ± 0.04 e | 0.86 ± 0.05 c | 1.29 ± 0.08 c |
| GR2 | 43.06 ± 0.03 h | 0.84 ± 0.05 cd | 1.73 ± 0.09 b |
| GR3 | 37.34 ± 0.26 g | 0.77 ± 0.04 d | 1.12 ± 0.07 d |
| SR1 | 44.54 ± 0.03 f | 0.88 ± 0.06 c | 2.49 ± 0.09 a |
| SR2 | 73.85 ± 0.09 c | 0.39 ± 0.02 e | 0.57 ± 0.04 e |
| SR3 | 72.62 ± 0.10 d | 0.99 ± 0.06 b | 1.43 ± 0.09 c |
| VR1 | 79.80 ± 0.06 b | 1.10 ± 0.07 a | 1.68 ± 0.11 b |
| VR2 | 72.77 ± 0.09 d | 0.84 ± 0.04 cd | 1.11 ± 0.07 d |
| VR3 | 86.71 ± 0.15 a | 0.99 ± 0.05 b | 1.39 ± 0.09 c |
| Mean | 64.20 ± 17.16 | 0.85 ± 0.20 | 1.42 ± 0.51 |
| Genotype | Moisture Content | Ash | TSC | Vitamine C | TPC | TTC | TFC | Alkaloids | TAC | Lycopene | Carotene | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 1 | 0.452 * | 0.23 | −0.319 | −0.248 | −0.570 ** | 0.409 * | 0.501 ** | 0.717 ** | −0.353 | 0.288 | −0.119 | −0.600 ** |
| Moisture content | 0.452 * | 1 | −0.526 ** | −0.303 | −0.572 ** | −0.157 | 0.561 ** | 0.09 | 0.452 * | −0.303 | 0.371 | 0.08 | −0.224 |
| Ash | 0.23 | −0.526 ** | 1 | −0.108 | 0.083 | 0.012 | 0.007 | 0.199 | 0.255 | 0.018 | −0.364 | −0.346 | 0.313 |
| TSC | −0.319 | −0.303 | −0.108 | 1 | 0.410 * | 0.456 * | 0.101 | 0.445 * | −0.269 | 0.307 | −0.073 | 0.447 * | −0.13 |
| Vitamin C | −0.248 | −0.572 ** | 0.083 | 0.410 * | 1 | −0.212 | −0.351 | 0.04 | −0.560 ** | −0.293 | −0.265 | 0.358 | 0.007 |
| TPC | −0.570 ** | −0.157 | 0.012 | 0.456 * | −0.212 | 1 | 0.391 * | 0.172 | 0.049 | 0.695 ** | −0.315 | −0.123 | 0.473 * |
| TTC | 0.409 * | 0.561 ** | 0.007 | 0.101 | −0.351 | 0.391 * | 1 | 0.687 ** | 0.705 ** | 0.076 | 0.026 | −0.018 | −0.029 |
| TFC | 0.501 ** | 0.09 | 0.199 | 0.445 * | 0.04 | 0.172 | 0.687 ** | 1 | 0.551 ** | 0.068 | 0.203 | 0.138 | −0.433 * |
| Alkaloids | 0.717 ** | 0.452 * | 0.255 | −0.269 | −0.560 ** | 0.049 | 0.705 ** | 0.551 ** | 1 | 0.025 | 0.184 | −0.358 | −0.246 |
| TAC | −0.353 | −0.303 | 0.018 | 0.307 | −0.293 | 0.695 ** | 0.076 | 0.068 | 0.025 | 1 | −0.37 | −0.395 * | −0.014 |
| Lycopene | 0.288 | 0.371 | −0.364 | −0.073 | −0.265 | −0.315 | 0.026 | 0.203 | 0.184 | −0.37 | 1 | 0.561 ** | −0.399 * |
| Carotene | −0.119 | 0.08 | −0.346 | 0.447 * | 0.358 | −0.123 | −0.018 | 0.138 | −0.358 | −0.395 * | 0.561 ** | 1 | −0.066 |
| DPPH | −0.600 ** | −0.224 | 0.313 | −0.13 | 0.007 | 0.473 * | −0.029 | −0.433 * | −0.246 | −0.014 | −0.399 * | −0.066 | 1 |
| Grădinile Genotypes | Studinita Genotypes | Vlădila Genotipes | RR | Assignation |
|---|---|---|---|---|
| (Skin/Pulp/Dried Fruit) | Pulp | |||
| 3266/3265/3257 | 3291/3266/3297 | 3259/3259/3243 | Stretching O–H, N–H; C–H, N–H asymmetric (asym) [42] | |
| 3009 | ν(=CH) | |||
| 2915/2938/2915 | 2915/2929/2916 | 2952, 2915/2951/2956, 2922 | 2918 | νasym C–H L |
| 2848/-/2850 | 2848/2852/2849 | 2847/2854/2849 | 2850 | νsym C–H L |
| 1730/1726/1737 | 1716/1716/1727 | -/-/1726 | 1730 | νC=O stretching due to lipids L |
| 1615/1632/1607 | 1616/1634/1686 | 1634/1634/1698 | 1653 | Amide I (disordered structure-non-hydrogen bonded) [40]; νC=O, Amide I, stretching of protein P |
| 1518/1516/1517 | 1522/1521/1519 | 1518/1518/1506 | Amide II | |
| 1454/1444/1439 | 1455/1443/1447 | 1444/1444/- | 1439 | δasym CH2 L |
| 1415/1417/1420 | 1417/1417/- | 1415/1418/1415 | 1417 | Stretching C–N, deformation N-H, deformation C–H L |
| 1373/1372/1318 | 1375/1371/1374 | 1371/1371/1370 | 1371 | Stretching C–N [43], deformation N–H, δ CH2 [44] P |
| 1246/1241/1235 | 1244/1240/1236 | 1243/1243/1229 | 1240 | Amide III P |
| 1160/1141/- | 1163/1140/- | 1146/1146/1144 | 1138 | νasym O–C–O stretching of polysaccharides C |
| 1104/1103/1100 | 1104/1104/1104 | 1104/1105/1104 | Stretching PO2− symmetric (phosphate II) | |
| 1048 | 1045/1052 | 1051/1057/1041 | 1099 | P—ring resonance [39]; stretching C–O deoxyribose, C—starch OH, cellulose |
| 1027/1017/1011 | -/1017/1028 | 1017/1019/1015 | 1016 | Vibrational frequency of -CH2OH groups of carbohydrates (including glucose, fructose) C, M—PO43− |
| -/-/970 | 992/-/- | 935/971/- | C–O ribose, C–C C | |
| 924/924/912 | 923/919/920 | 904/-/923 | Phosphodiester region [45] | |
| -/-/817 | 862/826/879 | 866/-/863 | C–C, C–O deoxyribose [45] | |
| 720/722/723 | 720/722/714 | 721/721/723 | 719 | ρ(CH2)n [40]; CH out-of-plane bending vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamin, F.D.; Vijan, L.E.; Topală, C.M.; Cosmulescu, S.N. The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits. Agronomy 2024, 14, 2847. https://doi.org/10.3390/agronomy14122847
Stamin FD, Vijan LE, Topală CM, Cosmulescu SN. The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits. Agronomy. 2024; 14(12):2847. https://doi.org/10.3390/agronomy14122847
Chicago/Turabian StyleStamin, Florin Daniel, Loredana Elena Vijan, Carmen Mihaela Topală, and Sina Niculina Cosmulescu. 2024. "The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits" Agronomy 14, no. 12: 2847. https://doi.org/10.3390/agronomy14122847
APA StyleStamin, F. D., Vijan, L. E., Topală, C. M., & Cosmulescu, S. N. (2024). The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits. Agronomy, 14(12), 2847. https://doi.org/10.3390/agronomy14122847







