The Bacterial Root Microbiome in Ecuadorian Andean Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Bacterial Microbiome Analysis
2.2.1. Sampling and Sample Processing
2.2.2. DNA Extraction and Sequencing
2.2.3. Diversity Analysis
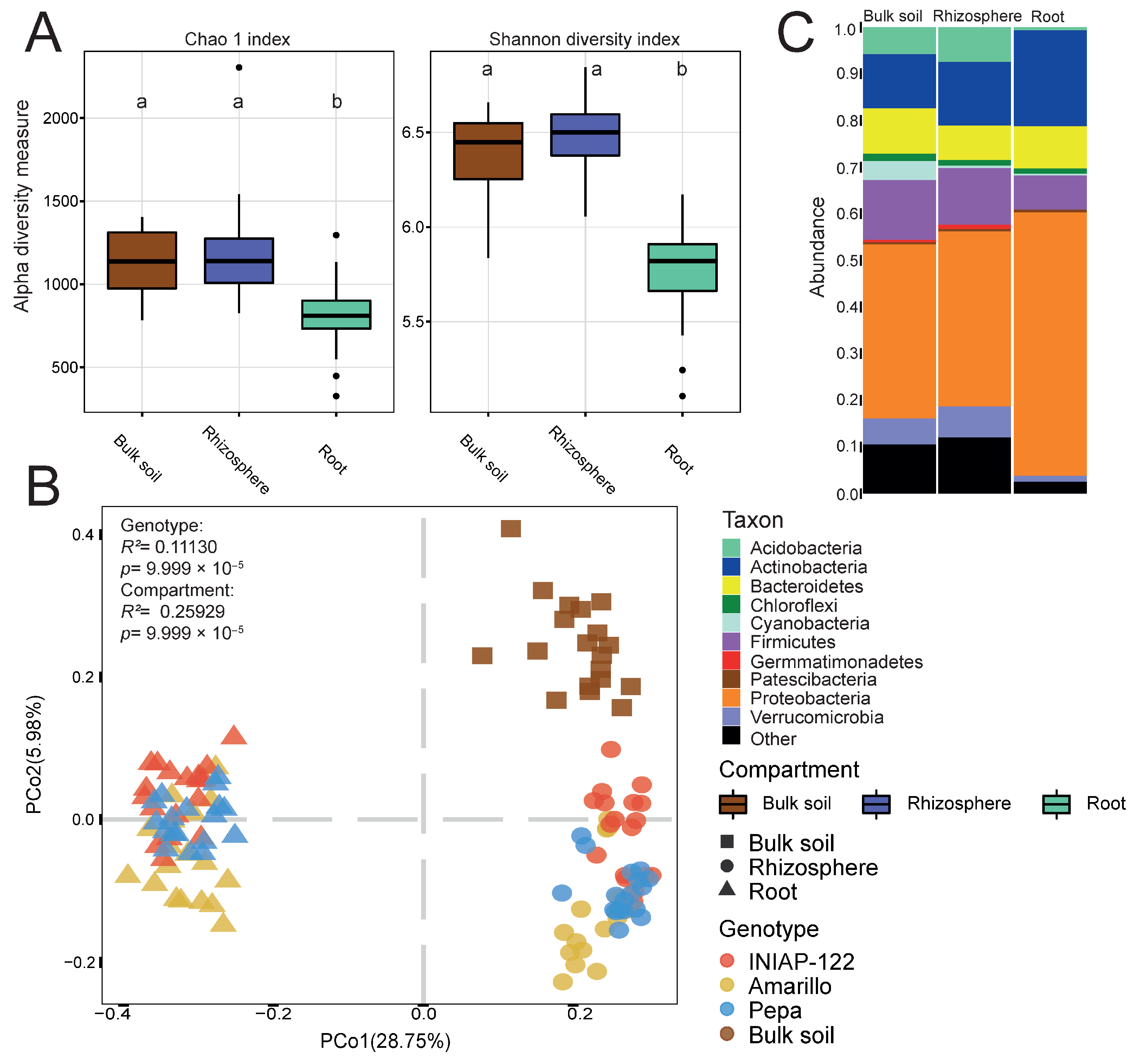
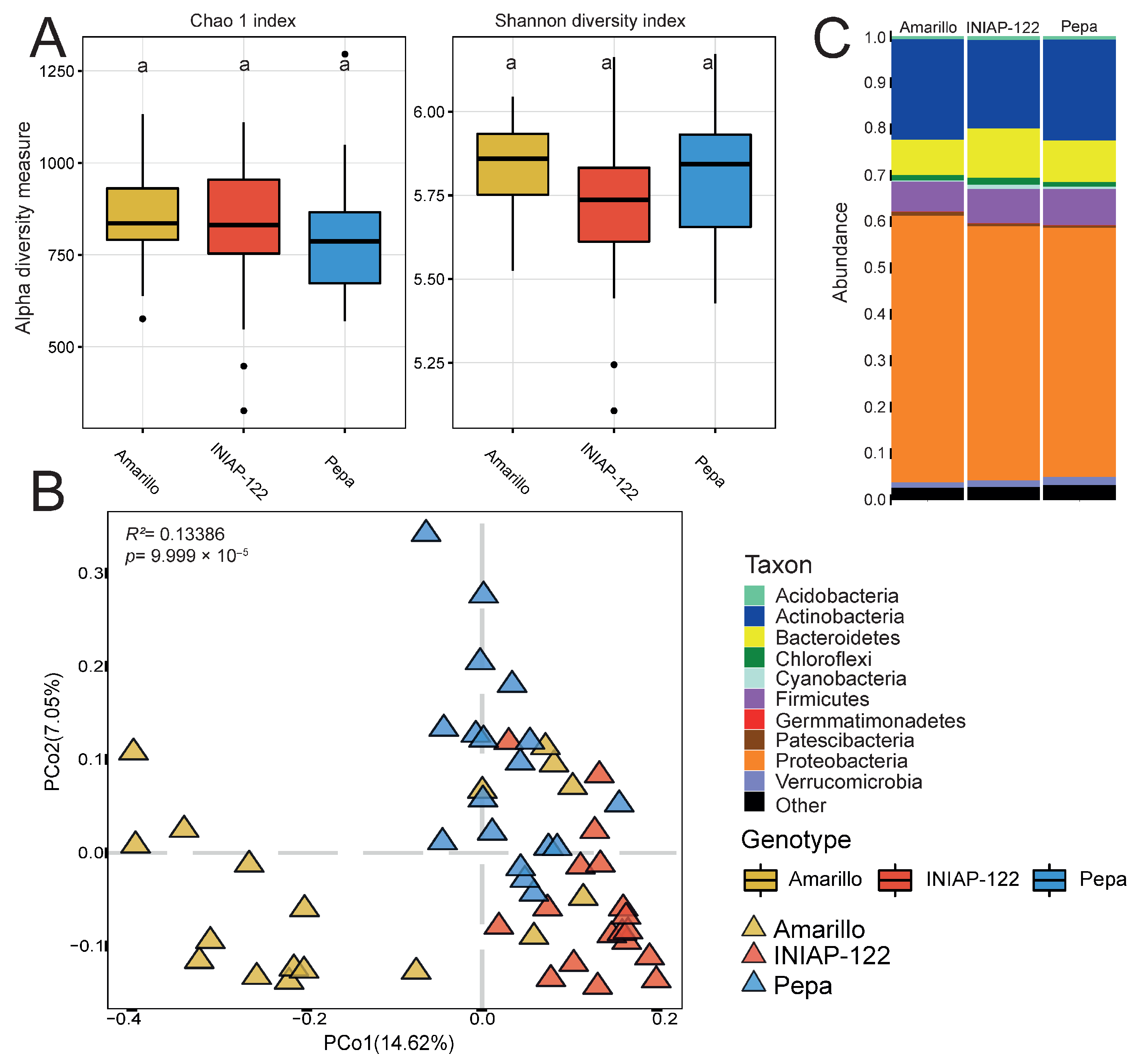
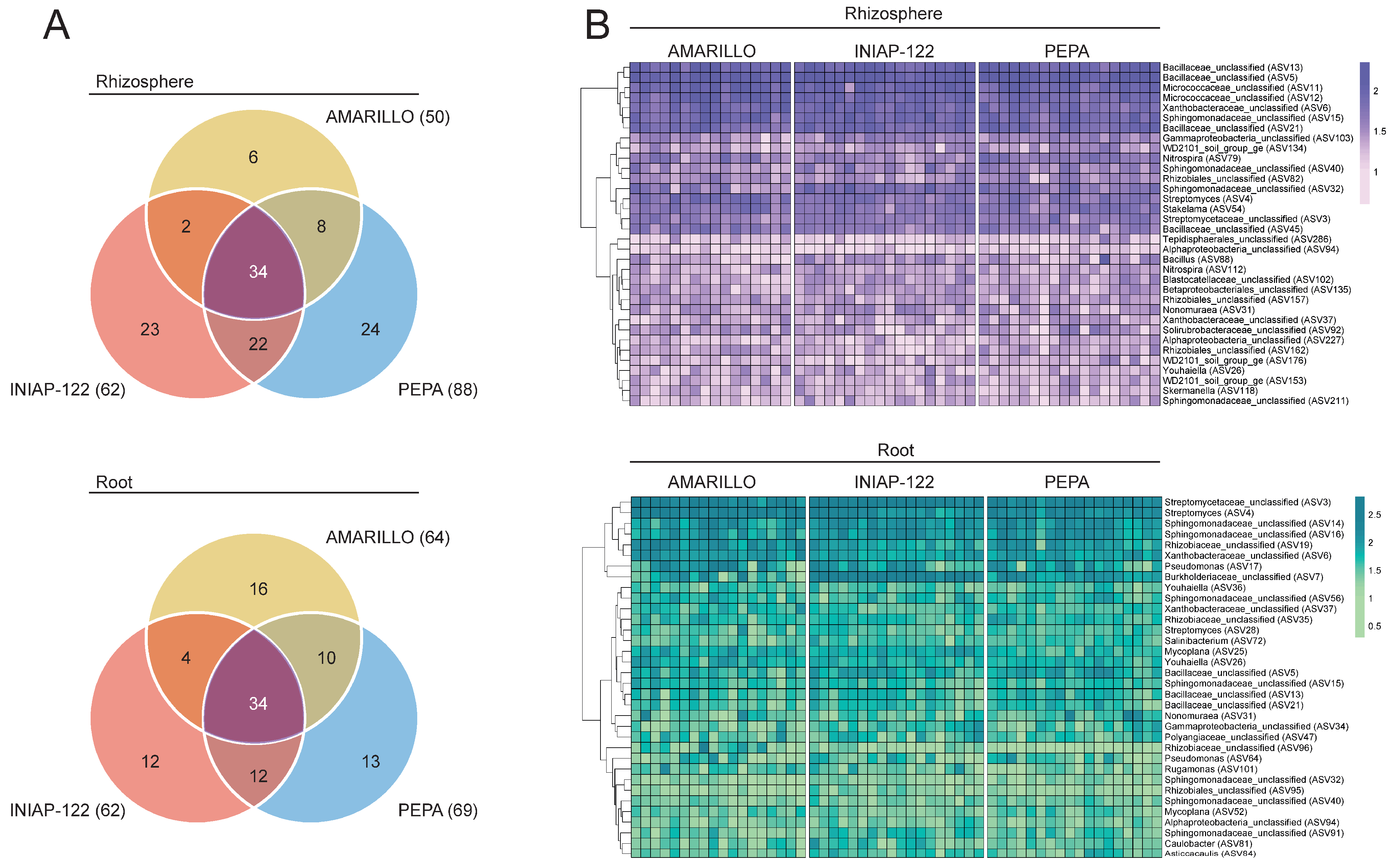
3. Results
3.1. Bacterial Microbial Communities of the Bulk Soil, Rhizosphere, and Root Differ Between Maize Genotypes
3.2. Effect of Plant Genetics on Bacterial Microbiome Assembly in Rhizosphere and Root
3.3. The Bacterial Core Microbiome Between Andean Maize Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Kaul, J.; Jain, K.; Olakh, D. An Overview on Role of Yellow Maize in Food, Feed and Nutrition Security. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3037–3048. [Google Scholar] [CrossRef]
- Perales, H.; Golicher, D. Mapping the Diversity of Maize Races in Mexico. PLoS ONE 2014, 9, e114657. [Google Scholar] [CrossRef] [PubMed]
- Tokatlidis, I.S. Adapting Maize Crop to Climate Change. Agron. Sustain. Dev. 2013, 33, 63–79. [Google Scholar] [CrossRef]
- Tiziani, R.; Miras-Moreno, B.; Malacrinò, A.; Vescio, R.; Lucini, L.; Mimmo, T.; Cesco, S.; Sorgonà, A. Drought, Heat, and Their Combination Impact the Root Exudation Patterns and Rhizosphere Microbiome in Maize Roots. Environ. Exp. Bot. 2022, 203, 105071. [Google Scholar] [CrossRef]
- Swift, J.F.; Kolp, M.R.; Carmichael, A.; Ford, N.E.; Hansen, P.M.; Sikes, B.A.; Kleiner, M.; Wagner, M.R. Environmental Legacy Effects Impact Maize Growth and Microbiome Assembly under Drought Stress. BioRxiv 2024, 2023.04.11.536405. Available online: http://biorxiv.org/content/early/2024/01/08/2023.04.11.536405.abstract (accessed on 27 August 2024).
- AL-Huqail, A.A.; El-Bondkly, A.M.A. Improvement of Zea mays L. Growth Parameters under Chromium and Arsenic Stress by the Heavy Metal-Resistant Streptomyces sp. NRC21696. Int. J. Environ. Sci. Technol. 2022, 19, 5301–5522. [Google Scholar] [CrossRef]
- Pooniya, V.; Zhiipao, R.R.; Biswakarma, N.; Kumar, D.; Shivay, Y.S.; Babu, S.; Das, K.; Choudhary, A.K.; Swarnalakshmi, K.; Choudhary, R.L.; et al. Conservation Agriculture Based Integrated Crop Management Sustains Productivity and Economic Profitability along with Soil Properties of the Maize-Wheat Rotation. Sci. Rep. 2022, 12, 1962. [Google Scholar] [CrossRef]
- Murphy, K.M.; Edwards, J.; Louie, K.B.; Bowen, B.P.; Sundaresan, V.; Northen, T.R.; Zerbe, P. Bioactive Diterpenoids Impact the Composition of the Root-Associated Microbiome in Maize (Zea mays). Sci. Rep. 2021, 11, 333. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Fan, W.; Deng, J.; Shao, L.; Jiang, S.; Xiao, T.; Sun, W.; Xiao, E. The Rhizosphere Microbiome Improves the Adaptive Capabilities of Plants under High Soil Cadmium Conditions. Front. Plant Sci. 2022, 13, 914103. [Google Scholar] [CrossRef] [PubMed]
- Coral Valenzuela, J.V.; Andrade Bolaños, H.J.; Pumisacho Gualoto, M.M.; Caicedo Chávez, J.D.; Salazar Vizuete, D.R. Caracterización Morfológica y Agronómica de dos Genotipos de Maíz (Zea mays L.) en la Zona Media de la Parroquia Malchinguí. ACI Av. En Cienc. E Ing. 2019, 11, 21–33. [Google Scholar] [CrossRef]
- Silva, E.; Dobronsky, J.; Heredia, J. INIAP-122 Chaucho Mejorado: Variedad de Maíz Amarillo Harinoso Semi-Precoz para la Provincia de Imbabura; INIAP: Quito, Ecuador, 1997. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Copenhagen, Denmark, 2018. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. 2012. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 August 2024).
- Xu, S.; Zhan, L.; Tang, W.; Wang, Q.; Dai, Z.; Zhou, L.; Feng, T.; Chen, M.; Wu, T.; Hu, E.; et al. MicrobiotaProcess: A Comprehensive R Package for Deep Mining Microbiome. Innovation 2023, 4, 100388. [Google Scholar] [CrossRef]
- Walters, D.R.; Scholes, J.D.; Goss, M.J.; Brown, J.F.; Bevan, M.W. Exploring the Maize Microbiome for Novel Insights into Plant-Microbe Interactions and Drought Tolerance. Plant Cell Environ. 2018, 41, 2877–2890. [Google Scholar]
- He, X.; Wang, D.; Jiang, Y.; Li, M.; Delgado-Baquerizo, M.; McLaughlin, C.; Marcon, C.; Guo, L.; Baer, M.; Moya, Y.A.T.; et al. Heritable microbiome variation is correlated with source environment in locally adapted maize varieties. Nat. Plants 2024, 10, 598–617. [Google Scholar] [CrossRef]
- Singh, R.; Goodwin, S.B. Exploring the Corn Microbiome: A Detailed Review on Current Knowledge, Techniques, and Future Directions. PhytoFrontiers™ 2022, 2, 158–175. [Google Scholar] [CrossRef]
- Jalloh, A.A.; Khamis, F.M.; Yusuf, A.A.; Subramanian, S.; Mutyambai, D.M. Long-term push–pull cropping system shifts soil and maize-root microbiome diversity paving way to resilient farming system. BMC Microbiol. 2024, 24, 92. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Chen, Y.; Wang, M.; Lu, Q.; Wang, K.; Dou, Z.; Chi, Z.; Qiu, W.; Dai, J.; et al. Microbiome convergence enables siderophore-secreting-rhizobacteria to improve iron nutrition and yield of peanut intercropped with maize. Nat. Commun. 2024, 15, 839. [Google Scholar] [CrossRef]
- Lanzavecchia, G.; Frascarelli, G.; Rocchetti, L.; Bellucci, E.; Bitocchi, E.; Di Vittori, V.; Sillo, F.; Ferraris, I.; Carta, G.; Delledonne, M.; et al. Genotype combinations drive variability in the microbiome configuration of the rhizosphere of maize/bean intercropping system. Int. J. Mol. Sci. 2024, 25, 1288. [Google Scholar] [CrossRef]
- Poppeliers, S.W.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to Support Plant Health: Understanding Bioinoculant Success in Complex Conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef] [PubMed]
- Beirinckx, S.; Viaene, T.; Haegeman, A.; Debode, J.; Amery, F.; Vandenabeele, S.; Nelissen, H.; Inzé, D.; Tito, R.; Raes, J.; et al. Tapping into the Maize Root Microbiome to Identify Bacteria That Promote Growth under Chilling Conditions. Microbiome 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wei, Q.; Wu, H.; Chen, X.; Xiao, C.; Ye, Y.; Liu, C.; Yu, H.; Guo, Y.; Sun, W.; et al. Bacillus species are core microbiota of resistant maize cultivars that induce host metabolic defense against corn stalk rot. Microbiome 2024, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Huang, S.; Li, L.; Gao, Q.; Wang, Y.; Zhang, S.; Huang, S.; Yuan, L.; Wen, Y.; et al. A highly conserved core bacterial microbiota with nitrogen-fixation capacity inhabits the xylem sap in maize plants. Nat. Commun. 2022, 13, 3361. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, D.; Li, F.; Dong, Y.; Jin, Z.; Liao, Y.; Li, X.; Peng, S.; Delgado-Baquerizo, M.; Li, X. Superiority of native soil core microbiomes in supporting plant growth. Nat. Commun. 2024, 15, 6599. [Google Scholar] [CrossRef]
- Tkacz, A.; Cheema, J.; Chandra, G.; Grant, A.; Poole, P.S. Stability and Succession of the Rhizosphere Microbiota Depends Upon Plant Type and Soil Composition. ISME J. 2015, 9, 2349–2359. [Google Scholar] [CrossRef]
- Leopold, D.R.; Busby, P.E. Host Genotype and Colonist Arrival Order Jointly Govern Plant Microbiome Composition and Function. Curr. Biol. 2020, 30, 3260–3266.e5. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]

| Variable | Ilustration | Treatments | |||||
|---|---|---|---|---|---|---|---|
| PEPA | Amarillo | INIAP-122 | |||||
| Average | SD | Average | SD | Average | SD | ||
| Leaf area (cm2) |    | 355 | 61.8 | 279.4 | 91.8 | 292.1 | 44 |
| Brace root BR number |    | 14.8 | 3.9 | 14 | 1.41 | 14 | 2.2 |
| Crown root CR number |    | 24. 2 | 6.3 | 16 | 2.2 | 15 | 2.9 |
| Brace root length (cm) |  | 20 | 1.8 | 19.8 | 7.4 | 21.9 | 4.2 |
| Crown root length (cm) |  | 24.6 | 3.3 | 32.8 | 7 | 39.1 | 7.1 |
| Relative density of laterals BR (RL/cm) |   | 7 | 1.4 | 6.5 | 0.6 | 7 | 0.8 |
| Relative density of laterals CR (RL/cm) |   | 8 | 0.8 | 7.5 | 1 | 8.5 | 0.6 |
| Relative content water (%) | 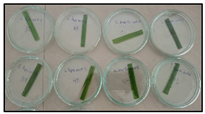 | 67.5 | 3.1 | 63.1 | 4.5 | 78.6 | 1.7 |
| Root biomass (g/plant) |  | 35.2 | 2.3 | 25.5 | 1.1 | 55.2 | 1 |
| Aerial biomass (g/plant) | 167.5 | 2.4 | 153.7 | 2.5 | 163.5 | 2.3 | |
| Grain yield (g/plant) |   | 31.8 | 0.5 | 54.8 | 2.2 | 47.5 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Garnica, M.A.; Lozano-Granda, A.F.; Ramirez-Villacis, D.X.; Barriga-Medina, N.; Urresta-Palacios, L.F.; Garzon-Proaño, B.A.; Proaño-Arias, S.; Andrade-Bolaños, H.J.; Leon-Reyes, A. The Bacterial Root Microbiome in Ecuadorian Andean Maize. Agronomy 2024, 14, 2878. https://doi.org/10.3390/agronomy14122878
Sanchez-Garnica MA, Lozano-Granda AF, Ramirez-Villacis DX, Barriga-Medina N, Urresta-Palacios LF, Garzon-Proaño BA, Proaño-Arias S, Andrade-Bolaños HJ, Leon-Reyes A. The Bacterial Root Microbiome in Ecuadorian Andean Maize. Agronomy. 2024; 14(12):2878. https://doi.org/10.3390/agronomy14122878
Chicago/Turabian StyleSanchez-Garnica, Manoella A., Angel F. Lozano-Granda, Dario X. Ramirez-Villacis, Noelia Barriga-Medina, Luis F. Urresta-Palacios, Brigitte A. Garzon-Proaño, Stefania Proaño-Arias, Hector J. Andrade-Bolaños, and Antonio Leon-Reyes. 2024. "The Bacterial Root Microbiome in Ecuadorian Andean Maize" Agronomy 14, no. 12: 2878. https://doi.org/10.3390/agronomy14122878
APA StyleSanchez-Garnica, M. A., Lozano-Granda, A. F., Ramirez-Villacis, D. X., Barriga-Medina, N., Urresta-Palacios, L. F., Garzon-Proaño, B. A., Proaño-Arias, S., Andrade-Bolaños, H. J., & Leon-Reyes, A. (2024). The Bacterial Root Microbiome in Ecuadorian Andean Maize. Agronomy, 14(12), 2878. https://doi.org/10.3390/agronomy14122878





