Abstract
Climate change has emerged as a challenge for soybean cultivation around the world, stimulating the development of technological alternatives that aim to mitigate the damage caused by water deficit. From this perspective, algae extract-based biostimulants have been tested to reduce water stress in several crops, but little is known about their effects on soybean. Thus, we hypothesize that a commercial biostimulant based on Ascophyllum nodosum can improve the physiological performance and water relations of Glycine max plants subjected to water deficit. To test this hypothesis, we set up an experiment in controlled conditions in a greenhouse, considering five treatments (control; application of biostimulant; water deficit (WD); WD + application of biostimulant; and WD + split application of biostimulant). The experiment was designed in completely randomized blocks with four replications per treatment and conducted in polyethylene pots containing 10 L of soil and three plants per pot. The irrigation was carried out daily; the water deficit was 50% soil moisture at field capacity, starting at the R1 stage (beginning of flowering, where there is at least one flower open at any node on the plant) and maintained for ten days. The biostimulant was applied concurrently with the onset of water deficit. We confirmed the hypothesis that foliar application of 1.0 L ha−1 of the biostimulant reduces the deleterious effects of the common water deficit at the beginning of the reproductive stage of soybean through the reduction of damage from oxidative stress (reduction of malondialdehyde synthesis by 31.2% in relation to the WD plants), maintenance of water potential and cellular homeostasis (10.2% increase in relative water content when compared with WD plants), and conservation of the contents of chlorophyll in leaves and stimulation of photosynthesis and carboxylation (68% increase in net photosynthetic rate and 49.3% increase in carboxylation efficiency in relation to WD plants). However, when applied in installments, the biostimulant was not efficient in reducing soybean water stress. Therefore, we conclude that the application of a biostimulant based on A. nodosum can help reduce the harmful effects of water deficit on soybean plants, opening up perspectives for the mass use of this extract in agricultural crops produced on a large scale.
1. Introduction
Drought is still the most limiting factor to crop development. Water limitation causes several morphophysiological disorders in plants [1]. Stomatal closure caused by water deficit results in reduced carbon uptake and fixation through photosynthesis, as well as increased production of reactive oxygen species [2]. In addition, the low availability of CO2 in cells results in increased photorespiration in C3 plants, such as soybean, which results in the loss of carbon available for photosynthesis [3,4].
As a result of water deficit, there is occurrence of chlorosis, necrosis and wilting of leaves, reduction of leaf area and size of plants, and abortion of leaves, flowers, and pods, with a consequent reduction in grain filling and productivity [5,6]. The water demand for soybean crops is in the 450 to 800 mm range throughout the cycle, with a greater need during the grain filling phase [7]. Thus, the occurrence of off-season summer periods during soybean reproductive stages has been the main cause of reduced crop yields in Brazil [8].
In order to reduce damage to crops exposed to environmental stress, new technologies have emerged with the potential to mitigate this problem, such as bioinoculants and biostimulants. Bioinoculants such as plant growth-promoting rhizobacteria (PGPRs) have demonstrated efficiency in mitigating biotic and abiotic stress on commercial plants [9,10,11]. Physicochemical modifications induced by rhizobacteria culminate in resistance and resilience to drought. These modifications include changes in phytohormone levels, metabolic adjustments, production of bacterial exopolysaccharides (EPS), biofilm formation and antioxidant resistance, and accumulation of many suitable organic solutes such as carbohydrates, amino acids, and polyamines [12]. Biostimulants fulfill the same purpose, and they are defined as a mixture of two or more plant growth regulators with other substances (such as amino acids and nutrients) [13,14]. This mixture is capable of altering the physiological processes of the plant, favoring the acquisition of nutrients [15] and vegetative and reproductive development [16,17], in addition to reducing the effects of abiotic stresses, as in the case of products based on extracts of the seaweed Ascophyllum nodosum [18,19,20,21].
This species, found in the waters of the North Atlantic under extreme conditions of temperature and agitation of the tides [22], manages to develop mechanisms for survival, accumulating a large quantity and variety of organic compounds such as amino acids, phytohormones, polyphenols, betaines, polysaccharides, fatty acids, steroids, and polyamines, in addition to macro- and micronutrients [23].
This content of organic compounds (not yet fully elucidated), when absorbed by plants, results in greater efficiency in controlling stomatal opening, greater CO2 conductance, greater stimulus of the production of antioxidant enzymes, and greater protection of photosystems [24,25], culminating in an increase in the efficiency of water use and a consequent reduction in water stress [26,27,28].
A. nodosum algae extract-based biostimulants have been widely used to reduce abiotic stresses in crops, especially for water stress [29,30,31,32]. However, little is known about the results of the use of these products on the soybean crop. As the productivity of this crop is highly sensitive to climate change [33,34], studies that test the use of biostimulants as a way of minimizing the impacts of water deficit in this crop should be encouraged. Soy is the main source of vegetable protein in the world, and studies have highlighted that climatic conditions, combined with genetic and management factors involving the use of inoculants, PGPRs, and biostimulants can define production capacity [35]. Here, we tested the foliar use of the commercial biostimulant Megafol®, which has already demonstrated growth-promoting effects and increased productivity in several crops, including soybean [36]. This product has also demonstrated potential for reducing damage caused by water deficit, improving fruit production and water use efficiency in stressed tomato plants [37].
Thus, we hypothesize that a biostimulant based on A. nodosum can improve the physiological performance and water relations of Glycine max plants subjected to water deficit. To test our hypothesis, we evaluated gas exchange, chlorophyll-a fluorescence, chlorophyll synthesis, and water potential, as well as the production of proline and malondialdehyde (MDA) in stressed plants treated with biostimulants. Our research aims to understand the effects of applying A. nodosum to soybean grown under water deficit, highlighting physiological and biochemical changes. This study contributes to the understanding of the mechanisms that involve the promotion of growth mediated by algal extracts, contributing to the large-scale use of these products.
2. Materials and Methods
2.1. Experimental Conditions
The experiment was carried out in controlled conditions in a greenhouse (with an average temperature of 27 °C and relative humidity of 65%) from November 2019 to February 2020. Soybean plants of the cultivar Foco 74I77RSF IPRO (maturation group 7.3 and indeterminate growth habit) were used.
A randomized block design consisting of five treatments was used (1—control; 2—application of biostimulant; 3—water deficit (WD); 4—water deficit + application of biostimulant (WD + biostimulant); and 5—water deficit + split application of biostimulant (WD + split biostimulant)) with four replications, resulting in 20 experimental plots. The purpose of the split application was to test two moments of stimulation to reduce the effects of water deficit. The first dose was intended for plant protection and the second for recovery from possible damage caused by stress.
Plots consisted of polyethylene pots with a capacity of 10 L, which were evenly and equidistantly distributed on benches. A mixture of soil, collected in a layer of 0 to 20 cm of a dystroferric red oxisol, and sand, in a 2:1 ratio, was used as the substrate for plant cultivation. The soil had the following chemical and physical properties: pH (CaCl2) 4.6; O.M. (g dm−3) 12.6; P (mg dm−3) 13.7; K (mg dm−3) 107.4; S (mg dm−3) 18.9; Ca (cmolc dm−3) 1.2; Mg (cmolc dm−3) 1.0; e H + Al (cmolc dm−3) 3.5; clay (%) 54, silt (%) 7, sand (%) 39. The substrate was previously subjected to liming (4.5 g pot−1) and plastering, and at the time of planting, it was fertilized as recommended for the crop [38], consisting of: S (2.92 g pot−1); KCl (2.92 g pot−1), MAP (2.92 g pot−1), and MgSO₄ (1.02 g pot−1). For each pot, five seeds were sown 2 cm deep, and only three plants remained at 7 days after emergence (DAE).
Irrigation was performed daily, with humidity control maintained by weighing the pots. The volume of available water was estimated taking into account the soil density and 90% moisture at field capacity. Water deficit (DH) started at the R1 stage (beginning of flowering) and was maintained for ten days. To determine the field capacity, the soil in the pots was initially dried in an oven to completely remove moisture and weighed to determine mass. The pots were then watered to fill 100% of the porosity and covered with a plastic bag to prevent water loss through evaporation. Subsequently, the macropores drained, as observed through water dripping at the bottom of the vessel. When the water stopped dripping, indicating the end of drainage, the pots were weighed. The difference between the mass of the wet substrate after drainage and the dry substrate is equivalent to the moisture at field capacity. Once this humidity was known, a value corresponding to 50% of the humidity was determined to establish water stress. In order to induce stress, a reduction in pot irrigation was carried out, maintaining water availability in the soil at 50% moisture at field capacity.
Megafol® biostimulant (marketed by Syngenta Biologicals) was used. This is a product derived from natural compounds and containing raw materials: urea, potassium acetate, vinasse, algae extract, and water. It consists of 9.0% Corg, 8.0% K2O, 3.0% N, and 78.9% inert ingredients [39].
The biostimulant was applied concurrently with the onset of water deficit using a CO2 pressurized backpack sprayer equipped with a spray bar with four spray nozzles, TT 110-02 with double fan, and a spray volume equivalent to 150 L ha−1 (equivalent to 60.7 L acre−1, 0.004 mL pot−1) when regulated at 2.5 kgf cm−2. The biostimulant was applied equivalent to 1.0 L ha−1. In the treatment in which the dose was split (0.5 L ha−1 + 0.5 L ha−1), the first application was carried out concomitantly with the onset of water deficit, and the second application was carried out five days after the first.
At the end of the ten days of water limitation, evaluations of gas exchange were conducted for the plants, as well as collection of material for evaluations of water relations and metabolism.
2.2. Assessment of Gas Exchange and Chlorophyll-a Fluorescence
Variables related to gas exchange and chlorophyll-a fluorescence were obtained by analyzing the central leaflet of the youngest fully expanded leaf. Evaluations were conducted between 8 a.m. and 11 a.m., without the presence of cloudiness, using an infrared gas analyzer (IRGA, LI-COR®—model LI-6800) with constant photosynthetically active radiation (1500 µmol m−2 s−1) and controlled CO2 concentration (400 ppm), constant temperature (25 °C), and constant relative humidity (50%). Gas exchanges were evaluated to record the net photosynthetic rate (A), transpiration rate (E), stomatal conductance of water vapor (Gs), and ratio of internal to external concentration of CO2 (Ci/Ca). The instantaneous efficiency of water use was calculated using the ratio of photosynthetic to transpiration rates (A/E). The carboxylation efficiency was calculated using the ratio of the photosynthetic rate to the internal carbon concentration in the leaf (A/Ci).
With the aid of a fluorometer coupled to the IRGA, measurements of chlorophyll-a fluorescence were obtained in the same area of the leaf where the gas exchange measurements were taken. The apparent electron transport rate (ETR) [40] and the effective quantum yield of PSII (ϕPSII) [41] were determined.
2.3. Evaluation of Water Status of Plants
The water relations of the plants were determined through evaluation of leaf relative water content (RWC) using the equation proposed by Catsky [42]; leaf water potential (Ψw) using a Scholander pressure pump [43]; and leaf osmotic potential (Ψs) obtained using a vapor pressure osmometer and calculated using the Van’t Hoff equation [44]. The osmotic adjustment of cells was estimated by evaluating the contents of proline (Pro) [45].
2.4. Determination of Damage to Cell Membranes
Damage to cell membranes was measured directly and indirectly by assessing lipid peroxidation by determining the concentration of malondialdehyde (MDA) [46].
2.5. Determination of Chloroplast Pigment Concentration
The concentration of chloroplast pigments was determined in three leaf discs of 0.5 cm in diameter that were immersed in 5 mL dimethyl sulfoxide (DMSO) and saturated with calcium carbonate (CaCO3) in flasks protected from light. After 6 h incubation of the samples at 60 °C, readings of the absorbance of the extract were taken at wavelengths of 665, 649, and 480 nm. Chlorophyll-a (Cla) content, chlorophyll-b (Clb) content, and total chlorophyll content (ClT) were determined. Concentrations of pigments were calculated according to the equations proposed by Wellburn [47], and the results are expressed in µg cm−2.
2.6. Statistical Analysis
Data were tested by analysis of variance using the F-test at 5% probability. Once significance was found, a Tukey’s test was applied to compare the treatment means. Subsequently, all variables that showed significant differences were jointly evaluated in a correlation matrix and associated using principal component analysis (PCA). Because these variables had different units of measurement, correlation PCA was performed using standardized data to obtain a mean of 0 and a standard deviation of 1. The number of principal components was defined according to the eigenvalues (>1.0) and explained variance (>70%). Statistical analysis was performed using R version 4.0.4 [48].
A matrix of similarities was compiled to determine the similarities or differences among plants subjected to different treatments. The similarity index was obtained using the Pearson correlation coefficient, with r values transformed by d = (1 − r) × 100 to estimate the distance (d) values. A dendrogram was then generated using the Unweighted Pair Group Method with arithmetic averages (UPGMA), with adjustment between the distance matrix and the dendrogram estimated using a cophenetic correlation coefficient [49]. This analysis used the DendroUPGMA software version 1.0 [50].
3. Results
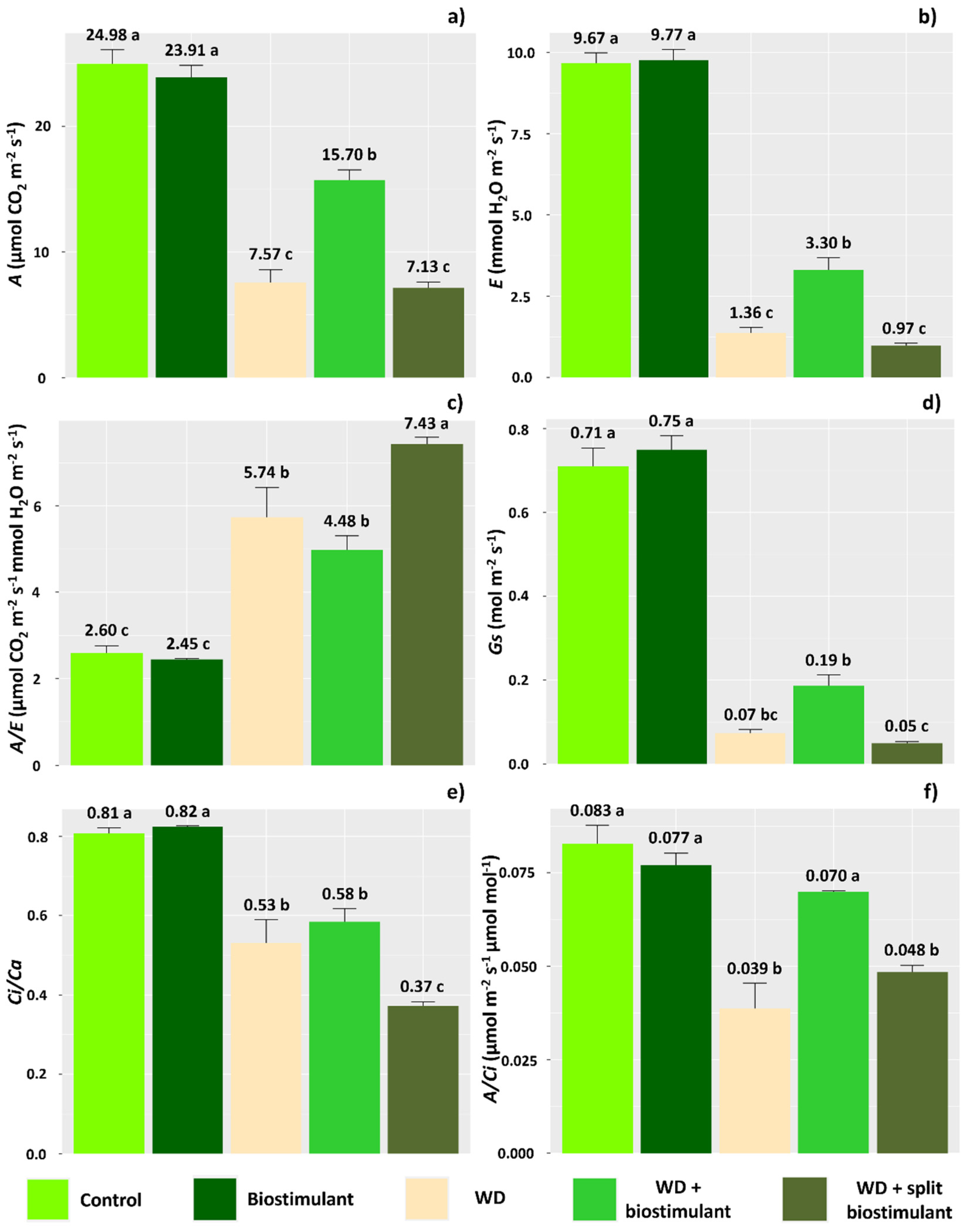
The imposition of water deficit resulted in changes in all the analyzed physiological parameters, causing reductions in most values (Figure 1). However, plants under WD treated with the biostimulant showed that this product can mitigate the effects of water stress on the photosynthetic rate (A) of soybean plants. Plants subjected to this treatment had a 107% increase in A compared with WD plants (Figure 1a). In these plants, the transpiration rate was maintained at higher levels than those observed in plants affected by WD (143% increase) (Figure 1b). The values verified for water use efficiency (A/E), however, did not differ between the WD plants and the WD + biostimulant plants (Figure 1c), but plants under the effect of water deficit were, on average, 132% more efficient in using water in relation to control treatment plants, since the reduction in transpiration provides a greater balance between water uptake and the amount of fixed CO2.
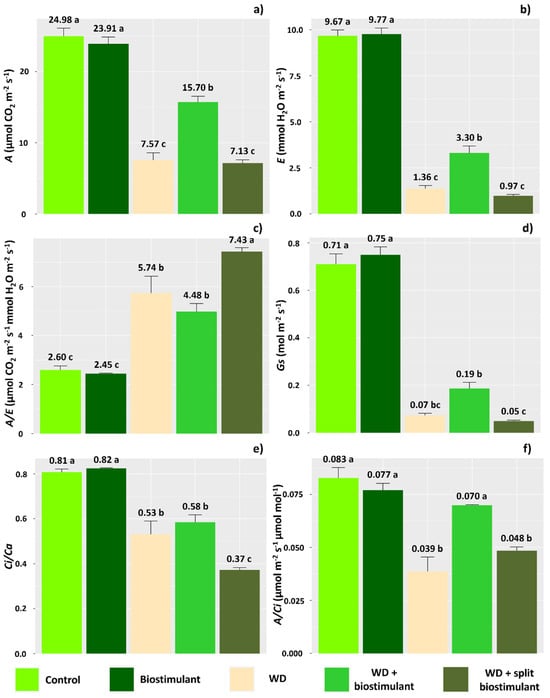
Figure 1.
Net photosynthetic rate (A) (a), transpiration rate (E) (b), water use efficiency (A/E) (c), stomatal conductance (Gs) (d), ratio of internal to external concentration of CO2 (Ci/Ca) (e), and carboxylation efficiency (A/Ci) (f) of soybean in the water stress reduction test through the application of algae extract-based biostimulant. Means followed by different letters are significantly different according to Tukey’s test at 5% probability, and error bars represent the standard error of the mean (SE).
The presence of biostimulant in plants subjected to WD guaranteed slightly higher stomatal conductance (Gs) than that observed in plants under WD (Figure 1d). The data obtained for the Ci/Ca ratio, however, were similar in plants under WD and WD + biostimulant plants (Figure 1e). The A/Ci ratio was improved in WD + biostimulant plants, compared with WD plants (increase of 79%), making the carboxylation efficiency in WD + biostimulant plants similar to that observed in plants from control treatments (Figure 1f). Additionally, the beneficial effect of 1.0 L ha−1 biostimulant on the efficiency of carboxylation in conditions of soybean water deficit is notable, given the absence of differences in relation to the control treatment when applied without the presence of water deficit. The photosynthetic parameters attested that the WD + split biostimulant treatment did not improve the photosynthetic rate, stomatal conductance, or carboxylation efficiency in soybean plants, with values similar to those observed in WD plants.
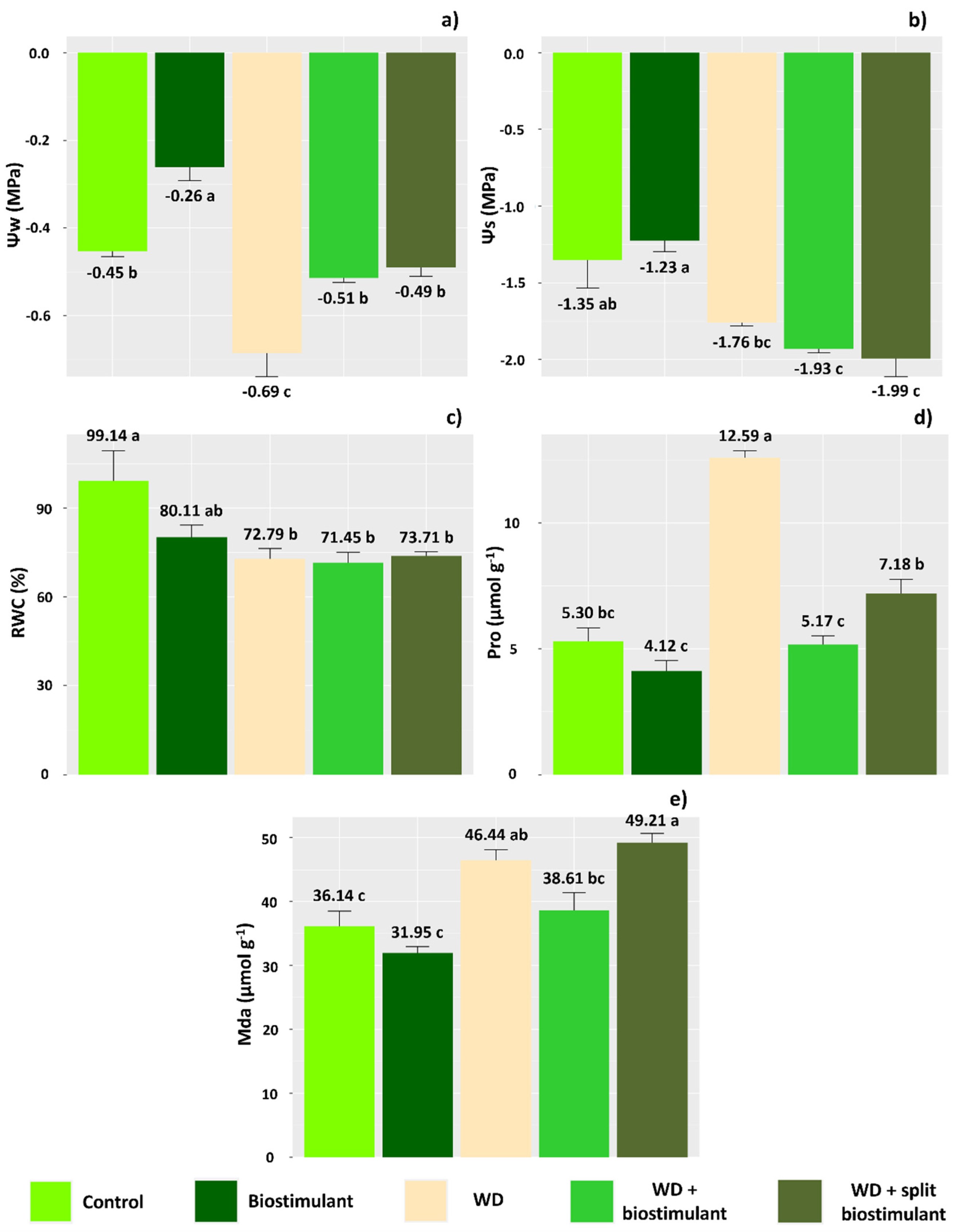
As for water relations, all parameters analyzed were affected by the treatments applied. In plants subjected to WD + biostimulant, there was an increase in water potential, which was similar to the control treatment (Figure 2a). These plants also had the lowest values of osmotic potential (Figure 2b). On the other hand, treatment without water deficit and application of 1.0 L ha−1 biostimulant resulted in the greatest water and osmotic potential. However, we did not observe an effect of biostimulant treatments on RWC (Figure 2c).
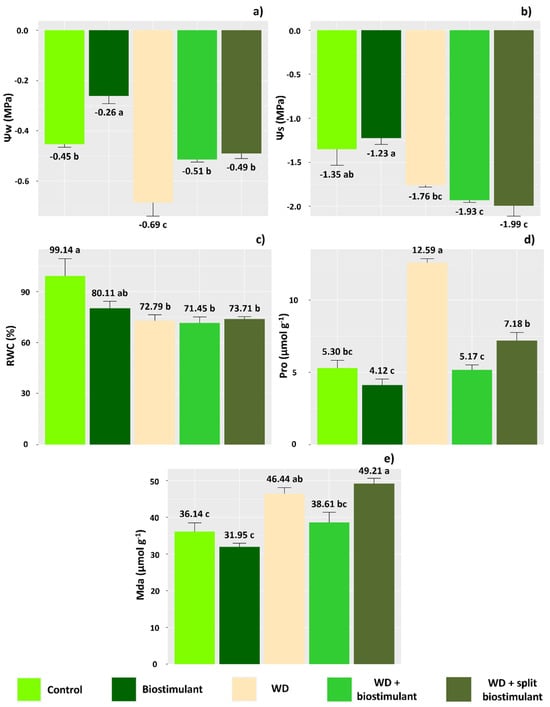
Figure 2.
Water potential (Ψw) (a), osmotic potential (Ψs) (b), relative water content (RWC) (c), proline content (Pro) (d), and malondialdehyde content (Mda) (e) of soybean in the water stress reduction test through the application of algae extract-based biostimulant. Means followed by different letters are significantly different according to Tukey’s test at 5% probability, and error bars represent the standard error of the mean (SE).
In plants under water deficit without application of the biostimulant, there was a significant increase in the content of proline (Figure 2d), which was not necessarily accompanied by a reduction in the osmotic potential (Figure 2b). On the other hand, proline concentration was similar between plants under water deficit that received application of the biostimulant and the control plants (Figure 2d). The MDA content of plants under water deficit and without application of biostimulant was higher than that in the control plants (Figure 2e), indicating an increase in lipid peroxidation due to the degradation of cell membranes because of oxidative stress. Otherwise, plants with WD + biostimulant showed similar MDA values to the control treatment, indicating greater protection of cells against the action of reactive oxygen species. The WD + split biostimulant treatment, however, caused MDA contents to increase in the plants, indicating damage to membranes. Thus, these results suggest that splitting the biostimulant dose in order to prevent water deficit in soybean is not beneficial.
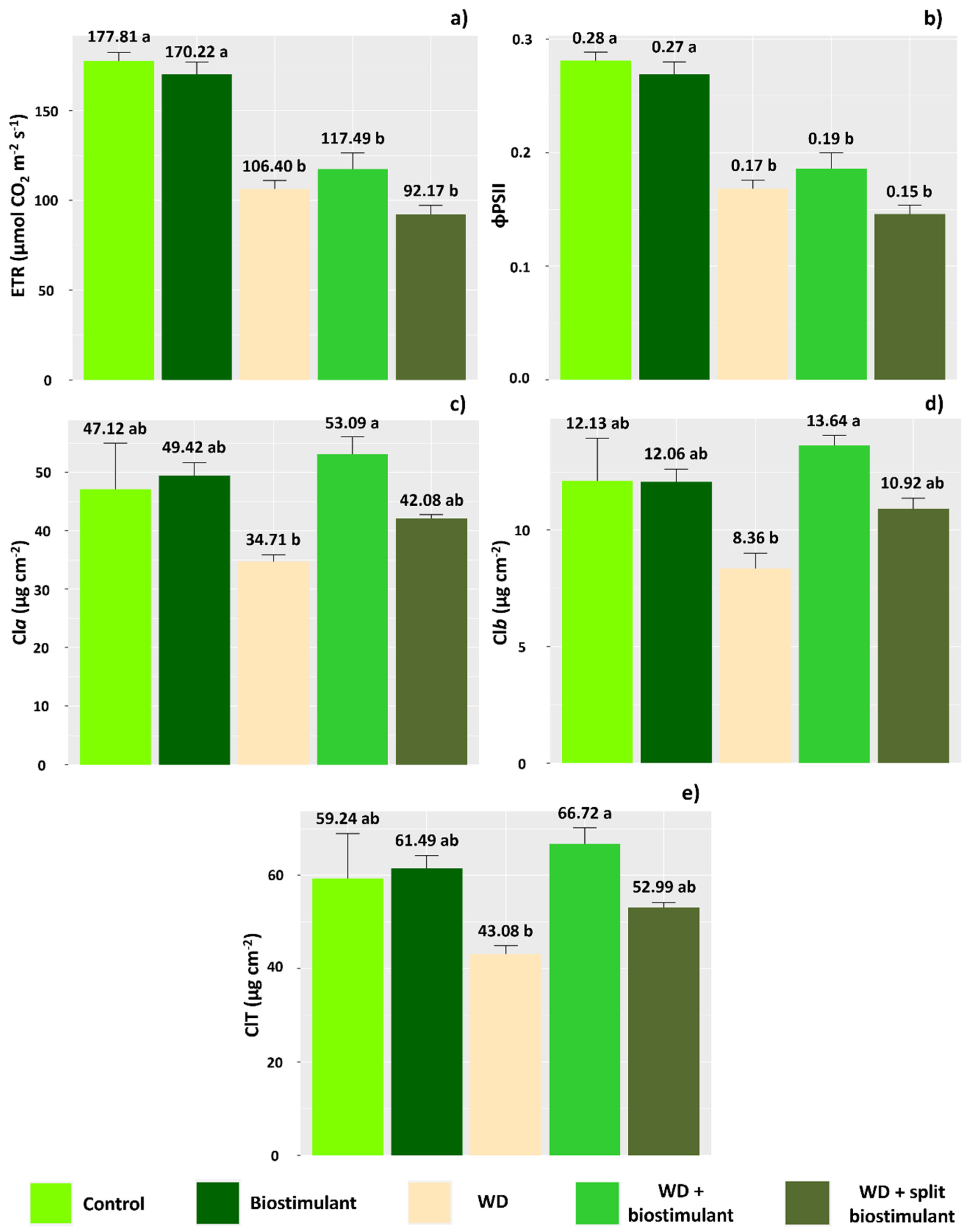
Biostimulant treatments did not improve ETR or ϕPSII in plants subjected to WD (Figure 3a,b,e), while lower levels of chlorophyll were observed in the WD without biostimulant treatment (Figure 3c, Figure 3d, and Figure 3e, respectively). On the other hand, in the WD + biostimulant treatment, chlorophyll values were similar to those of the control and higher than the WD treatment, indicating that the product stimulates an increase in the chlorophyll content of leaves.
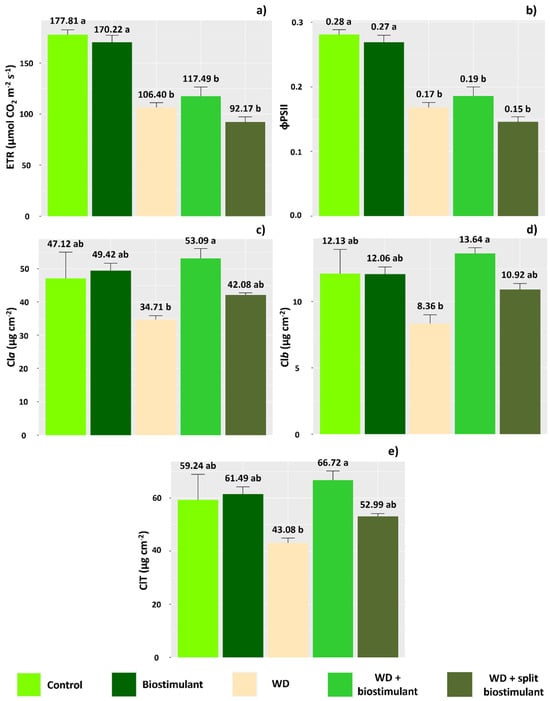
Figure 3.
Electron transport rate (ETR) (a), quantum efficiency of photosystem II (ϕPSII) (b), chlorophyll-a content (Cla) (c), chlorophyll-b content (Clb) (d), and total chlorophyll content (ClT) (e) of soybean in the water stress reduction tests that applied algae extract-based biostimulant. Means followed by different letters are significantly different according to Tukey’s test at 5% probability, and error bars represent the standard error of the mean (SE).
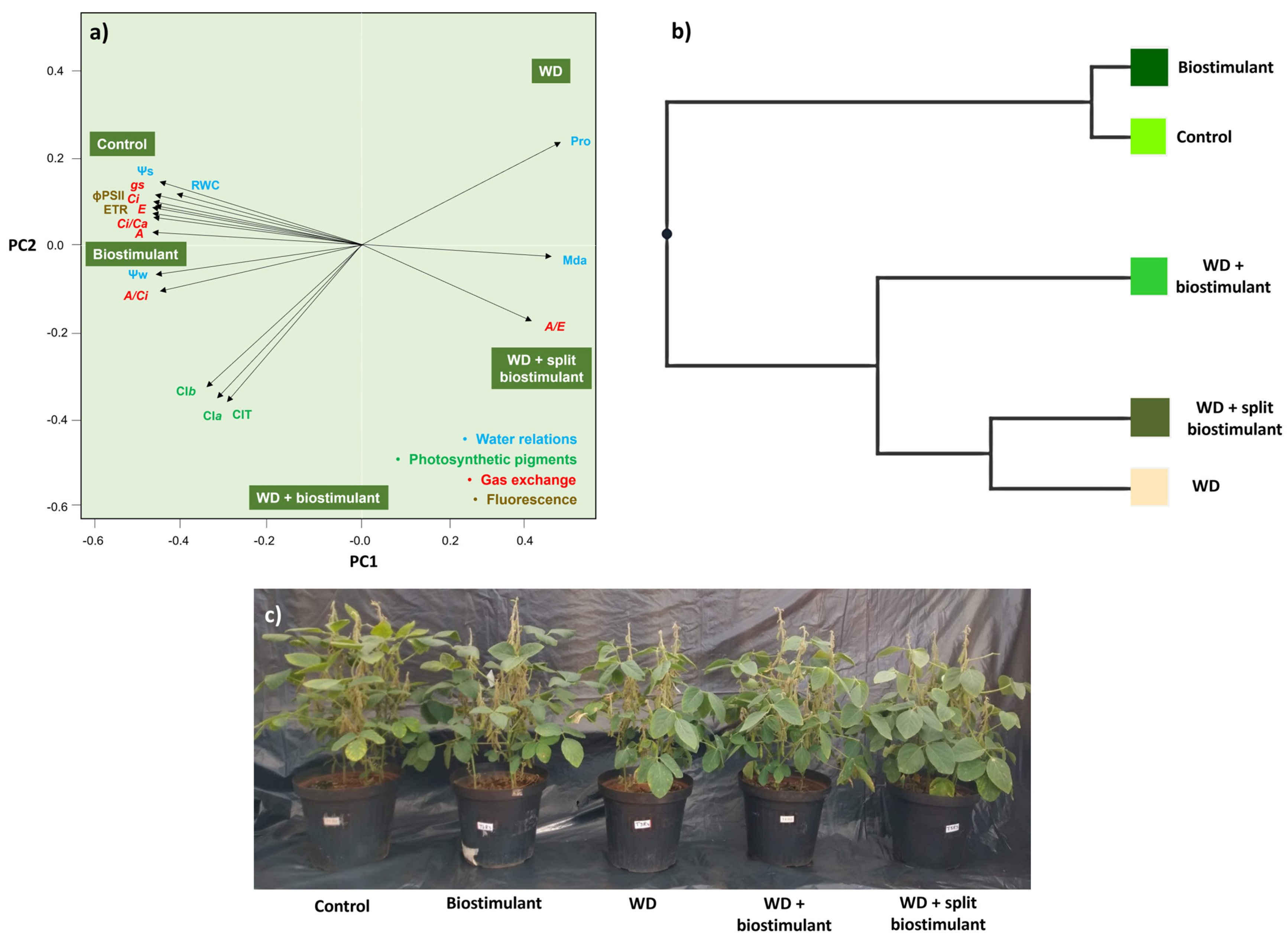
The relationship between the response variables and the treatments revealed that the plants in the control treatment and those in the biostimulant treatment without WD were similar and defined the variations observed in the gas exchange and fluorescence parameters (Gs, Ci, E, A, Ci/Ca, A/Ci, ϕPSII, and ETR) (Figure 4a). WD + biostimulant plants, however, had the highest averages for photosynthetic pigments (Cla, Clb, and ClT) and were close to plants treated with biostimulant without WD, given the high carboxylation efficiency (A/Ci) and Ψw. Cluster analysis reinforced the similarity between plants from control treatments and those subjected to the application of biostimulant without WD. The WD + biostimulant plants differ from these plants, but they also differ from the group consisting of the WD and WD + split biostimulant plants (Figure 4b). Thus, WD + biostimulant appears to be an intermediate treatment between water supply and water stress. The effects caused by the treatments on soybean plants included reduced growth in plants subjected to WD and growth similar to the control in WD + biostimulant plants (Figure 4c).
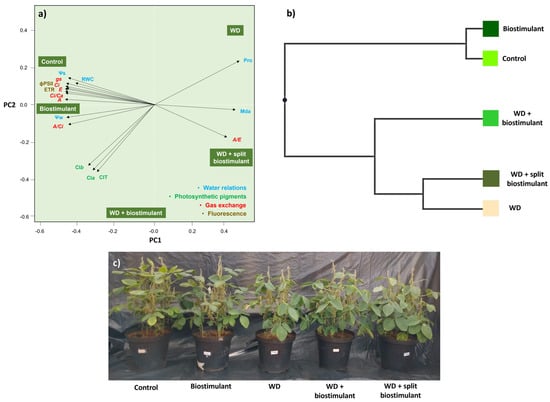
Figure 4.
Principal component analysis of water relations, photosynthetic pigment concentrations, gas exchange, and chlorophyll-a fluorescence (a), cluster analysis (b), and visual characteristics (c) of soybean in the water stress reduction test through the application of algae extract-based biostimulant. Ψw = water potential, Ψs = osmotic potential, RWC = relative water content, Pro = proline content, MDA = malondialdehyde, A = net photosynthetic rate, E = transpiration rate, A/E = water use efficiency, Gs = stomatal conductance, Ci = substomatal CO2 concentration, Ci/Ca = ratio of internal to external concentration of CO2, A/Ci = carboxylation efficiency, ETR = electron transport rate, ϕPSII = quantum efficiency of photosystem II, Cla = chlorophyll-a content, Clb = chlorophyll-b content, and ClT = total chlorophyll content.
4. Discussion
The water deficit resulted in changes in all the analyzed physiological parameters. This behavior was expected, since the restriction in the amount of water in the soil leads to a reduction in gas exchange owing to stomatal closure [5,51]. During soil drying, stomatal regulation is controlled by the hydraulic conductance of roots and soil [52]. Reduced hydraulic conductance affects the stomatal closure, which leads to a drop in transpiration. Consequently, there is a decrease in the internal concentration of CO2 in the leaves, causing a reduction in net photosynthesis [53].
The decrease in Rubisco activity leads to a reduction in the electron transport rate in the chloroplast transport chain and lower efficiency of photosystem II [54,55,56]. Despite the limitation to gas exchange observed in treatments under water deficit, in plants that received an application of 1.0 L ha−1 of the biostimulant, there was an increase in the photosynthetic efficiency, indicating a better ability to uptake the CO2 available in the leaf. This may be related to greater protection and activation of Rubisco provided by the algae extract [30] with stimulus to carboxylase activity to the detriment of oxygenase [57].
Therefore, the application of biostimulants is expected to mainly affect C3 plants, such as soybean, where the competition between photosynthesis and photorespiration is intense [3,58]. Under conditions of stress, this situation is even more accentuated, as the availability of CO2 decreases, leading to an increase in photorespiration and, consequently, a drop in net photosynthesis [2]. We proved that under these conditions, the application of an algae-based biostimulant can improve the physiological performance of soybean plants, suppressing the damage caused by water deficit.
The application of biostimulant increased the water potential of soybean plants, indicating stimulation of solute accumulation as an osmoprotective strategy for the cell against water loss caused by water deficit [59]. This demonstrates the ability of the biostimulant to maintain cell turgor under conditions of water deficit [60], ensuring water balance and cellular homeostasis. Santaniello et al. [30] demonstrated that the treatment of Arabidopsis plants with A. nodosum can induce, under water deficit conditions, a partial stomatal closure associated with changes in the expression levels of genes involved in ABA-responsive and antioxidant system pathways. The pre-activation of these pathways results in a stronger ability of A. nodosum-treated plants to maintain a better photosynthetic performance compared with untreated plants throughout the dehydration period, combined with a higher capacity to dissipate the excess of energy as heat in the reaction centers of PSII. In a similar study, [61] investigated the physiological and whole-genome transcriptome responses of Arabidopsis thaliana to drought stress after treatment with A. nodosum. A. nodosum strongly decreased drought-induced damage. Accumulation of reactive oxygen species (ROS), which typically stifle plant growth during drought, was reduced in A. nodosum-primed plants. Relative water contents remained high in A. nodosum -treated plants, whilst ion leakage, a measure of cell damage, was reduced compared with controls.
Elansary et al. [60] showed that the application of commercial biostimulants containing protein hydrolysates, humic acid, and especially brown algae extracts (Ascophyllum nodosum) mitigated the negative effects of drought stress on mint by increasing the antioxidant activity of key enzymes such as catalase and superoxide dismutase and reducing the accumulation of H2O2 in leaf tissue. This happens because algae-based biostimulants affect root development, increasing water and nutrient uptake, as well as resistance to abiotic stresses [62]. Phytohormones synthesized by algae act on cell elongation (via activation of plasma H + ATPase), chlorophyll synthesis, chloroplast development, phloem differentiation, apical dominance, tropisms, initiation of root formation, and stress tolerance (drought, salinity, and heat) [63]. This explains the improved fitness of soybean plants under water deficit conditions but protected by the application of the biostimulant. Our results are similar to those verified by Do Rosário Rosa et al. [27], where the use of a biostimulant based on A. nodosum + fulvic acids provided higher photosynthetic rates, more efficient mechanisms for dissipating excess energy, and greater activities of antioxidant enzymes.
We found that the use of the biostimulant in soybean plants subjected to WD reduces proline synthesis, prioritizing other physiological pathways, such as chlorophyll production. This attests to the ability of A. nodosum extract to mitigate the effects caused by a lack of water on the physiology of soybean plants. Our results can be explained by the cultivation conditions and can contribute to understanding the way in which A. nodosum operates by reducing stress, as plants treated with the extract of this alga and exposed to low temperatures accumulated high concentrations of proline [64]. Contrary to what we observed for soybean, other species such as tomato and Inga edulis appear to increase proline synthesis when treated with A. nodosum [19,65].
Despite the positive physiological results observed in plants inoculated with 1.0 L ha−1 of the biostimulant, plants treated with a split dose showed similar or even worse responses than those seen in WD plants without biostimulant. In these plants, the highest production of MDA was observed, indicating that the extract, when sprayed in a subdose (0.5 L ha−1), was unable to minimize the damage caused by WD to the membranes. These results draw attention to the conscious use of biostimulants in agriculture. We know that modern agriculture puts pressure on the market to increase the availability of microalgae-based bioinputs [66] and that future perspectives are that these inputs are routinely incorporated into organic and also into conventional and integrated agriculture [67]. As a market with a broad trend, it is estimated that revenue from biostimulants will reach almost 5 billion dollars by 2025 [68]. Therefore, we suggest that efforts should also be directed towards indications of the correct management of these products, like doses and stage of application, in order to avoid any different effects than expected for these plant growth-stimulating products.
5. Conclusions
We confirmed the hypothesis that foliar application of 1.0 L ha−1 of the biostimulant reduces the deleterious effects of the common water deficit at the beginning of the reproductive stage of soybean through a reduction in damage from oxidative stress (reduction of malondialdehyde synthesis by 31.2% in relation to the WD plants), maintenance of water potential and cellular homeostasis (10.2% increase in relative water content when compared with WD plants), conservation of the contents of chlorophyll in leaves and stimulation of photosynthesis and carboxylation (68% increase in net photosynthetic rate and 49.3% in carboxylation efficiency in relation to WD plants). However, when applied in installments, the biostimulant was not efficient in reducing soybean water stress. Future research should be conducted to evaluate the use of doses of less than 1.0 L ha−1 of the biostimulant, and studies under field conditions will be able to ensure the effectiveness of the use of A. nodosum extract on a large scale. Our work contributes to the portfolio of techniques that can be applied to soybean cultivation to reduce productivity losses in the face of water instability.
Author Contributions
Conceptualization, G.B.M. and A.G.d.S.; methodology, M.R., A.C.d.C. and C.R.R.; formal analysis, G.B.M. and A.A.d.S.; investigation, G.B.M., M.R. and L.A.B.; resources, G.B.M., M.R. and L.A.B.; writing—original draft preparation, G.B.M.; writing—review and editing, L.C.V. and L.A.B.; visualization, L.C.V.; supervision, L.C.V.; project administration, A.G.d.S.; funding acquisition, A.G.d.S. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data relevant to this manuscript are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), the Foundation for Research Support of the State of Goiás (FAPEG), the IFGoiano, Rio Verde campus, and UNIRV for the infrastructure and the students involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.O.N.J.A.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Vanloocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, L.; Wang, C.; Zhu, X.; Wang, Z.; Xing, B. Photosynthetic response mechanisms in typical C3 and C4 plants upon La2O3 nanoparticle exposure. Environ. Sci. Nano 2020, 7, 81–92. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef] [PubMed]
- Berlato, M.A.; Matzenauer, R.; Bergamaschi, H. Evapotranspiração máxima da soja, relações com a evapotranspiração calculada pela equação de Penman, evaporação de tanque “classe A” e radiação solar global. Agron. Sulriograndense 1986, 22, 243–259. [Google Scholar]
- Farias, J.R.B.; Nepomuceno, A.L.; Neumaier, N. Ecofisiologia da Soja; Embrapa Soja: Londrina, Brazil, 2007; p. 10. [Google Scholar]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Biostimulant application, under reduced nutrient supply, enhances quality and sustainability of ornamental containerized transplants. Agronomy 2023, 13, 765. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M.A. Biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; Mckeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; De Camargo, P.R.; Gaziola, S.A.; Azevedo, R.A. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comun. Sci. 2018, 9, 292–297. [Google Scholar] [CrossRef]
- Do Rosário Rosa, V.; Dos Santos, A.L.F.; Da Silva, A.A.; Sab, M.P.V.; Germino, G.H.; Cardoso, F.B.; De Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; VanderWeide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Marques, P.A.A.; Rezende, R.; Wenneck, G.S.; Terassi, D.D.S.; Andrean, A.F.B.A.; Matumoto-Pintro, P.T. Deficit irrigation with Ascophyllum nodosum extract application as a strategy to increase tomato yield and quality. Agronomy 2023, 13, 1853. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Verdeguer, M. Effect of two biostimulants, based on Ascophyllum nodosum extracts, on strawberry performance under mild drought stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L. Modelling the impacts of climate change and crop management measures on soybean phenology in China. J. Clean. Prod. 2020, 262, 121271. [Google Scholar] [CrossRef]
- Ramteke, R.; Gupta, G.K.; Singh, D.V. Growth and yield responses of soybean to climate change. Agric. Res. 2015, 4, 319–323. [Google Scholar] [CrossRef]
- Grassini, P.; La Menza, N.C.; Edreira, J.I.R.; Monzón, J.P.; Tenorio, F.A.; Specht, J.E. Soybean. In Crop Physiology Case Histories for Major Crops; Sadras, V., Calderini, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 282–319. [Google Scholar]
- de Andrade, C.L.L.; da Silva, A.G.; Melo, G.B.; Ferreira, R.V.; Moura, I.C.S.; Siqueira, G.G. Biostimulants derived from Ascophyllum nodosum associated of glyphosate in agronomic characteristics of soybean RR®. Rev. Bras. Herbic. 2018, 17. [Google Scholar] [CrossRef]
- Esfahani, Z.; Barzegar, T.; Ghahremani, Z.; Nikbakht, J. Effects of foliar application of Megafol on yield, fruit quality and water use efficiency of tomato Cv. Rio Grande under water deficit stress. J. Crop Improv. 2018, 19, 995–1009. [Google Scholar] [CrossRef]
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed.; Embrapa: Brasília, Brazil, 2004; p. 416. [Google Scholar]
- Valagro. Bioestimulantes. Available online: www.valagro.com/brazil/pt/produtos/farm/bioestimulante/megafol/ (accessed on 28 February 2019).
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Catsky, J. Water saturation deficit (relative water content). In Methods of Studying Plant Water Relations; Slavik, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 136–154. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.R.; Freire, M.B.G.S.; Cunha, K.P.V.; Nascimento, C.W.A.; Ruiz, H.A.; Lins, C.M.T. Biomass, anatomical change sand osmotic potential in Atriplex numularia L. cultivated in sodic saline soil under water stress. Environ. Exp. Bot. 2012, 82, 20–27. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco friendlier protocol for quantifying proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 27 March 2023).
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Palau, J.; Romeu, A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol. Biol. Evol. 1999, 16, 1125–1134. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef]
- Abdalla, M.; Ahmed, M.A.; Cai, G.; Wankmüller, F.; Schwartz, N.; Litig, O.; Carminati, A. Stomatal closure during water deficit is controlled by below-ground hydraulics. Ann. Bot. 2022, 129, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Gu, L.; Shi, Y.; Chen, H.; Liu, Y.; Lu, F.; Zhou, G. Plant hydraulic conductivity determines photosynthesis in rice under PEG-induced drought stress. Pak. J. Bot. 2021, 53, 409–417. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.; Safdar, M.E.; Brestic, M.; Liu, J. Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme system under a split-root system. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef]
- Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem. Soc. Trans. 2021, 49, 2007–2019. [Google Scholar] [CrossRef]
- Wijewardene, I.; Shen, G.; Zhang, H. Enhancing crop yield by using Rubisco activase to improve photosynthesis under elevated temperatures. Stress Biol. 2021, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, G.H. The carboxylation and oxygenation of ribulose 1, 5-bisphosphate: The primary events in photosynthesis and photorespiration. Ann. Rev. Plant Physiol. 1981, 32, 349–382. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy 2019, 10, 6. [Google Scholar] [CrossRef]
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a seaweed extract strongly improves drought tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Santos, C.C.; Silva, A.A.S.; Carvalho de Oliveira, C.H.; Silverio, J.M.; dos Santos Dias, A.; Linné, J.A.; Scalon, S.P.Q.; Alovisi, A.M.T. Ascophyllum nodosum seaweed extract in Inga edulis seedlings under drought and the potential of phenotypic plasticity. J. Appl. Phycol. 2023, 35, 3123–3135. [Google Scholar] [CrossRef]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as biofertilizer in modern agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Biostimulants Market by Active Ingredient (Humic Substances, Amino Acids, Seaweed Extracts, Microbial Amendments), Crop Type (Fruits & Vegetables, Cereals, Turf & Ornamentals), Application Method, Form, and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html?gclid=CjwKCAjw4_H6BRALEiwAvgfzq1LVX47L4C4O0v0leN5GfYGuk0xW2oF25JDZhWGs03E3I2rL1kEwGxoCnsAQAvD_BwE (accessed on 11 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).