Biochar Influences Polyethylene Microplastic-Contaminated Soil Properties and Enzyme Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Soybean Growth Determination
2.4. Measurement of Soil Nutrients, Microbial Biomass, and Enzymes
2.5. Statistical Analysis
3. Results
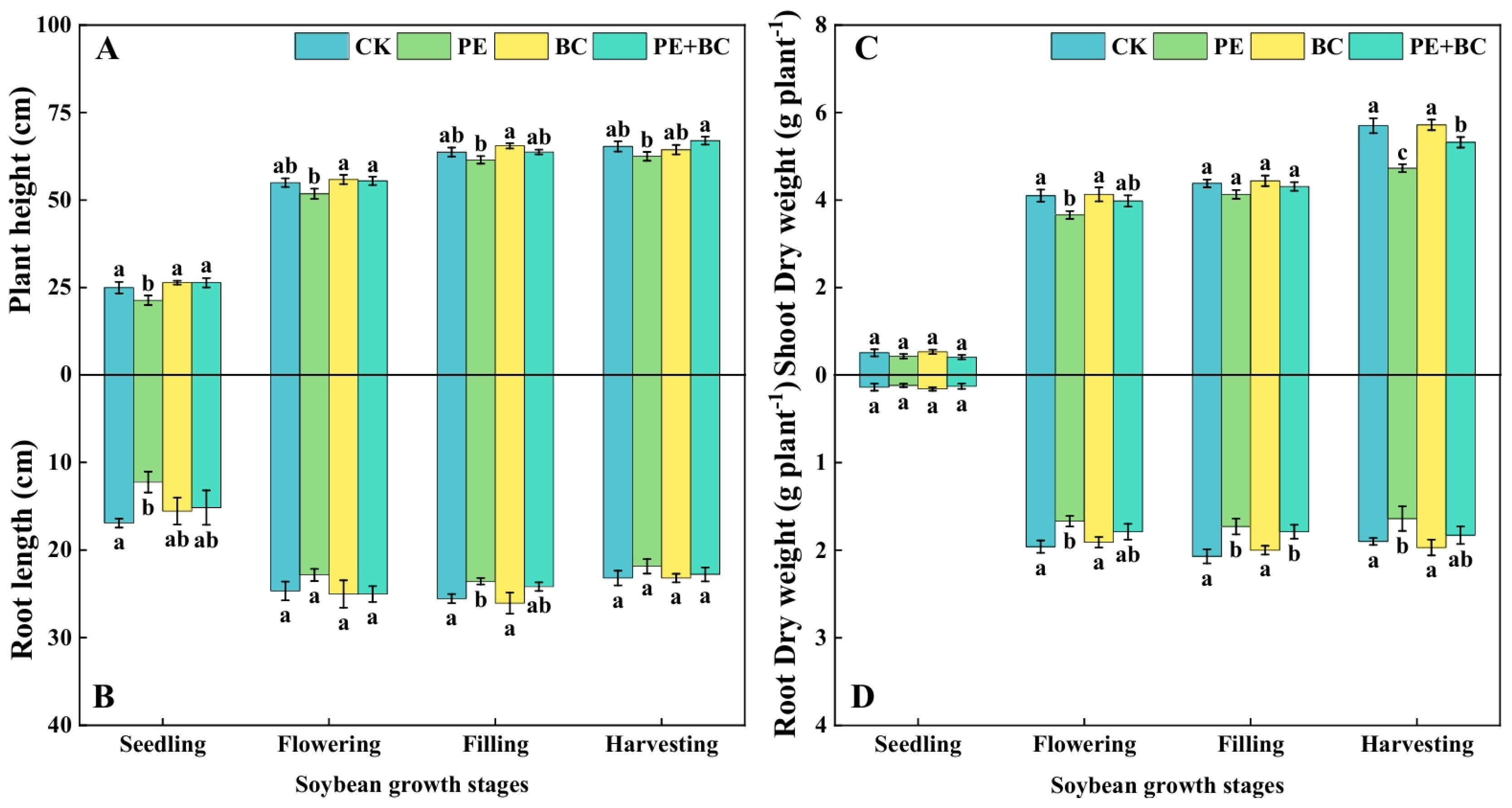
3.1. MPs and Biochar Effects on Soybean Growth
3.2. MPs and Biochar Effects on Soil Chemical Properties
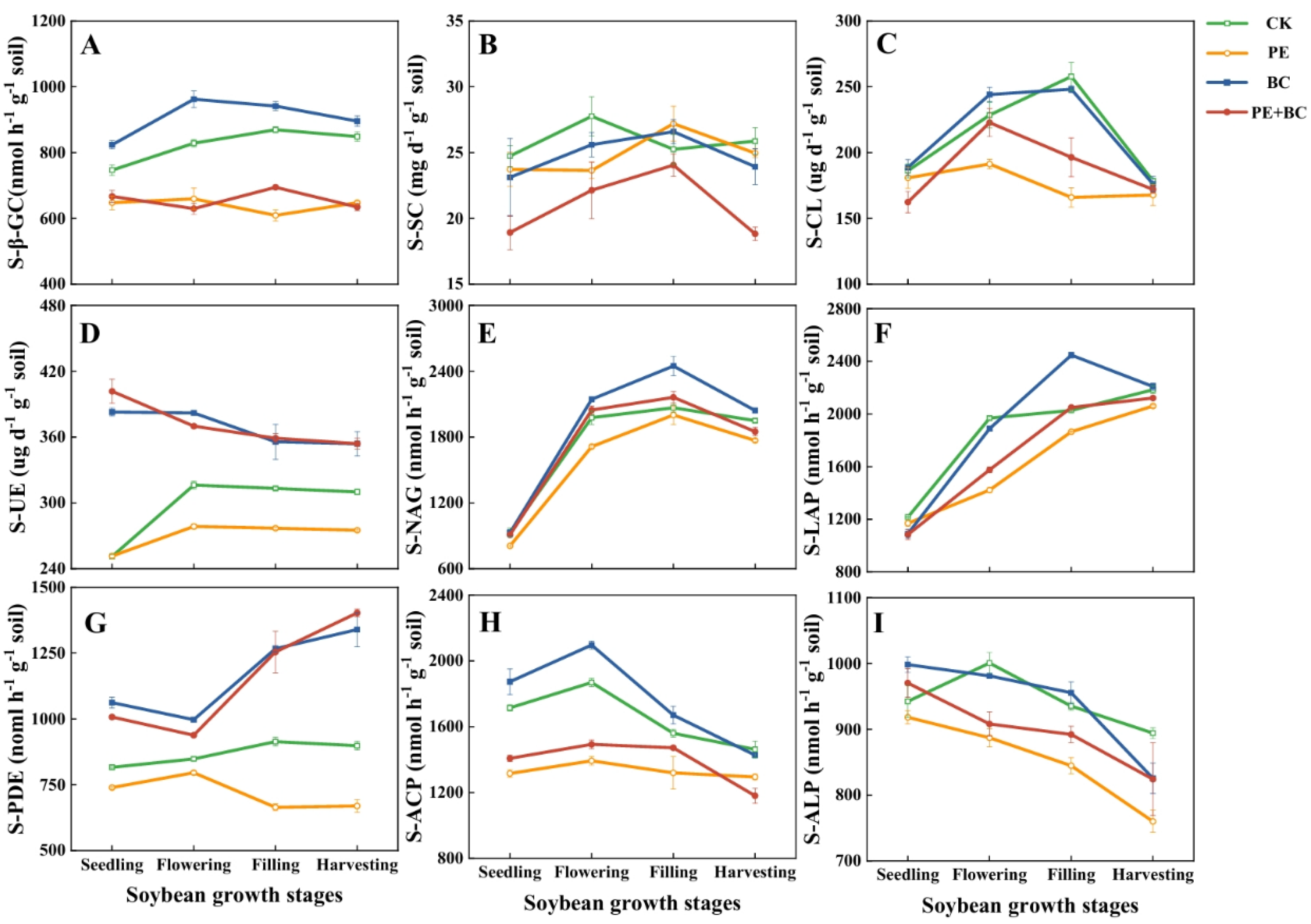
3.3. MPs and Biochar Effects on Soil Enzyme Activity
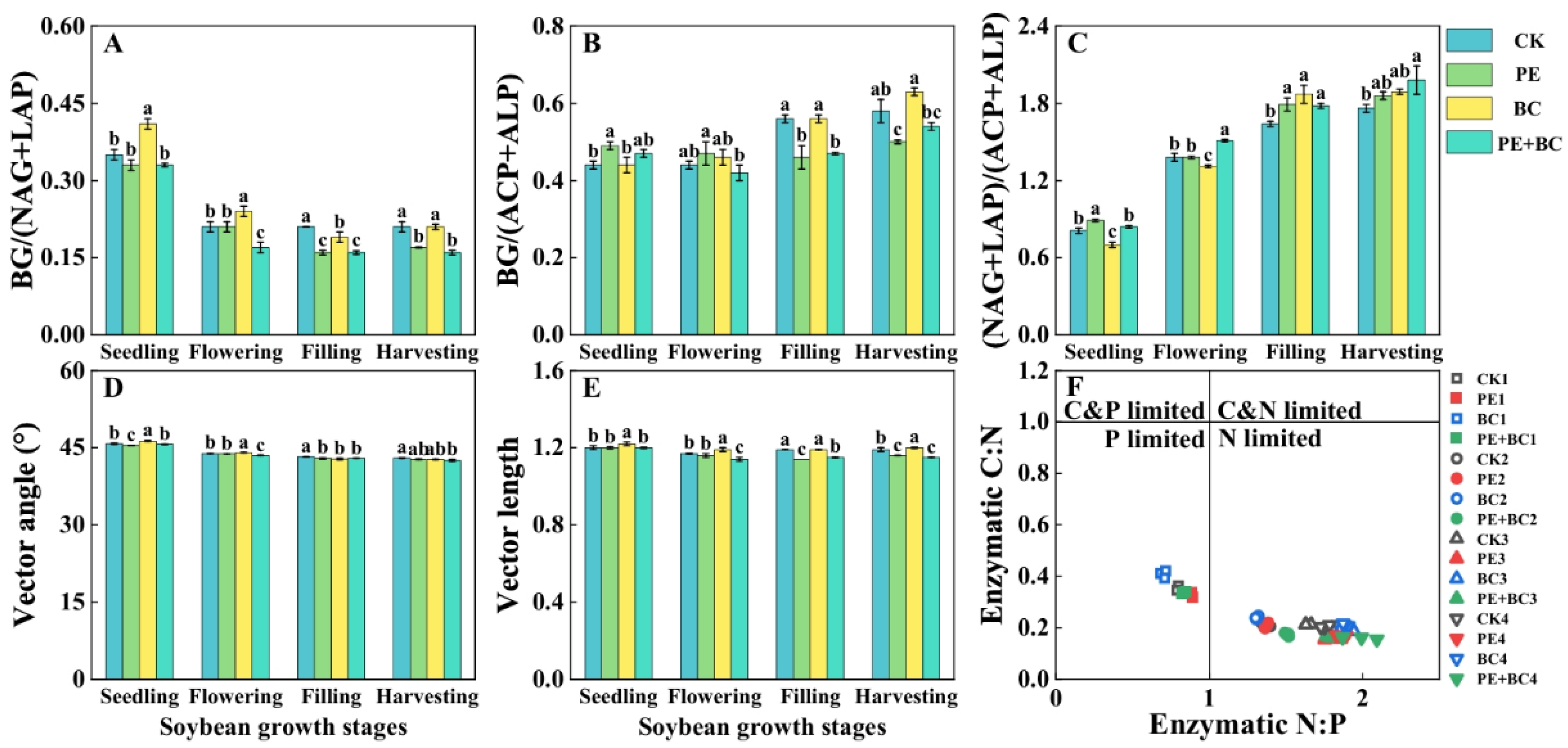
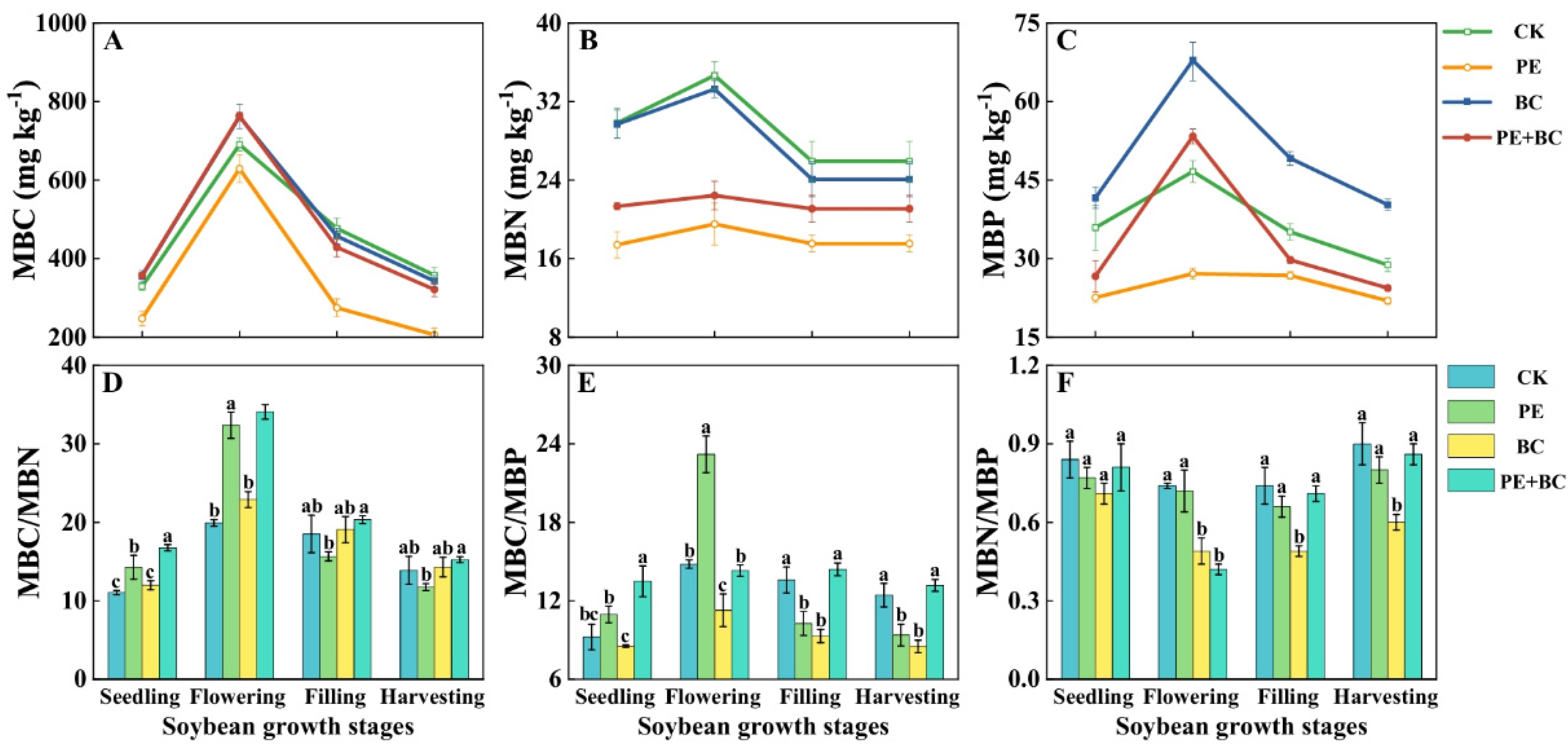
3.4. MPs and Biochar Effects on Soil Microbial C-N-P Characteristics
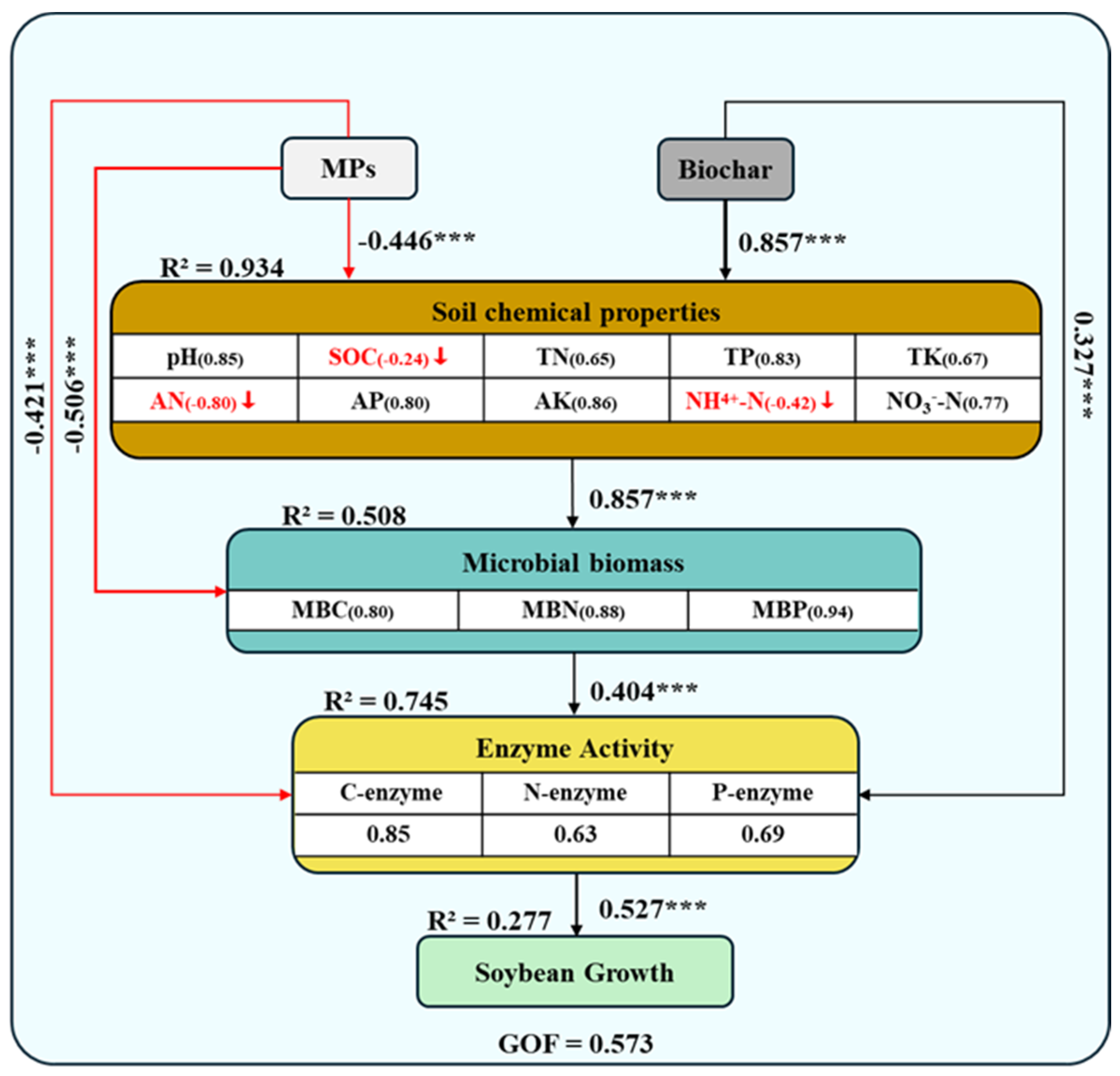
3.5. MPs Effects on the Soil–Microbial–Soybean System
4. Discussion
4.1. MPs and Biochar Affect Soybean Growth
4.2. MPs and Biochar Affect Soil Chemical Properties
4.3. Biochar Effects on Microbial Biomass and Enzyme Activity in MP-Contaminated Soil
4.4. Alterations in Microbial Biomass and Enzyme Activity Under MPs and Biochar Effect on Soybean Growth
5. Conclusions
- (1)
- The addition of microplastics inhibited soybean sprout height and root length during the observed growth stage.
- (2)
- Soil nutrients and enzyme activities were significantly reduced by microplastics, leading to a notable decline in soil microbial biomass.
- (3)
- In soils contaminated with microplastics, biochar had a positive impact on soybean growth, microbial biomass, and nutrient cycling enzyme activities.
- (4)
- Biochar effectively mitigated the adverse effects of microplastics on soil properties, thereby enhancing ecosystem functionality.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| PE | polyethylene |
| PP | polypropylene |
| PVC | polyvinyl chloride |
| RC | rubber crumb |
| BC | biochar |
| S-β-GC | soil β-glucosidase |
| S-SC | soil Saccharase |
| S-CL | soil Cellulase |
| S-UE | soil Urease |
| S-NAG | soil N-acetyl-β-glycosaminidase |
| S-LAP | soil Leucine-aminopeptidase |
| S-PDE | soil phosphodiesterase |
| S-ACP | soil Acid phosphatase |
| S-ALP | soil Alkaline phosphatase |
References
- Kim, Y.; Yoon, J.; Kim, K. Microplastic contamination in soil environment—A review. Soil. Sci. Annu. 2021, 71, 300–308. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S.; et al. Current research trends on micro- and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production; usage; disposal; adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Ng, E.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastics in soils—A review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950–17959. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Ren, S.; Kong, S.; Ni, H. Contribution of mulch film to microplastics in agricultural soil and surface water in China. Environ. Pollut. 2021, 291, 118227. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta, L.E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Z.; Sun, J.; Li, D.; Cheng, Z.; Niu, Y.; Guo, H.; Zhou, J.; Wang, T. Microplastics affect C, and P cycling in natural environments: Highlighting the driver of soil hydraulic properties. J. Hazard. Mater. 2023, 459, 132326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ng, E.L.; Hu, W.; Wang, H.; Galaviz, P.; Yang, H.; Sun, W.; Li, C.; Ma, X.; Fu, B.; et al. Plastic pollution in croplands threatens long-term food security. Global Chang. Biol. 2020, 26, 3356–3367. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic Addition Alters the Microbial Community Structure and Stimulates Soil Carbon Dioxide Emissions in Vegetable-Growing Soil. Environ. Toxicol. Chem. 2021, 40, 352–365. [Google Scholar] [CrossRef]
- Jiao, K.; Yang, B.; Wang, H.; Xu, W.; Zhang, C.; Gao, Y.; Sun, W.; Li, F.; Ji, D. The individual and combined effects of polystyrene and silver nanoparticles on nitrogen transformation and bacterial communities in an agricultural soil. Sci. Total Environ. 2022, 820, 153358. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Xia, Y.; Tao, R.; Zhang, M.; Mei, Y.; Qu, M. Simulation of the effects of microplastics on the microbial community structure and nitrogen cycle of paddy soil. Sci. Total Environ. 2022, 818, 151768. [Google Scholar] [CrossRef]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between microplastics and soil fauna: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, J.; Liu, Z.; Wei, H.; Zhang, J. Polyethylene microplastics alter soil microbial community assembly and ecosystem multifunctionality. Environ. Int. 2024, 183, 108360. [Google Scholar] [CrossRef]
- Yu, F.; Fu, M.; Tang, C.; Mo, C.; Li, S.; Luo, S.; Qin, P.; Zhao, Y.; Li, Y. Potential impact of polyethylene microplastics on the growth of water spinach (Ipomoea aquatica F.): Endophyte rhizosphere effects. Chemosphere 2023, 330, 138737. [Google Scholar] [PubMed]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil. 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Liu, R.; Liang, J.; Yang, Y.; Jiang, H.; Tian, X. Effect of polylactic acid microplastics on soil properties, soil microbials and plant growth. Chemosphere 2023, 329, 138504. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotox Environ. Safe 2021, 211, 111899. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Chen, X.; Lu, X.; Yan, J.; Wang, W.; Zou, W.; Yan, L. Application of organic manure as a potential strategy to alleviate the limitation of microbial resources in soybean rhizospheric and bulk soils. J. Integr. Agric. 2024, 23, 2065–2082. [Google Scholar]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar]
- Dominchin, M.F.; Verdenelli, R.A.; Berger, M.G.; Aoki, A.; Meriles, J.M. Impact of N-fertilization and peanut shell biochar on soil microbial community structure and enzyme activities in a Typic Haplustoll under different management practices. Eur. J. Soil Biol. 2021, 104, 103298. [Google Scholar] [CrossRef]
- Liao, X.; Kang, H.; Haidar, G.; Wang, W.; Malghani, S. The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: A meta-analysis. Geoderma 2022, 411, 115692. [Google Scholar] [CrossRef]
- Yang, L.; Shen, P.; Liang, H.; Wu, Q. Biochar relieves the toxic effects of microplastics on the root-rhizosphere soil system by altering root expression profiles and microbial diversity and functions. Ecotox Environ. Safe 2024, 271, 115935. [Google Scholar]
- Li, J.; Yu, Y.; Chen, X.; Yu, S.; Cui, M.; Wang, S.; Song, F. Effects of biochar on the phytotoxicity of polyvinyl chloride microplastics. Plant Physiol. Bioch 2023, 195, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J. Harnessing the Potential of Forage Legumes. Alfalfa, Soybean, and Cowpea for Sustainable Agriculture and Global Food Security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Liu, J.; Han, S.; Li, Y.; Xu, T.; Xi, J.; Hou, L.; Lin, Y. Effect of conventional and biodegradable microplastics on the soil-soybean system: A perspective on rhizosphere microbial community and soil element cycling. Environ. Int. 2024, 190, 108781. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.R.; Shah, T.; Asad, M.; Ali, A.; Samee, E.; Adnan, F.; Bhatti, M.F.; Marhan, S.; Kammann, C.I.; Haider, G. Biochar alleviated the toxic effects of Pvc microplastic in a soil-plant system by upregulating soil enzyme activities and microbial abundance. Environ. Pollut. 2023, 332, 121810. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Wang, Y.; Zhang, Y.; Xu, T.; Zhang, Y.; Xi, J.; Hou, L.; Li, L.; Zhang, Z.; et al. Negative effects of poly(butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome. J. Hazard. Mater. 2022, 437, 129294. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Go, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Zhou, Y.; An, Z.; Cai, Y.; Chang, S.X. Microplastics affect the ecological stoichiometry of plant, soil and microbes in a greenhouse vegetable system. Sci. Total Environ. 2024, 924, 171602. [Google Scholar] [CrossRef]
- Wang, C.; Yao, X.; Li, X.; Wang, Q.; Wang, J.; Zhu, L.; Wang, J. Effects of dibutyl phthalate on microbial community and the carbon cycle in salinized soil. J. Clean. Prod. 2023, 404, 136928. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; LI, R.; LI, M.; Zhang, H.; Huang, S. Effects of a decade of organic fertilizer substitution on vegetable yield and soil phosphorus pools, phosphatase activities, and the microbial community in a greenhouse vegetable production system. J. Integr. Agric. 2022, 21, 2119–2133. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, X.; Zhu, Z.; Qin, Q.; Song, K.; Sun, L.; Sun, Y.; Zhang, Y.; Lv, W.; Xue, Y. Effects of fertilizer application on phthalate ester pollution and the soil microbial community in plastic-shed soil on long-term fertilizer experiment. Chemosphere 2022, 308, 136315. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C. N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Shah, T.; Ali, A.; Haider, G.; Asad, M.; Munsif, F. Microplastics alter soil enzyme activities and microbial community structure without negatively affecting plant growth in an agroecosystem. Chemosphere 2023, 322, 138188. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Duan, P.; Tian, X.; Huang, J.; Ji, J.; Chen, Z.; Yang, J.; Yu, H.; Zhang, W. Polystyrene microplastics sunlight-induce oxidative dissolution, chemical transformation and toxicity enhancement of silver nanoparticles. Sci. Total Environ. 2022, 827, 154180. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.; Zeng, Z.; Jones, D.L.; Zang, H. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Ding, L.; Zhang, G.; Bai, B.; Han, Y.; Xiao, L.; Song, Y.; Li, Y.; Wan, S.; et al. Microplastics reduce nitrogen uptake in peanut plants by damaging root cells and impairing soil nitrogen cycling. J. Hazard. Mater. 2023, 443, 130384. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, X. Systemic Stress and Recovery Patterns of Rice Roots in Response to Graphene Oxide Nanosheets. Environ. Sci. Technol. 2017, 51, 2022–2030. [Google Scholar] [CrossRef]
- Wen, B.; Li, C.; Fu, X.; Li, D.; Li, L.; Chen, X.; Wu, H.; Cui, X.; Zhang, X.; Shen, H.; et al. Effects of nitrate deficiency on nitrate assimilation and chlorophyll synthesis of detached apple leaves. Plant Physiol. Bioch 2019, 142, 363–371. [Google Scholar] [CrossRef]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere’s microbial community and health. Chemosphere 2022, 294, 133710. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, J.; Xue, J. Polyethylene microplastic and soil nitrogen dynamics: Unraveling the links between functional genes, microbial communities, and transformation processes. J. Hazard. Mater. 2023, 458, 131857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Ren, X.; Li, Y.; Zhang, Y.; Zhang, H.; Han, H.; Chen, Z. Plant growth-promoting bacteria modulate gene expression and induce antioxidant tolerance to alleviate synergistic toxicity from combined microplastic and Cd pollution in sorghum. Ecotox Environ. Safe 2023, 264, 115439. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z. The pollution of microplastics in sediments: The ecological risk assessment and pollution source analysis. Sci. Total Environ. 2023, 859, 160323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Liu, J.; Wu, H. Discrepant responses of bacterial community and enzyme activities to conventional and biodegradable microplastics in paddy soil. Sci. Total Environ. 2024, 909, 168513. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. Ldpe microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Yu, H.; Pu, Z.; Wang, S.; Chen, Y.; Wang, C.; Wan, Y.; Dong, Y.; Wang, J.; Wan, S.; Wang, D.; et al. Mitigating microplastic stress on peanuts: The role of biochar-based synthetic community in the preservation of soil physicochemical properties and microbial diversity. Sci. Total Environ. 2024, 932, 172927. [Google Scholar] [CrossRef]
- Shi, L.; Hou, Y.; Chen, Z.; Bu, Y.; Zhang, X.; Shen, Z.; Chen, Y. Impact of polyethylene on soil physicochemical properties and characteristics of sweet potato growth and polyethylene absorption. Chemosphere 2022, 302, 134734. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Al Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land. Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, Q.; Wang, Q.; Wang, T.; Kang, Z.; Zeng, Y.; Yang, X.; Song, N.; Zhang, S.; Han, X.; et al. Effects of polyethylene microplastics on properties, enzyme activities, and the succession of microbial community in Mollisol: At the aggregate level. Environ. Res. 2023, 237, 116976. [Google Scholar] [CrossRef] [PubMed]
- Daughtridge, R.C.; Nakayama, Y.; Margenot, A.J. Sources of abiotic hydrolysis of chromogenic substrates in soil enzyme assays: Storage, termination, and incubation. Soil. Biol. Biochem. 2021, 158, 108245. [Google Scholar] [CrossRef]
- Liu, Y.; Shahbaz, M.; Ge, T.; Zhu, Z.; Liu, S.; Chen, L.; Wu, X.; Deng, Y.; Lu, S.; Wu, J. Effects of root exudate stoichiometry on CO2 emission from paddy soil. Eur. J. Soil. Biol. 2020, 101, 103247. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Chen, Y.; Liu, G.; Xue, S. Responses of soil enzyme activity and soil organic carbon stability over time after cropland abandonment in different vegetation zones of the Loess Plateau of China. Catena 2021, 196, 104812. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Shahbaz, M.; Hu, Z.E.; Zhu, Z.; Lu, S.; Yu, Y.; Yao, H.; Chen, J.; Ge, T. Microplastics in soil can increase nutrient uptake by wheat. J. Hazard. Mater. 2022, 438, 129547. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Hu, R.; Wang, X.; Xu, J.; Zhang, Y.; Pan, Y.; Su, X. The mechanism of soil nitrogen transformation under different biocrusts to warming and reduced precipitation: From microbial functional genes to enzyme activity. Sci. Total Environ. 2020, 722, 137849. [Google Scholar] [CrossRef]
- Neemisha; Sharma, S. Soil Enzymes and Their Role in Nutrient Cycling; Varma, A., Giri, B., Giri, B., Kapoor, R., Wu, Q., Wu, Q., Kapoor, R., Varma, A., Eds.; Springer: Singapore, 2022; pp. 173–188. [Google Scholar]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. Ldpe microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Oladele, S.O.; Ojokole, W.; Oladele, B.B. Microplastics in agricultural soil: Polystyrene fragments inhibit soil microbial and enzymatic activities but promote nutrient concentration of Cowpea (Vigna unguiculata). J. Hazard. Mater. Adv. 2023, 10, 100263. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, N.; Li, Y.; Dou, S.; Liu, Z.; Chen, Y.; Ma, C.; Zhang, H. Responses of maize (Zea mays L.) seedlings growth and physiological traits triggered by polyvinyl chloride microplastics is dominated by soil available nitrogen. Ecotox Environ. Safe 2023, 252, 114618. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Kublik, S.; Gschwendtner, S.; Magritsch, T.; Radl, V.; Rillig, M.C.; Schloter, M. Microplastics in soil induce a new microbial habitat, with consequences for bulk soil microbiomes. Front. Env. Sci. 2022, 10, 989267. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Chang, Z.; Li, Z.; Joseph, J.; Yusuf, A.A.; Luo, X.; Hou, E. Biochar rate-dependent regulation of extended nitrogen supply by modifying stable aggregates-N and microbial responses. Carbon Res. 2023, 2, 22. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Iqbal, B.; Tariq, M.; Rehman, A.; Khan, I.; Du, D. Soil nutrient levels regulate the effect of soil microplastic contamination on microbial element metabolism and carbon use efficiency. Ecotox Environ. Safe 2023, 267, 115640. [Google Scholar] [CrossRef]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect evaluation of microplastics on activated sludge nitrification and denitrification. Sci. Total Environ. 2020, 707, 135953. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Zhu, Y.; Chen, X.; Lu, X.; Yan, J.; Yan, L.; Zou, W. Biochar Influences Polyethylene Microplastic-Contaminated Soil Properties and Enzyme Activities. Agronomy 2024, 14, 2919. https://doi.org/10.3390/agronomy14122919
Su J, Zhu Y, Chen X, Lu X, Yan J, Yan L, Zou W. Biochar Influences Polyethylene Microplastic-Contaminated Soil Properties and Enzyme Activities. Agronomy. 2024; 14(12):2919. https://doi.org/10.3390/agronomy14122919
Chicago/Turabian StyleSu, Jie, Yuanchen Zhu, Xu Chen, Xinchun Lu, Jun Yan, Lei Yan, and Wenxiu Zou. 2024. "Biochar Influences Polyethylene Microplastic-Contaminated Soil Properties and Enzyme Activities" Agronomy 14, no. 12: 2919. https://doi.org/10.3390/agronomy14122919
APA StyleSu, J., Zhu, Y., Chen, X., Lu, X., Yan, J., Yan, L., & Zou, W. (2024). Biochar Influences Polyethylene Microplastic-Contaminated Soil Properties and Enzyme Activities. Agronomy, 14(12), 2919. https://doi.org/10.3390/agronomy14122919






