Effects of Root Trace Nitrogen Reduction in Arid Areas on Sucrose–Starch Metabolism of Flag Leaves and Grains and Yield of Drip-Irrigated Spring Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
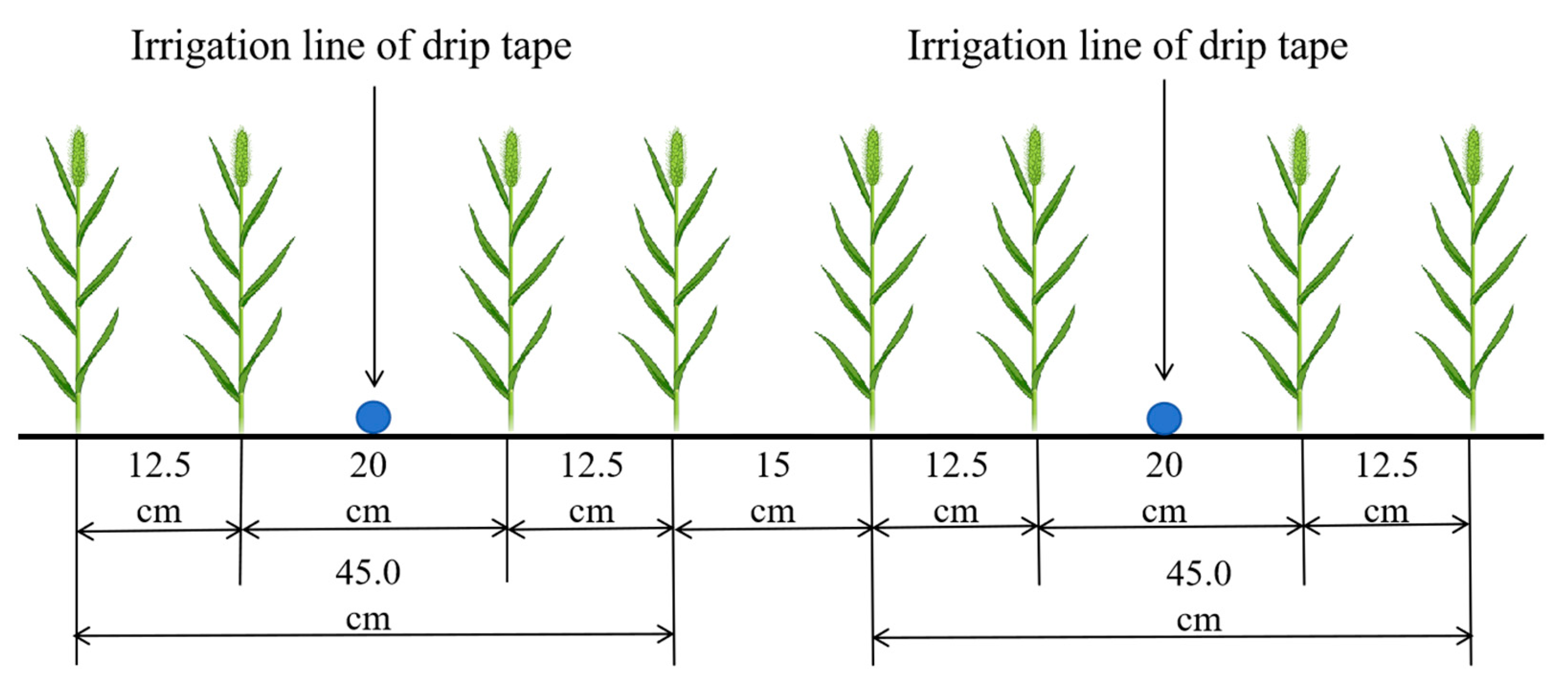
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Sample Collection
2.3.2. Determination of Key Enzyme Activities in Carbon Metabolism of Flag Leaves and Grains
2.3.3. Determination of Soluble Sugar and Sucrose Content in Flag Leaves and Grains, Starch and Component Content in Grains
2.3.4. Yield Measurement
2.4. Data Analysis
3. Results
3.1. Effect of Nitrogen Supply on Carbon Metabolism in Flag Leaves of Spring Wheat
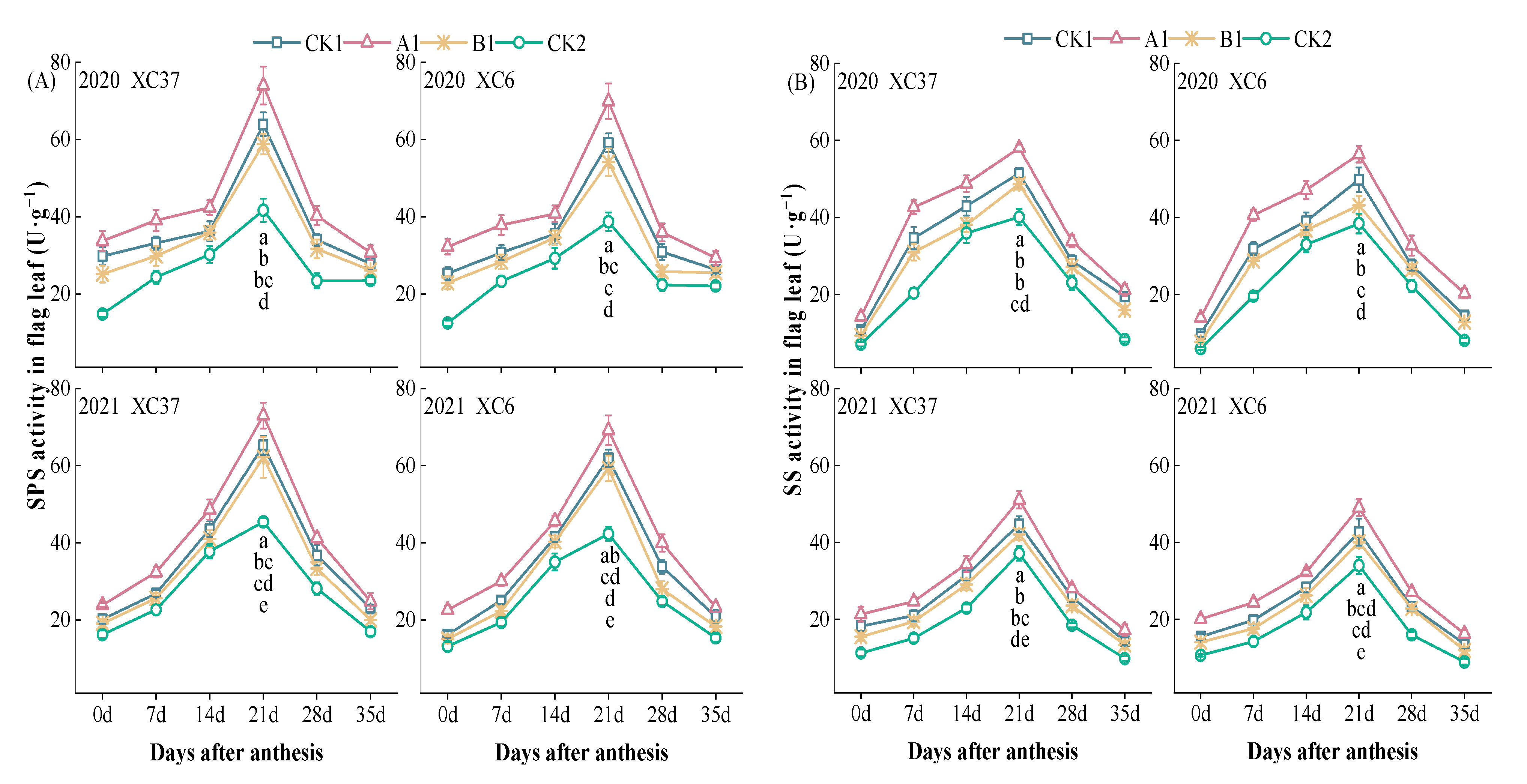
3.1.1. Changes in the Activity of Key Enzymes Involved in Sucrose Metabolism in Flag Leaves
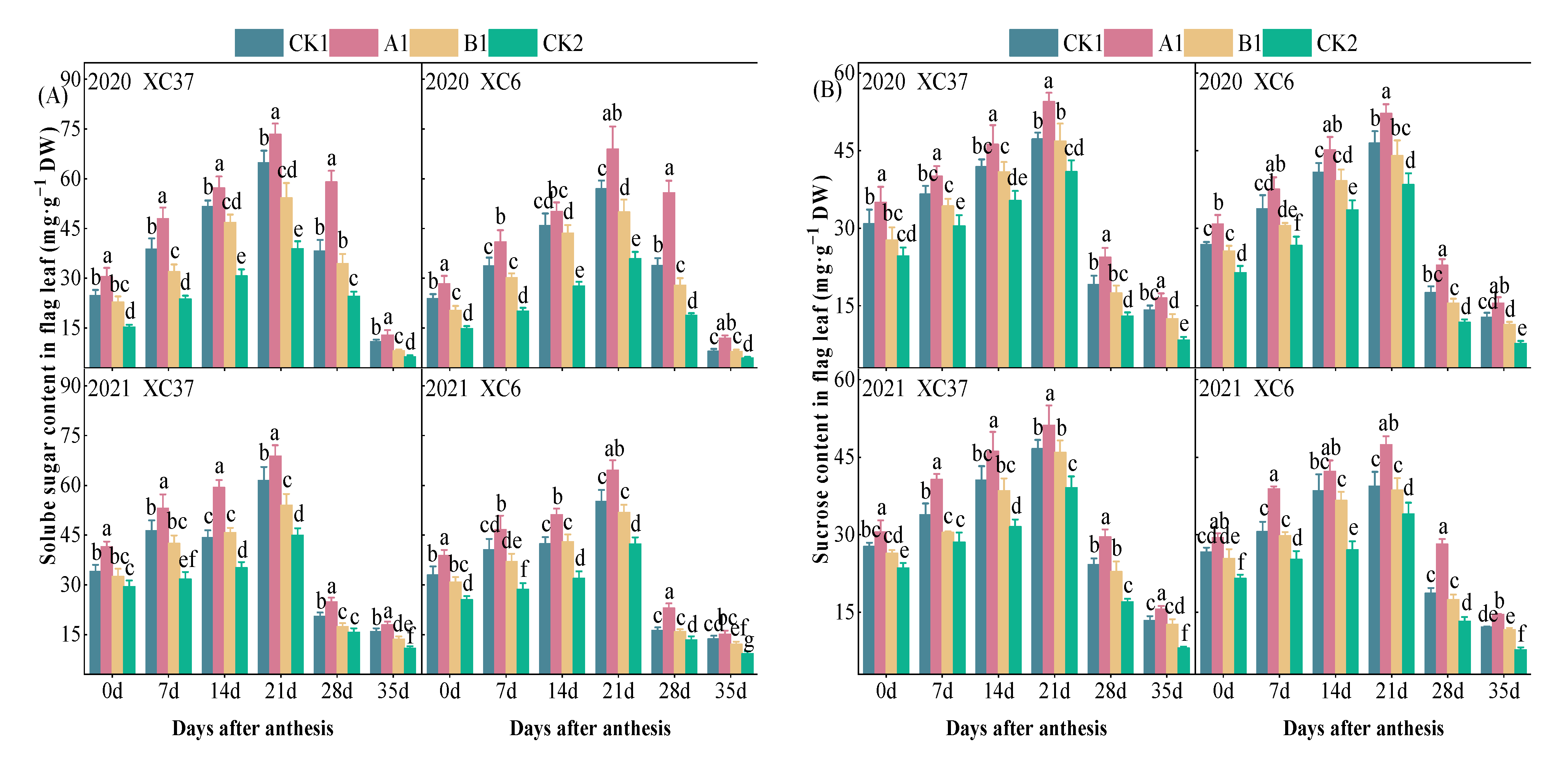
3.1.2. Changes in Soluble Sugar and Sucrose Content in Flag Leaves
3.2. Effect of Nitrogen Supply on Carbon Metabolism in Grains of Spring Wheat
3.2.1. Changes in the Activity of Key Enzymes Involved in Sucrose Metabolism in Grains
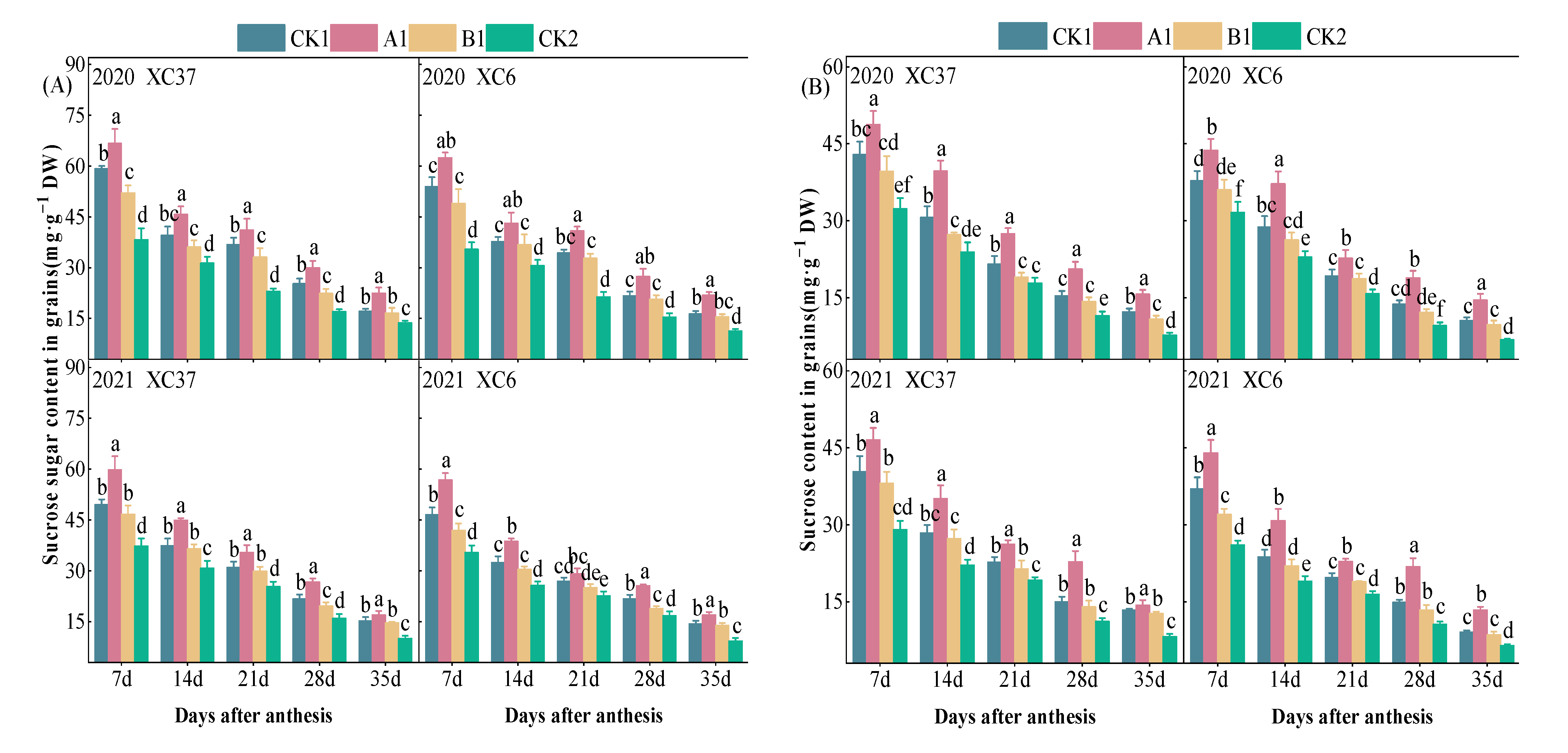
3.2.2. Changes in Soluble Sugar and Sucrose Content in Grains
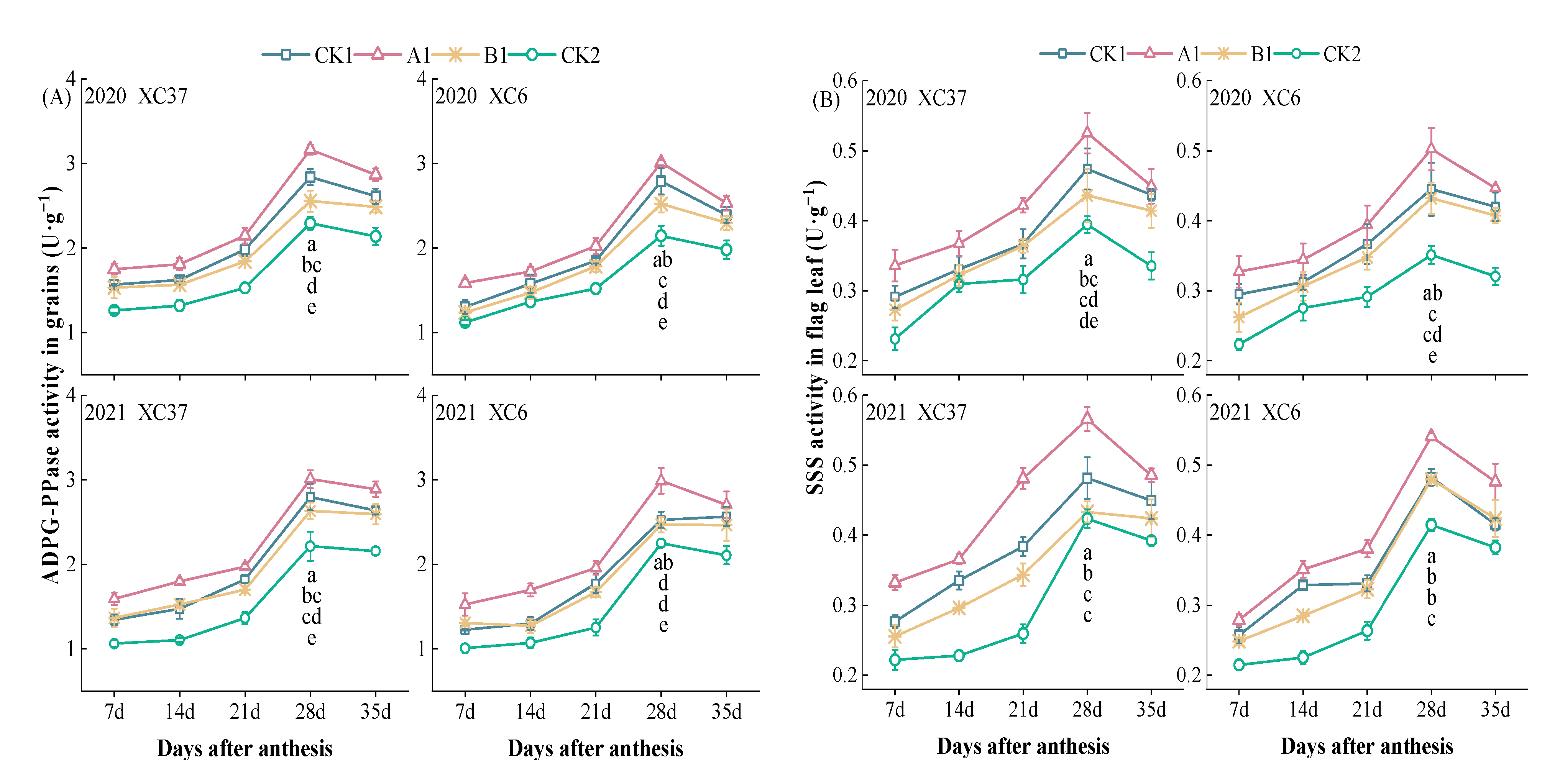
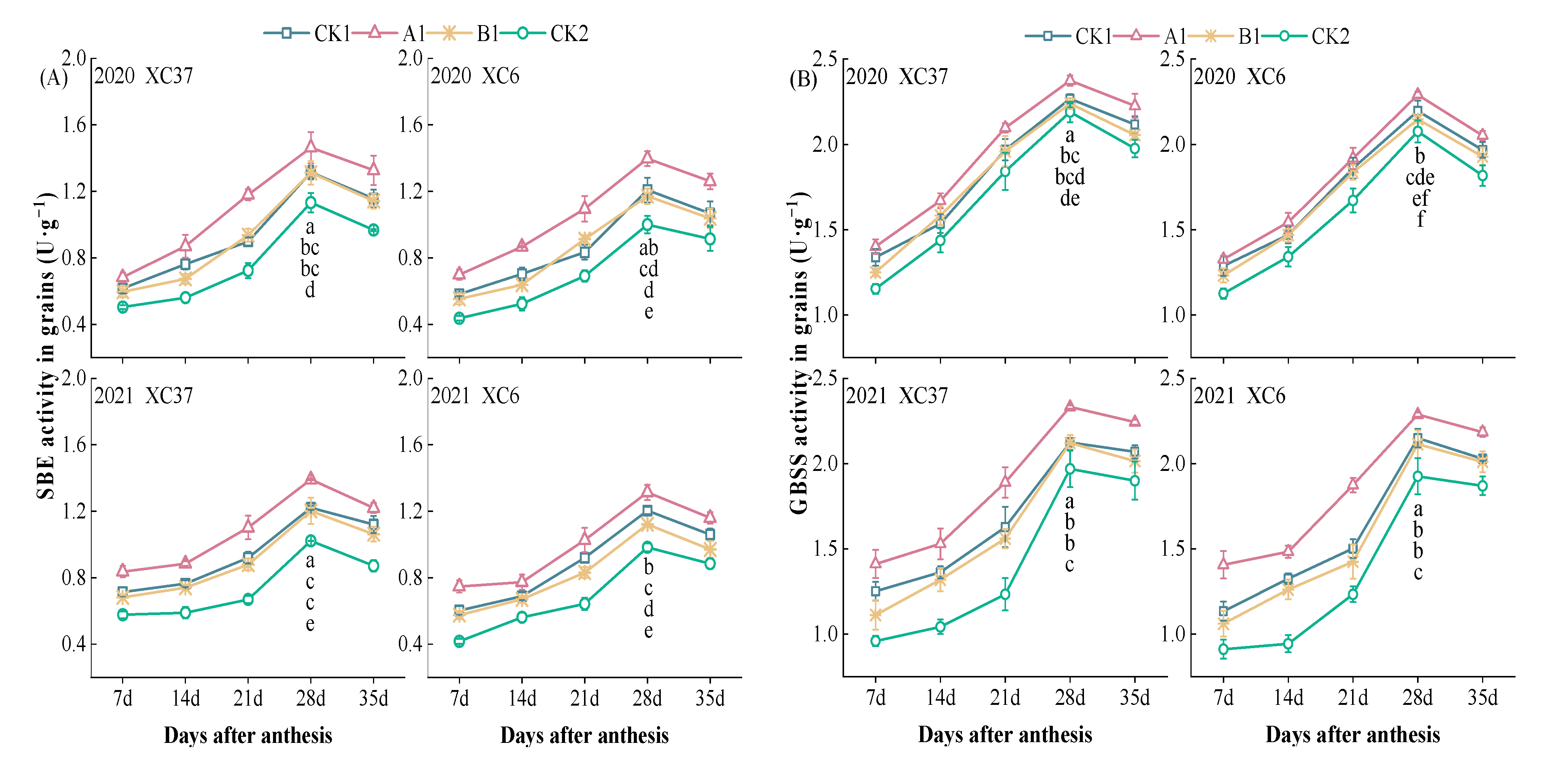
3.2.3. Changes in Key Enzyme Activity of Grain Starch
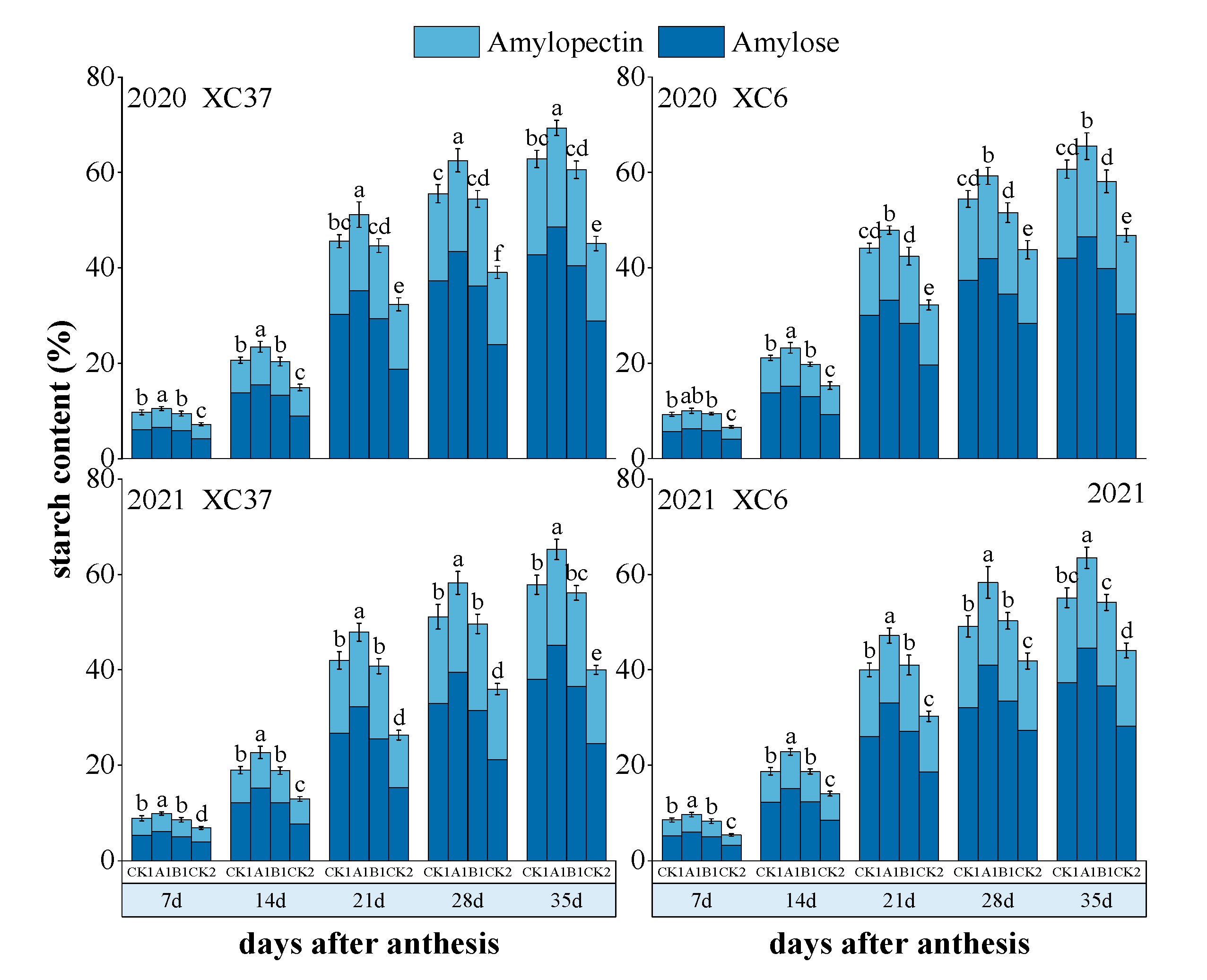
3.3. Effect of Root-Layer Nitrogen Reduction on Starch Composition and Content in Grains
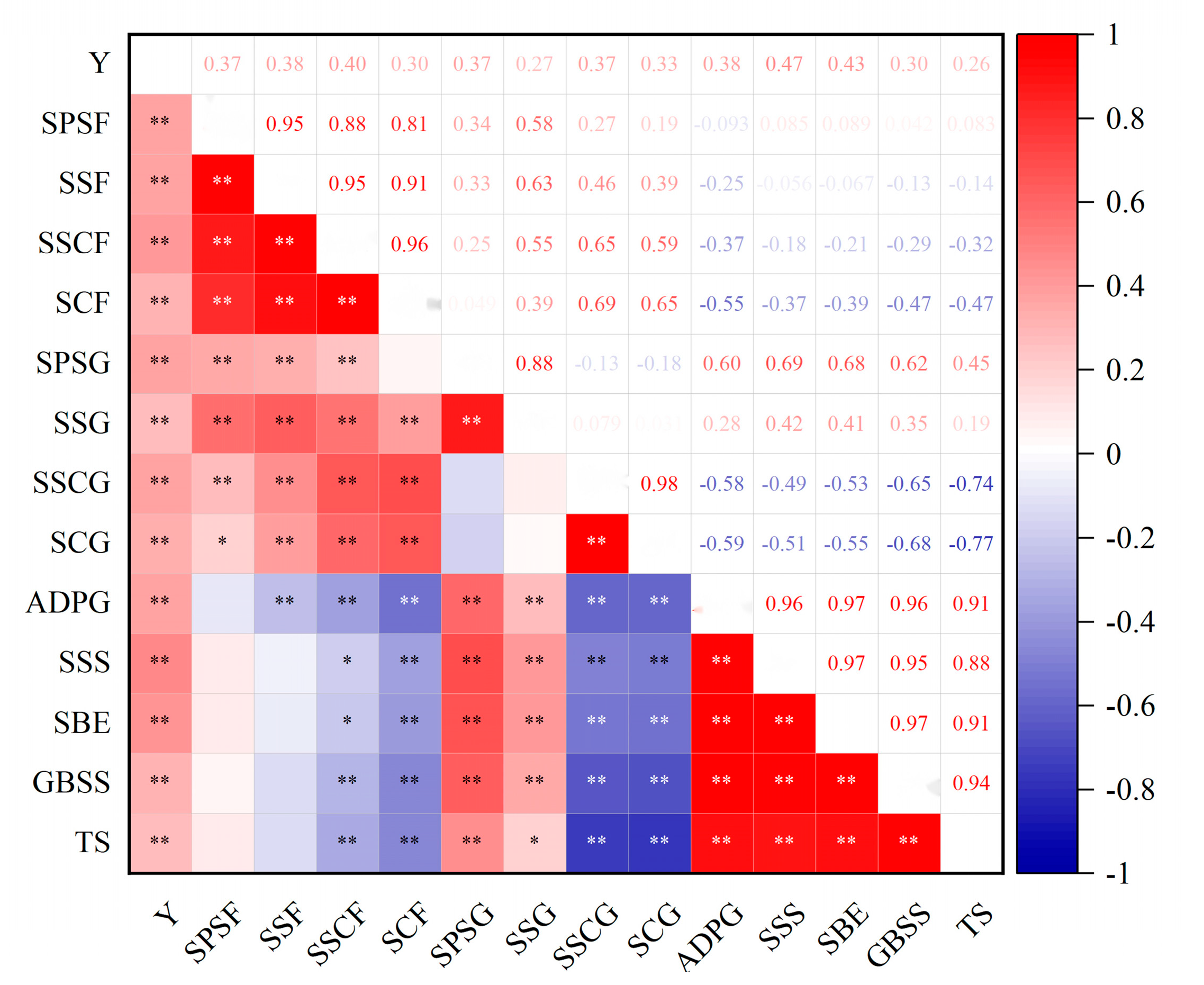
3.4. Correlation Analysis between Yield and Carbon Metabolism Parameters of Flag Leaves and Grains under Root-Layer Nitrogen Reduction
4. Discussion
4.1. Effect of Nitrogen Application Rate on Sucrose Metabolism in Flag Leaves and Grains of Spring Wheat
4.2. Effect of Nitrogen Application Rate on Starch Metabolism and Synthesis in Spring Wheat Grains
4.3. Effect of Nitrogen Application Rate on Spring Wheat Yield and Its Composition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Sun, J.; Zhang, Z.; Xu, P.; Shangguan, Z. Winter wheat grain yield in response to different production practices and soil fertility in northern China. Soil Tillage Res. 2018, 176, 10–17. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Zhao, X.; Wang, Z. Growth, yield, and nitrogen use in the wheat/maize intercropping system in an arid region of northwestern China. Field Crops Res. 2014, 167, 19–30. [Google Scholar] [CrossRef]
- Shibata, H.; Galloway, J.N.; Leach, A.M.; Cattaneo, L.R.; Cattell Noll, L.; Erisman, J.W.; Gu, B.J.; Liang, X.; Hayashi, K.; Ma, L.; et al. Nitrogen footprints: Regional realities and options to reduce nitrogen loss to the environment. Ambio 2017, 46, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, Z.J.; Wang, J.C.; Pan, X.J.; Zhang, D.; Xu, Y.L. Effect of Water and Nitrogen Coupling on N Absorption, Translocation and Yield of Winter Wheat under Drip Irrigation. Xinjiang Agric. Sci. 2018, 55, 44–56. [Google Scholar] [CrossRef]
- Mahdy, R.E.; Alghamdi, S.A.; Amro, A.; Tammam, S.A. Changes in Carbon and Nitrogen Metabolites before, at, and after Anthesis for Wheat Cultivars in Response to Reduced Soil Water and Zinc Foliar Application. Plants 2022, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Bhardwaj, R.D.; Singla, P.; Kaur, S.; Kaur Grewal, S. Drought Stress-Induced Alterations in Source–Sink Relationships in Barley During Grain Development. Gesunde Pflanz. 2023, 75, 2401–2412. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, Z.; Zhang, Y.; Ma, L.; Qin, B.; Liu, Q.; Zhang, W.; Ma, S.; Ma, C.; Huang, Z. Pre-anthesis night warming improves post-anthesis physiological activity and plant productivity to post-anthesis heat stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2022, 197, 104819. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, Z.Z.; Gu, F.X.; Mou, H.M.; Wang, Y.; Duan, J.Z.; Feng, W.; Wang, Y.; Guo, T. Effects of nitrogen fertilizer and plant density on carbon metabolism, nitrogen metabolism and grain yield of two winter wheat varieties. Sci. Agric. Sin. 2021, 54, 4070–4083. [Google Scholar] [CrossRef]
- Jiang, D.; Fan, X.; Dai, T.; Cao, W. Nitrogen fertiliser rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant Soil 2008, 304, 301–314. [Google Scholar] [CrossRef]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.G.; Qi, J.C.; Hui, H.S.; Lin, L.H.; Wang, F. Starch accumulation in hulless barley during grain filling. Bot. Stud. 2017, 58, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dai, T.; Jiang, D.; Cao, W. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. J. Agron. Crop Sci. 2008, 194, 47–54. [Google Scholar] [CrossRef]
- Yang, G.D.; Hu, Z.Y.; Huang, R.D.; Hao, Z.Y.; Li, J.H.; Wang, Q.; Ming, X.; Zhou, Y.F. Effect of nitrogen on the starch formation and yield of high-density sorghum [Sorghum bicolor (L.) Moench] in northern China. Appl. Ecol. Environ. Res. 2020, 18, 5727–5741. [Google Scholar] [CrossRef]
- Xin, L.; Fu, Y.; Ma, S.L.C.; Wang, H.; Gao, Y.; Wang, X. Effects of Post-Anthesis Irrigation on the Activity of Starch Synthesis-Related Enzymes and Wheat Grain Quality under Different Nitrogen Conditions. Plants 2023, 12, 4086. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Liu, Y.; Xie, J.; Anwar, S.; Fudjoe, S.K. Nitrogen supply affects yield and grain filling of maize by regulating starch metabolizing enzyme activities and endogenous hormone contents. Front. Plant Sci. 2022, 12, 798119. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Wan, C.; Wang, P.; Eeckhout, M.; Gao, J. Suitable nitrogen fertilizer application drives the endosperm development and starch synthesis to improve the physicochemical properties of common buckwheat grain. Int. J. Biol. Macromol. 2023, 235, 123837. [Google Scholar] [CrossRef]
- Ran, L.; Yu, X.; Li, Y.; Zou, J.; Deng, J.; Pan, J.; Xiong, F. Analysis of development, accumulation and structural characteristics of starch granule in wheat grain under nitrogen application. Int. J. Biol. Macromol. 2020, 164, 3739–3750. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Li, Z.K.; Li, E.P.; Wang, W.L.; Yuan, L.M.; Zhang, H.; Liu, L.J.; Wang, Z.Q.; Gu, J.F.; Yang, J.C. Optimization of nitrogen fertilization improves rice quality by affecting the structure and physicochemical properties of starch at high yield levels. J. Integr. Agric. 2022, 21, 1576–1592. [Google Scholar] [CrossRef]
- Wang, X.Y.; He, M.R.; Li, F.; Liu, Y.H.; Zhang, H.H.; Liu, C.G. Coupling effects of irrigation and nitrogen fertilizer on grain protein and starch quality of strong-gluten winter wheat. J. Plant Nutr. Fertil. 2007, 13, 361–367. [Google Scholar] [CrossRef]
- Khan, G.R.; Akma, M. Nitrogen application rate and timing management for improved grain quality parameters of wheat crop. Pak. J. Agric. Sci. 2021, 58, 1141–1153. [Google Scholar] [CrossRef]
- Jing, J.; Li, Z.; Qian, F.; Chang, X.; Li, W. Effects of Different Drip Irrigation Patterns on Grain Yield and Population Structure of Different Water-and Fertilizer-Demanding Wheat (Triticum aestivum L.) Varieties. Agronomy 2023, 13, 3018. [Google Scholar] [CrossRef]
- Douglas, C.D.; Tsung, M.K.; Frederick, C.F. 1988. Enzymes of sucrose and hexose metabolism indevelopment kernels of two inbreds of maize. Plant Physiol. 1988, 86, 1013–1019. [Google Scholar] [CrossRef]
- Keller, F.; Ludlow, M.M. Carbohydrate metabolism in drought-stressed leaves of pigeonpea (Cajanus cajan). J. Exp. Bot. 1993, 44, 1351–1359. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.Y.; Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Vila, F.; Sanz, A. A proposal for evaluating laboratory instruction in a plant physiology course. Theor. Exp. Plant Physiol. 2018, 30, 4002–4008. [Google Scholar] [CrossRef]
- Jin, Y.H.; Zhang, K.L.; Zhang, X.C.; Du, J.H. Determination of straight chain and branched chain starch content in wheat and wheat sprouts using dual wavelength method. J. Chin. Cereals Oils Assoc. 2009, 24, 137–140. (In Chinese) [Google Scholar]
- Verma, A.K.; Upadhyay, S.K.; Verma, P.C.; Solomon, S.; Singh, S.B. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. 2011, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Zhao, C.; Zhang, M.; Sun, C.; Liu, X.; Hu, J.; Zeeshan, M.; Zaid, A.; Dai, T.; Tian, Z. Nitrogen enhances the effect of pre-drought priming against post-anthesis drought stress by regulating starch and protein formation in wheat. Physiol. Plant. 2023, 175, e13907. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Hu, Y.X.; Tung, S.A.; Yang, L.; Wang, Y.; Zhou, X.B. Evaluating the Effects of Water-Nitrogen Interactions on Carbon and Nitrogen Accumulation as Well as Related Metabolic Enzymes Activity in Autumn Maize. J. Soil Sci. Plant Nutr. 2023, 23, 5245–5256. [Google Scholar] [CrossRef]
- Yu, H.R.; Guo, Y.; Zhu, A.M.; Lu, F.Y.; Wang, L.; Zhang, Y.X. Effects of nitrogen fertilizer level on non-structural carbon and nitrogen metabolite levels in oats grown in sandy desert soil. Acta Prataculturae Sin. 2018, 27, 61. [Google Scholar] [CrossRef]
- Jiang, D.; Cao, W.; Dai, T.; Qi, J. Activities of key enzymes for starch synthesis in relation to growth of superior and inferior grains on winter wheat (Triticum aestivum L.) spike. Plant Growth Regul. 2003, 41, 247–257. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Cheng, F.; Liu, Y.; Zhang, G. The differences in grain weight and quality within a rice (Oryza sativa L.) panicle as affected by panicle type and source-sink relation. J. Agron. Crop Sci. 2007, 193, 63–73. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Lyu, D.; Su, F.; Liu, S.; Li, H.; Wang, X.; Liu, Z.; Hu, L. Effects of different nitrogen application rates on starch accumulation, starch synthase gene expression and enzyme activity in two distinctive potato cultivars. Potato Res. 2018, 61, 309–326. [Google Scholar] [CrossRef]
- Li, G.; Hu, Q.; Shi, Y.; Cui, K.; Nie, L.; Huang, J.; Peng, S. Low nitrogen application enhances starch-metabolizing enzyme activity and improves accumulation and translocation of non-structural carbohydrates in rice stems. Front. Plant Sci. 2018, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- De Lucena Marinho, J.; Silva, S.R.; de Batista Fonseca, I.C.; Zucareli, C. Nitrogen use efficiency and yield of wheat genotypes affected by nitrogen fertilizing and environmental conditions in Southern Brazil. Int. J. Plant Prod. 2022, 16, 495–510. [Google Scholar] [CrossRef]
- Yuan, S.; Ling, Y.; Xiong, Y.; Zhang, C.G.; Sha, L.N.; You, M.H.; Lei, X.; Bai, S.Q.; Ma, X. Effect of nitrogen fertilizer on seed yield and quality of Kengyilia melanthera (Triticeae, Poaceae). PeerJ 2022, 10, e14101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Shen, C.C.; Xu, Q.X.; Zafar, S.; Du, B.; Dang, D.Y. Grain yield, nitrogen use efficiency and antioxidant enzymes of rice under different fertilizer n inputs and planting density. Agronomy 2022, 12, 430. [Google Scholar] [CrossRef]
- Si, Z.; Zain, M.; Mehmood, F.; Wang, G.; Gao, Y.; Duan, A. Effects of nitrogen application rate and irrigation regime on growth, yield, and water-nitrogen use efficiency of drip-irrigated winter wheat in the North China Plain. Agric. Water Manag. 2020, 231, 106002. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Pan, M.; Wang, H.; Sun, J.; Chen, Y.; Cai, J.; Zhou, Q.; Wang, X.; Jiang, D. Insights into the Functional Components in Wheat Grain: Spatial Pattern, Underlying Mechanism and Cultivation Regulation. Plants 2023, 12, 2192. [Google Scholar] [CrossRef] [PubMed]



| Year | Total Nitrogen Content/ (g·kg−1) | Availine Hydrolysis Nitrogen Content/ (mg·kg−1) | Available Phosphorus Content/ (mg·kg−1) | Available Potassium Content/ (mg·kg−1) | Organic Matter Content/ (g·kg−1) | pH |
|---|---|---|---|---|---|---|
| 2020 | 1.20 | 58.30 | 14.24 | 137.01 | 13.43 | 7.8 |
| 2021 | 1.27 | 55.71 | 15.96 | 132.02 | 12.84 | 7.7 |
| Treatment | Nitrogen Application Amount | Base Fertilizer (20%) | Top Dressing | |||||
|---|---|---|---|---|---|---|---|---|
| Two-Leaf One-Hearted Stage (8%) | Tillering Stage (8%) | Jointing Stage (32%) | Booting Stage (16%) | Flowering Stage (12%) | Milky Maturity Stage (4%) | |||
| CK1 | 300 | 60 | 24.0 | 24.0 | 96.0 | 48.0 | 36.0 | 12.0 |
| A1 | 255 | 51 | 20.4 | 20.4 | 81.6 | 40.8 | 30.6 | 10.2 |
| B1 | 210 | 42 | 16.8 | 16.8 | 67.2 | 33.6 | 25.2 | 8.4 |
| CK2 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Variety (V) | Treatment (N) | 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1000-Grain Weight/(g) | Spike Number/(×104·ha−2) | Grains per Spike | Grain Yield /(kg·ha−1) | 1000-Grain Weight·(g) | Spike Number/ (×104·ha−2) | Grains per Spike | Grain Yield /(kg·ha−1) | ||
| XC37 | CK1 | 46.83 a | 415.16 bc | 36.08 b | 6932.75 c | 45.67 abcd | 415.64 b | 36.63 ab | 6895.81 c |
| A1 | 47.83 a | 431.61 a | 38.78 a | 7257.5 a | 47.09 a | 435.75 a | 37.39 ab | 7170.56 a | |
| B1 | 45.34 bc | 413.27 bc | 37.76 ab | 6818.6 d | 46.62 ab | 410.95 bc | 38.03 a | 6782.11 d | |
| CK2 | 43.08 d | 389.08 d | 33.38 c | 5444.33 f | 44.67 cd | 393.95 cd | 34.49 c | 5664.87 f | |
| XC 6 | CK1 | 46.46 ab | 413.08 bc | 36.04 b | 6881.06 c | 45.14 bcd | 410.27 bc | 36.34 b | 6648.67 e |
| A1 | 47.08 a | 423.76 ab | 36.61 b | 7091.44 b | 46.84 a | 427.62 ab | 37.04 ab | 7053.33 b | |
| B1 | 44.97 c | 408.77 c | 37.42 ab | 6736.09 e | 46.21 abc | 407.15 bc | 37.69 ab | 6697.73 de | |
| CK2 | 42.76 d | 384.07 d | 32.91 c | 5364.23 g | 44.24 d | 382.15 d | 34.07 c | 5383.47 g | |
| F | V | ns | * | ns | ** | ns | ** | ns | ** |
| N | ** | ** | ** | ** | ** | ** | ** | ** | |
| N × V | * | * | * | * | * | * | * | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, H.; Liu, J.; Wang, R.; Che, Z. Effects of Root Trace Nitrogen Reduction in Arid Areas on Sucrose–Starch Metabolism of Flag Leaves and Grains and Yield of Drip-Irrigated Spring Wheat. Agronomy 2024, 14, 312. https://doi.org/10.3390/agronomy14020312
Ma Y, Wang H, Liu J, Wang R, Che Z. Effects of Root Trace Nitrogen Reduction in Arid Areas on Sucrose–Starch Metabolism of Flag Leaves and Grains and Yield of Drip-Irrigated Spring Wheat. Agronomy. 2024; 14(2):312. https://doi.org/10.3390/agronomy14020312
Chicago/Turabian StyleMa, Yilin, Haiqi Wang, Jianguo Liu, Rongrong Wang, and Ziqiang Che. 2024. "Effects of Root Trace Nitrogen Reduction in Arid Areas on Sucrose–Starch Metabolism of Flag Leaves and Grains and Yield of Drip-Irrigated Spring Wheat" Agronomy 14, no. 2: 312. https://doi.org/10.3390/agronomy14020312




