How Does Irrigation with Wastewater Affect the Physical Soil Properties and the Root Growth of Sugarcane under Subsurface Drip?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Area and Cultivation Soil
2.2. Experimental Design and Treatments
2.3. Cultivation, Planting, and Treatments
2.4. Fertilization and Irrigation
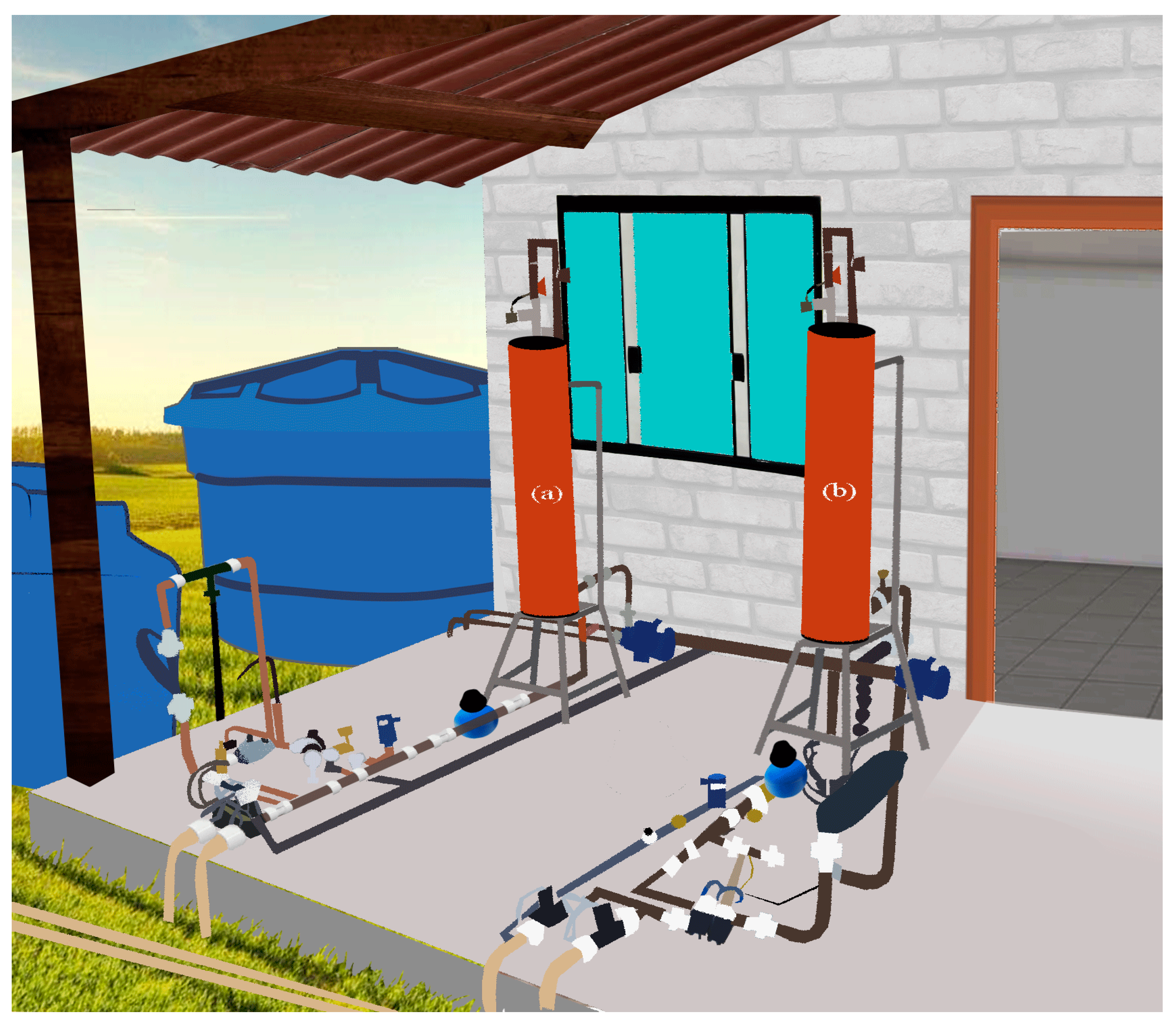
2.5. Irrigation System and Management
2.6. SRW and TSE Quality
2.7. Post-Cultivation Evaluation of Soil Physical Properties
2.8. Sugarcane Root System
2.9. Data Analysis
3. Results
3.1. Univariate Data Analysis
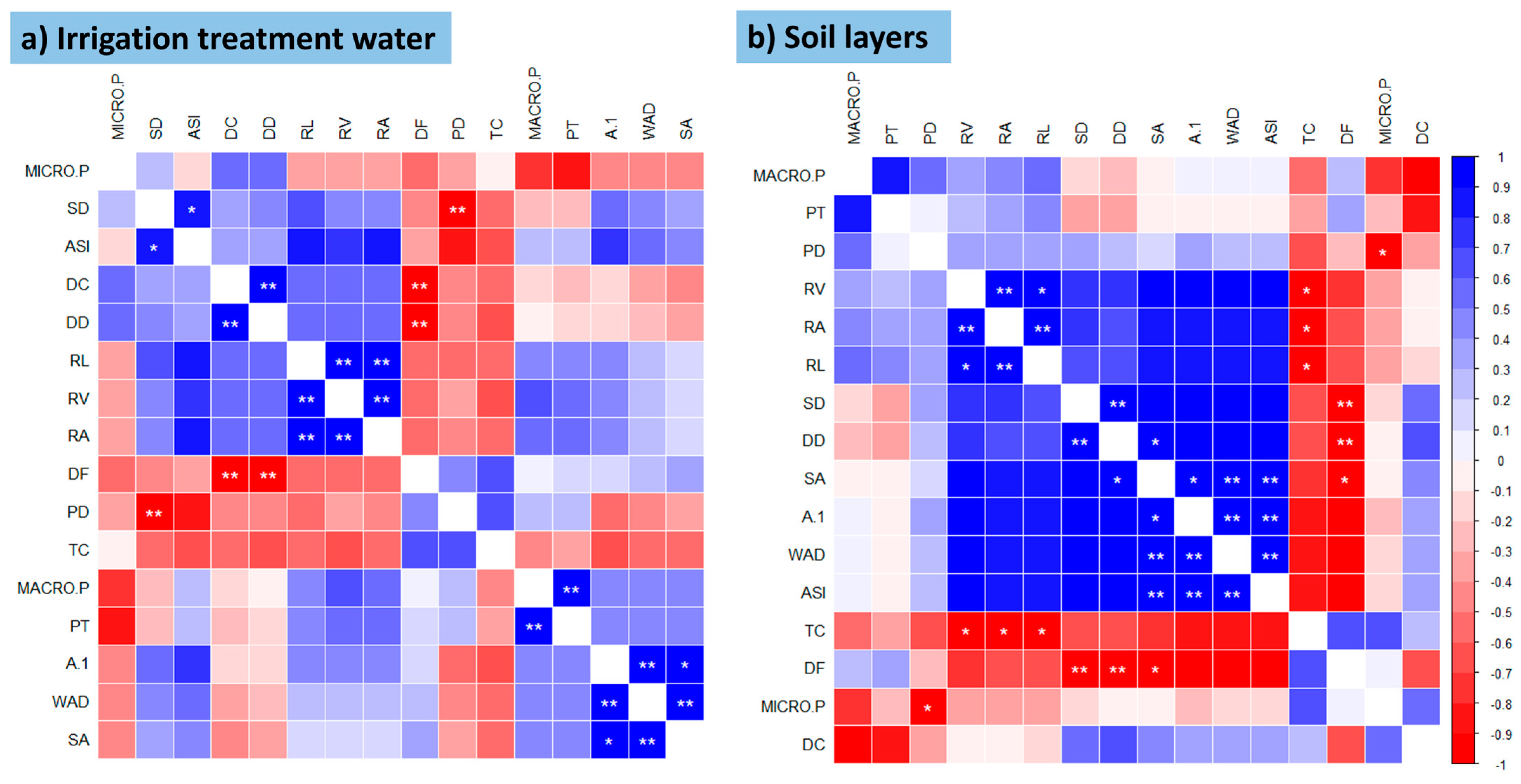
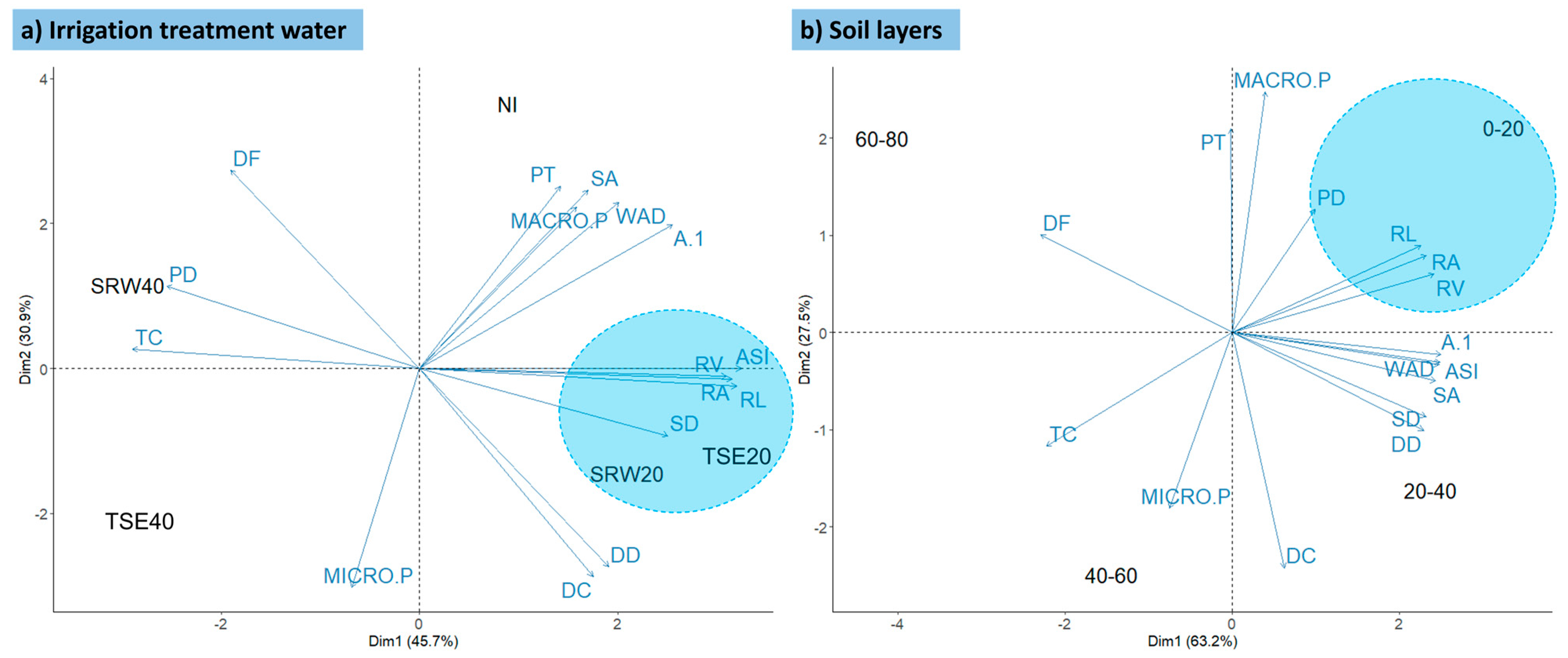
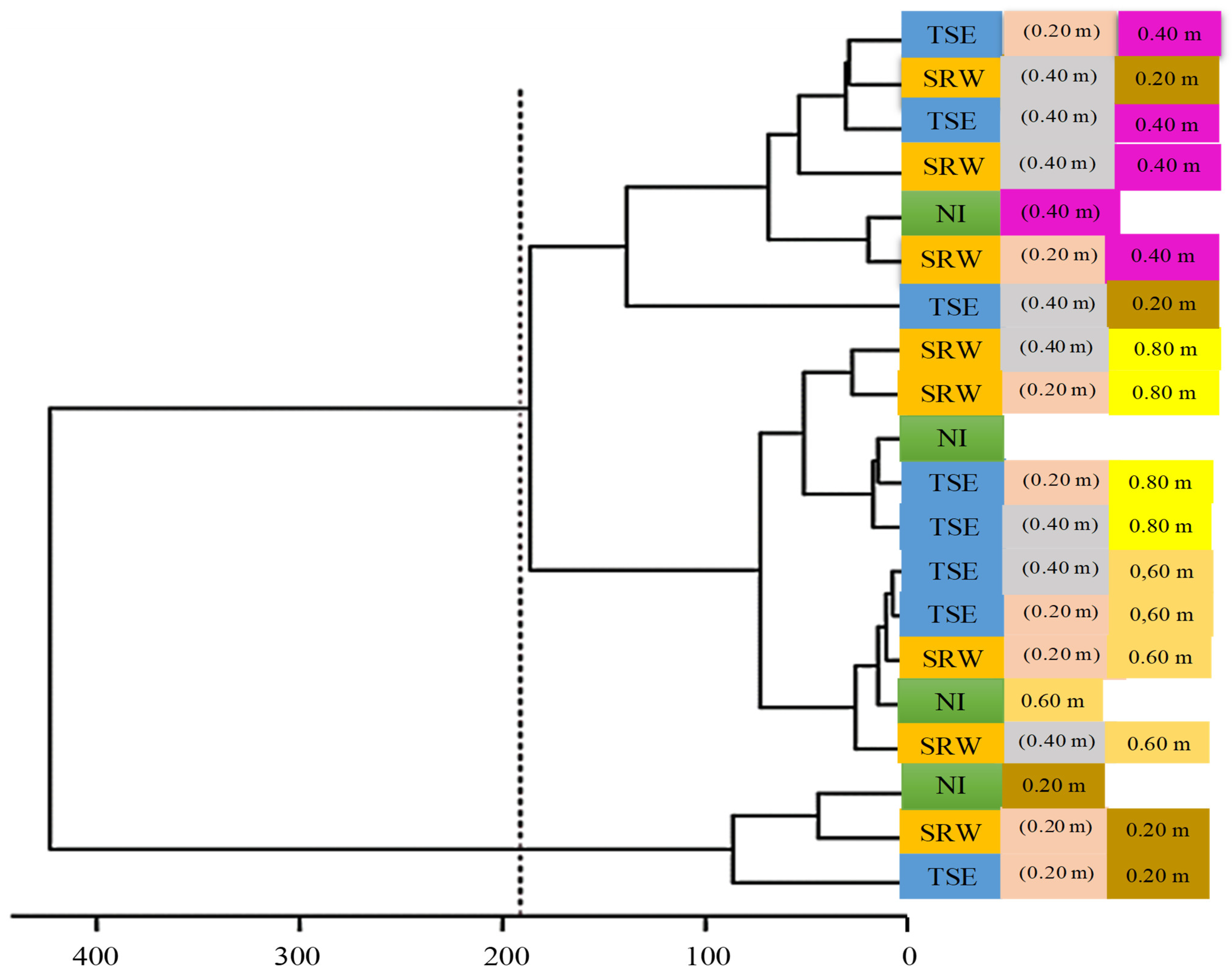
3.2. Pearson Correlation and Multivariate Analysis
4. Discussion
4.1. Sugarcane Irrigation with TSE and SRW at the Shallowest Layer Improved Root Development but Increased Soil Density
4.2. TSE and SRW Can Be Used to Irrigate Sugarcane without Affecting Root Formation at Shallower Layers, but We Highlight the Need to Monitor Compaction, Especially at Deeper Layers
4.3. Sugarcane Irrigation with TSE and SRW Improves Soil Physical Qualities in Shallower Depth Layers, with the Opposite Result in Deeper Layers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes Sobrinho, O.P.; Santos, L.N.S.; Santos, G.O.; Cunha, F.N.; Soares, F.A.L.; Teixeira, M.B. Balanço hídrico climatológico mensal e classificação climática de Köppen e Thornthwaite para o município de Rio Verde, Goiás. RBCLima 2020, 27, 19–33. [Google Scholar] [CrossRef]
- Gonçalves, I.Z.; Barbosa, E.A.A.; Santos, L.N.S.; Nazário, A.A.; Feitosa, D.R.C.; Tuta, N.F.; Matsura, E.E. Nutritional balance and production of sugarcane irrigated with treated wastewater through subsurface drip. Irrig. Sci. 2019, 37, 207–217. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Treated wastewater irrigation—A review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Ganjegunte, G.; Ulery, A.; Niu, G.; Wu, Y. Organic carbon, nutrient, and salt dynamics in saline soil and switchgrass (Panicum virgatum L.) irrigated with treated municipal wastewater. Land Degrad. Dev. 2018, 29, 80–90. [Google Scholar] [CrossRef]
- Urbano, V.R.; Mendonça, T.G.; Bastos, R.G.; Souza, C.F. Effects of treated wastewater irrigation on soil properties and lettuce yield. Agric. Water Manag. 2017, 181, 108–115. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, T.; Seong, C.; Park, S. Assessing nitrogen fertilizer rates and split applications using the DSSAT model for rice irrigated with urban wastewater. Agric. Water Manag. 2014, 141, 1–9. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, R.; Kumar, M. Use of treated sewage or wastewater as an irrigation water for agricultural purposes-Environmental, health, and economic impacts. Total Environ. Res. Themes 2023, 6, 100051. [Google Scholar] [CrossRef]
- Owusu, S.; Cofie, O.; Mul, M.; Barron, J. The significance of small reservoirs in sustaining agricultural landscapes in dry areas of West Africa: A review. Water 2022, 14, 1440. [Google Scholar] [CrossRef]
- Lamm, F.R.; Aysrw, J.E.; Nakayama, F.S. Microirrigation for Crop Production: Design, Operation and Management, 1st ed.; Lamm, F.R., Aysrw, J.E., Nakayama, F.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–618. [Google Scholar]
- Pinto, J.; da Cunha, F.F.; Adão, A.d.S.; de Paula, L.B.; Ribeiro, M.C.; Neto, J.R.R.C. Strawberry production with different mulches and wetted areas. Horticulturae 2022, 8, 930. [Google Scholar] [CrossRef]
- Vanella, D.; Cuesta, J.M.R.; Minnolo, G.L.; Longo, D.; D’emilio, A.; Consoli, S. Identifying soil-plant interactions in a mixed-age orange orchard using electrical resistivity imaging. Plant Soil 2023, 483, 181–197. [Google Scholar] [CrossRef]
- Namdarian, D.; Naseri, A.A.; BoroomandNasab, S.; Parvizialmani, M. Effect of subsurface drip and furrow irrigation system on growth and yield indices in sugarcane cultivation. Iran J. Soil Water Res. 2020, 51, 1515–1527. [Google Scholar] [CrossRef]
- Sheini-Dashtgol, A.; Kermannezhad, J.; Ghanbari-Adivi, E.; Hamoodi, M. Evaluating moisture distribution and salinity dynamics in sugarcane subsurface drip irrigation. Water Conserv. Sci. Eng. 2022, 7, 227–245. [Google Scholar] [CrossRef]
- Singh, K.; Mishra, S.K.; Brar, A.S. Optimizing sugarcane and water productivity through surface and subsurface drip fertigation in subtropical India. Sugar Tech 2024, 26, 63–76. [Google Scholar] [CrossRef]
- Starikov, A.V.; Gribanov, A.A.; Starikova, A.A. Automation of combined irrigation system control in greenhouses with electrochemically activated water. In Proceedings of the 2022 International Ural Conference on Electrical Power Engineering (UralCon), Magnitogorsk, Russia, 23–25 September 2022; pp. 284–289. [Google Scholar]
- Nazário, A.A.; Gonçalves, I.Z.; Barbosa, E.A.A.; dos Santos, L.N.S.; Feitosa, D.R.C.; Matsura, E.E. Impact of the application of domestic wastewater by subsurface drip irrigation on the soil solution in sugarcane cultivation. Appl. Environ. Soil Sci. 2019, 2019, 8764162. [Google Scholar] [CrossRef]
- Barbosa, E.A.A.; Gonçalves, I.Z.; Santos, L.N.S.; Nazario, A.A.; Feitosa, D.R.C.; Matsura, E.E. Greenhouse gas emission of sugarcane irrigated with treated domestic sewage by subsurface drip, in Southeast Brazil. Irrig. Drain. 2023, 72, 1053–1065. [Google Scholar] [CrossRef]
- Kul, R.; Ekinci, M.; Turan, M.; Ors, S.; Yildirim, E. How abiotic stress conditions affects plant roots. In Plant Root; InTech Open: London, UK, 2020; pp. 6–10. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Pareek, S.L.S.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol. Plant 2022, 174, e13651. [Google Scholar] [CrossRef] [PubMed]
- Pissolato, M.D.; da Cruz, L.P.; Silveira, N.M.; Machado, E.C.; Ribeiro, R.V. Sugarcane regrowth is dependent on root system size: An approach using young plants grown in nutrient solution. Bragantia 2021, 80, e4321. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.; Lu, G.; Zeng, R.; Que, Y. Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects. Biology 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Woodin, S.J.; Pakeman, R.J.; Johnson, D.; Wal, R.V.D. Root traits predict decomposition across a landscape-scale grazing experiment. New Phytol. 2014, 203, 851–862. [Google Scholar] [CrossRef]
- Santos, L.N.S.; Barbosa, E.A.A.; Nazário, A.A.; Gonçalves, I.Z.; Ohashi, A.Y.P.; Matsura, E.E.; Pires, R.C.M. Root growth of sugarcane irrigated with wastewater through subsurface drip system. CIGR J. 2017, 19, 16–25. [Google Scholar]
- Ohashi, A.Y.P.; Pires, R.C.d.M.; Silva, A.L.B.d.O.; Santos, L.N.S.; Matsura, E.E. Minirhizotron as an in-situ tool for assessing sugarcane root system growth and distribution. Agric. Res. Technol. 2019, 22, 556182. [Google Scholar] [CrossRef]
- Scarpare, F.V.; Lier, Q.d.J.V.; de Camargo, L.; Pires, R.C.M.; Corrêa, S.T.R.; Bezerra, A.H.F.; Gava, G.J.C.; Dias, C.T.S. Tillage effects on soil physical condition and root growth associated with sugarcane water availability. Soil Tillage Res. 2019, 187, 110–118. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. (Eds.) Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements, 1st ed.; FAO. Irrigation and Drainage Paper, 56; FAO: Rome, Italy, 1998; pp. 1–281. [Google Scholar]
- Segato, S.V.; Mattiuz, C.F.M.; Mozambani, A.E. Aspectos Fenológicos da Cana-de-Açúcar. Atualização em Produção de Cana-de-Açúcar, Piracicaba-SP, 1st ed.; Segato, S.V., Pinto, A.S., Jendiroba, E., Nóbrega, J.C.M., Eds.; Livroceres: Piracicaba, Brazil, 2006; Volume 1, 36p. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- CEPAGRI—Centro de Pesquisas Meteorológicas e Climáticas Aplicadas à Agricultura. Tempo e Clima. 2014. Available online: https://www.cpa.unicamp.br (accessed on 19 January 2024).
- Dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; EMBRAPA: Brasília, Brazil, 2018; 355p. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014; 372p.
- FAO—Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015; 192p. [Google Scholar]
- Raij, B.V.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendação de adubação e calagem para o Estado de São Paulo, 2nd ed.; IAC: Campinas, Brazil, 1996; 285p. [Google Scholar]
- Camargo, O.A.; Moniz, A.C.; Jorge, J.A.; Valadares, J.M. Métodos de Análise Química, Mineralógica e Física de Solos do Instituto Agronômico de Campinas; Boletim Técnico, 106; Instituto Agronômico: Campinas, Brazil, 2009; 77p.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; EMBRAPA: Brasília, Brazil, 2017; 57p. [Google Scholar]
- Meneghetti, A.M. Manual de Procedimentos de Amostragem e Análise Química de Plantas, Solo e Fertilizantes; EDUTFPR: Curitiba, Brazil, 2018; 251p. [Google Scholar]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Washington: United states salinity laboratory staff, united states department of agriculture. In Agriculture Handbook; United States Salinity Laboratory Staff, United States Department of Agriculture: Washington, DC, USA, 1954; Volume 60, pp. 84–156. [Google Scholar]
- Silva, M.d.A.; Arantes, M.T.; Rhein, A.F.d.L.; Gava, G.J.C.; Kolln, O.T. Potencial produtivo da cana-de-açúcar sob irrigação por gotejamento em função de variedades e ciclos. Rev. Bras. Eng. Agric. Ambient. 2014, 18, 241–249. [Google Scholar] [CrossRef]
- Rodolfo, F., Jr.; Ribeiro, W.Q., Jr.; Ramos, M.L.G.; Batista, F.P.d.S.; de Lima, C.A.; Rocha, O.C. Biometric responses of third ratoon sugarcane varieties under variable water regime. Comum. Sci. 2018, 9, 81–92. [Google Scholar] [CrossRef]
- Magro, C.R.; Laca-Buendía, J.P. Efeito da profundidade de plantio no perfilhamento da cana-de-açúcar. Fazu Em Rev. 2010, 7, 48–54. [Google Scholar]
- De Azevedo, M.C.; Silva, E.d.S.; Almeida, L.J.d.M.; Rosendo, B.H.B.; Ribeiro, J.E.d.S.; Simões Neto, D.E.; Mielezrski, F. Produtividade de genótipos de cana de açúcar em resposta à aplicação de calcário em microclima do semiárido brasileiro. Res. Soc. Dev. 2021, 10, e34710716784. [Google Scholar] [CrossRef]
- Rossetto, R.; Dias, F.L.F.; Vitti, A.C. Fertilidade do Solo, Nutrição e Adubação, 1st ed.; Dinardo-Miranda, L.L., Vasconcelos, A.C.M., Landell, M.G.A., Eds.; Instituto Agronômico: Campinas, Brazil, 2010; 882p.
- Haag, H.P.; Dechen, A.R.; Carmello, Q.A.C. Nutrição Mineral da Cana-de-Açúcar; Paranhos, S.B., Ed.; Cultivo e Utilização; Fundação Cargill: Campinas, Brazil, 1987; Volume 1, pp. 88–162. [Google Scholar]
- NETAFIM. Tubo Gotejador Integral Autocompensado, DripNet PC™ AS. 2022. Available online: http://www.netafim.com.br/product/dripnet-pc-as-thick-walled-dripperlines (accessed on 19 January 2024).
- Souza, C.F.; Folegatti, M.V.; Matsura, E.E.; Ou, D. Calibração da reflectometria no domínio do tempo (TDR) para a estimativa da concentração da solução no solo. Eng. Agric. 2006, 26, 282–291. [Google Scholar] [CrossRef]
- Mestas, R.M.; Roque, M.W.; Matsura, E.E.; Bizari, D.R.; Paz, A. Variabilidad espacial de los atributos físico-hídricos del suelo y de la productividad del cultivo de fréjol (Phaseolus vulgaris L.) irrigado bajo un sistema de siembra directa. Rev. Bras. Cienc. Agrar. 2010, 33, 307–313. [Google Scholar] [CrossRef]
- Souza, C.F.; Matsura, E.E.; Testezlaf, R. Experiência do Laboratório de Irrigação e Drenagem da Faculdade de Engenharia Agrícola/Unicamp no uso da técnica de TDR. In Aplicações da Técnica de TDR na Agricultura, 1st ed.; Matsura, E.E., Ed.; Biblioteca da Área de Engenharia (BAE), Universidade Estadual de Campinas: Campinas, Brazil, 2001; 178p. [Google Scholar]
- Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA/AWWA/WPCF: Washington, DC, USA, 2023; 1624p. [Google Scholar]
- USEPA—United States Environmental Protection Agency. Test Methods. 2013. Available online: https://www.epa.gov/emc/emc-other-test-methods (accessed on 19 January 2024).
- Yoder, R.E. A direct method of aggregate analysis of soils and a study of the physical nature of erosion losses. Agron. J. 1936, 28, 337–351. [Google Scholar] [CrossRef]
- Kiehl, E.J. Manual de Edafologia: Relações Solo-Planta; Ceres: São Paulo, Brazil, 1979; 262p. [Google Scholar]
- Libardi, P.L. Dinâmica da Água no Solo, 3rd ed.; Editora da Universidade de São Paulo: São Paulo, Brazil, 2018; 352p. [Google Scholar]
- Fujiwara, M.; Kurachi, S.A.H.; Arruda, F.B.; Pires, R.C.M.; Sakai, E. A Técnica de Estudo de Raízes Pelo Método do Trado; Boletim Técnico, 153; Instituto Agronômico: Campinas, Brazil, 1994; 9p.
- Jorge, L.A.d.C.; Rodrigues, A.F.d.O. Safira: Sistema de Análise de Fibras e Raízes; Boletim de Pesquisa e Desenvolvimento—EMBRAPA: São Carlos, Brazil, 2008; 20p. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. Package ‘ExpDes. pt’; R Package Version 1.2.; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 19 January 2024).
- Ossani, P.C.; Cirillo, M.A. MVar.pt: Analise multivariada (Brazilian Portuguese). 2017. Available online: https://cran.r-project.org/web/packages/MVar.pt/MVar.pt.pdf (accessed on 19 January 2024).
- Azevedo, A.M. Package ‘Multivariate Analysis’. 2021. Available online: https://cran.rproject.org/web/packages/MultivariateAnalysis/MultivariateAnalysis.pdf (accessed on 19 January 2024).
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 18, 6019–6034. [Google Scholar] [CrossRef] [PubMed]
- Fromm, H. Root plasticity in the pursuit of water. Plants 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Roumet, C.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; de Deyn, G.B.; Johnson, D.; Klimešová, J.; Lukac, M.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Gregory, P.J. Russell Review Are plant roots only “in” soil or are they “of” it? Roots, soil formation and function. Eur. J. Soil Sci. 2022, 73, e13219. [Google Scholar] [CrossRef]
- Ngasoh, F.G.; Ahmadu, A.A.; Lingbuin, H.G. Treatment and use of sewage effluent and sludge for irrigation: A review. Int. J. Innov. Sci. Res. Technol. 2020, 5, 87–94. [Google Scholar]
- Pereira, L.R.M.; da Fonseca, A.F.; Herpin, U.; Melfi, A.J. Agricultural utilization of treated sewage effluent: Experience from Brazil. Isr. J. Plant Sci. 2011, 59, 235–248. [Google Scholar]
- Costa, M.C.G.; Coutinho, I.A.C. Root systems of agricultural crops and their response to physical and chemical subsoil constraints. In Subsoil Constraints for Crop Production; Oliveira, T.S., Bell, R.W., Eds.; Springer: Cham, Swizterland, 2022; pp. 225–261. [Google Scholar] [CrossRef]
- Shoaib, M.; Banerjee, B.P.; Hayden, M.; Kant, S. Roots’ drought adaptive traits in crop improvement. Plants 2022, 11, 2256. [Google Scholar] [CrossRef] [PubMed]
- Antwerpen, R.V.; Heerden, P.D.R.; Keeping, M.G.; Titshall, L.W.; Jumman, A.; Tweddle, P.B.; Antwerpen, T.V.; Ramouthar, P.V.; Campbell, P.L. Chapter Two—A review of field management practices impacting root health in sugarcane. Adv. Agron. 2022, 173, 79–162. [Google Scholar] [CrossRef]
- Bano, C.; Amist, N.; Singh, N.B. Morphological and anatomical modifications of plants for environmental stresses. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: Hoboken, NJ, USA, 2019; pp. 29–44. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of soil water deficit on growth and development of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Werner, A.D.; Li, Y.L. Root-induced changes of soil hydraulic properties—A review. J. Hydrol. 2020, 589, 125203. [Google Scholar] [CrossRef]
- Regassa, A.; Tsehai, K.K.; Selassie, Y.G.; Kiflu, A.; Tena, W. Soil Properties. In The Soils of Ethiopia; Springer International Publishing: Cham, Switzerland, 2023; pp. 111–156. [Google Scholar] [CrossRef]
- Kumi, F.; Obour, P.B.; Arthur, E.; Moore, S.E.; Asare, P.A.; Asiedu, J.; Angnuureng, D.B.; Atiah, K.; Amoah, K.K.; Amponsah, S.K.; et al. Quantifying root-induced soil strength, measured as soil penetration resistance, from different crop plants and soil types. Soil Tillage Res. 2023, 233, 105811. [Google Scholar] [CrossRef]
- Nogueira, V.H.B.; Diotto, A.V.; Thebaldi, M.S.; Colombo, A.; Silva, Y.F.; Lima, E.M.d.C.; Resende, G.F.L. Variation in the flow rate of drip emitters in a subsurface irrigation system for different soil types. Agric. Water Manag. 2021, 243, 106485. [Google Scholar] [CrossRef]
- Shiqi, F.; Panyue, Z.; Jianying, Y.; Weijie, B.; Wenzhang, M.; Ben, Z.; Jiajing, L. Analysis of Salinization Characteristics of Artificial Greenland Stratified Soils and Their Influencing Factors in the Eastern Part of the Arid Desert Region of Northwest China. J. Ecol. 2023, 14, 856–867. [Google Scholar] [CrossRef]
- Watanabe, K.; Thienyaem, T.; Poniyom, K.; Saensupo, S.; Sriroth, K.; Jaiphong, T. Effects of fertilizer application depth on the above-and belowground growth of sugarcane under different water regimes and machinery performance. Sugar Tech 2023, 25, 1092–1101. [Google Scholar] [CrossRef]
- Bian, J.; Toyota, M.; Morokuma, M. Effect of flood and drip irrigation and difference of previous crop residue input on morphological and physiological traits in rice root. Plant Prod. Sci. 2023, 26, 249–258. [Google Scholar] [CrossRef]
- Ferreira, C.J.B.; Tormena, C.A.; Severiano, E.D.C.; Zotarelli, L.; Betioli Júnior, E. Soil compaction influences soil physical quality and soybean yield under long-term no-tillage. Arch Agron. Soil Sci. 2021, 67, 383–396. [Google Scholar] [CrossRef]
- Keller, T.; Colombi, T.; Ruiz, S.; Schymanski, S.J.; Weisskopf, P.; Koestel, J.; Sommer, M.; Stadelmann, V.; Breitenstein, D.; Kirchgessner, N.; et al. Soil structure recovery following compaction: Short-term evolution of soil physical properties in a loamy soil. Soil Sci. Soc. Am. J. 2021, 85, 1002–1020. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Fernandes, R.B.A. Physical subsoil constraints of agricultural and forestry land. In Subsoil Constraints for Crop Production; Oliveira, T.S., Bell, R.W., Eds.; Springer: Cham, Switzerland, 2022; pp. 125–160. [Google Scholar] [CrossRef]
- Corrêa, S.T.R.; Barbosa, L.C.; Menandro, L.M.S.; Scarpare, F.V.; Reichardt, K.; de Moraes, L.O.; Hernandes, T.A.D.; Franco, H.C.J.; Carvalho, J.L.N. Straw removal effects on soil water dynamics, soil temperature, and sugarcane yield in south-central Brazil. Bioenergy Res. 2019, 12, 749–763. [Google Scholar] [CrossRef]
- Lovera, H.L.; de Souza, Z.M.; Esteban, D.A.A.; de Oliveira, I.N.; Farhate, C.V.V.; Lima, E.d.S.; Panosso, A.R. Sugarcane root system: Variation over three cycles under different soil tillage systems and cover crops. Soil Tillage Res. 2021, 208, 104866. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.; Sidhu, J.S.; Ajmera, I.; Castañeda, T.G.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Li, J.; Cao, M.; Wang, Z.; Li, J.; Meng, D.; Cao, P.; Duan, S.; Zhang, M.; et al. Mechanism of intermittent deep tillage and different depths improving crop growth from the perspective of rhizosphere soil nutrients, root system architectures, bacterial communities, and functional profiles. Front. Microbiol. 2022, 12, 759374. [Google Scholar] [CrossRef]
- Benites, V.d.M.; Schaefer, C.E.G.R.; Machado, P.L.O.A.; Polidoro, J.C. The Soils of Brazil; World Soils Book Series; Springer: Cham, Switzerland, 2023; pp. 471–486. [Google Scholar] [CrossRef]
- Gurjar, O.P.; Meena, R.; Latare, A.M.; Rai, S.; Kant, S.; Kumar, A.; Sheshama, M. Effects of sewage wastewater irrigation compare to ground water irrigation on soil physico-chemical properties. Int. J. Chem. Stud. 2017, 5, 265–267. [Google Scholar]
- Leuther, F.; Schlüter, S.; Wallach, R.; Vogel, H.J. Structure and hydraulic properties in soils under long-term irrigation with treated wastewater. Geoderma 2019, 333, 90–98. [Google Scholar] [CrossRef]
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water scarcity and waste water reuse in crop irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated wastewater reuse for irrigation: Pros and cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Gal, A.B.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Mannina, G.; Gulhan, H.; Ni, B.J. Water reuse from wastewater treatment: The transition towards circular economy in the water sector. Bioresour Technol. 2022, 363, 127951. [Google Scholar] [CrossRef]
- Kadhim, Y.M.; Al-Adhamii, R.A.; Fattah, M.Y. Geotechnical properties of clayey soil improved by sewage sludge ash. J. Air Waste Manag. Assoc. 2022, 72, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, M.C.; Ferreira, S.R.; Delgado, J.M.; Silva, F.A.; Oliveira, J.T.; Oliveira, P.E.; Azevedo, A.C. Sewage sludge valorization for collapsible soil improvement. Buildings 2023, 13, 338. [Google Scholar] [CrossRef]
- Helmecke, M.; Fries, E.; Schulte, C. Regulating water reuse for agricultural irrigation: Risks related to organic micro-contaminants. Environ. Sci. Eur. 2020, 32, 4. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.R.; Badawai, M.; Lima, E.C.; Makinia, J. Wastewater treatment for reuse in agriculture: Prospects and challenges. Environ. Res. 2023, 23, 116711. [Google Scholar] [CrossRef]
- Easton, Z.M. Soil and Soil Water Relationships; Virginia Cooperative Extension: Petersburg, VA, USA, 2021. [Google Scholar]
- Ganat, T.A.O. Fundamentals of Reservoir Rock Properties; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Brown, S.; Biswas, A.; Caron, J.; Dyck, M.; Si, B. Soil Physics; Digging into Canadian Soils: Saskatoon, SK, Canada, 2021. [Google Scholar]
- Seehusen, T.; Mordhorst, A.; Riggert, R.; Fleige, H.; Horn, R.; Riley, H. Subsoil compaction of a clay soil in South-East Norway and its amelioration after 5 years. Int. Agrophys. 2021, 35, 145–157. [Google Scholar] [CrossRef]
- Sartori, F.; Piccoli, I.; Polese, R.; Berti, A. Transition to conservation agriculture: How tillage intensity and covering affect soil physical parameters. Soil 2022, 8, 213–222. [Google Scholar] [CrossRef]
- Blanco, H.; Kumar, S.; Anderson, S.H. Soil health and soil water. In Soil Hydrology in a Changing Climate; CSIRO: Canberra, Australia, 2022; p. 39. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, H.; Liu, Y.; Li, H.; Ren, R.; Si, B. Rock water use by apple trees affected by physical properties of the underlying weathered rock. Agric. Water Manag. 2023, 287, 108413. [Google Scholar] [CrossRef]
- De Moraes, M.T.; Debiasi, H.; Franchini, J.C.; Mastroberti, A.A.; Levien, R.; Leitner, D.; Schnepf, A. Soil compaction impacts soybean root growth in an Oxisol from subtropical Brazil. Soil Tillage Res. 2020, 200, 104611. [Google Scholar] [CrossRef]
- Vizioli, B.; Polizeli, K.M.V.C.; Tormena, C.A.; Barth, G. Effects of long-term tillage systems on soil physical quality and crop yield in a Brazilian Ferralsol. Soil Tillage Res. 2021, 209, 104935. [Google Scholar] [CrossRef]
- Yue, L.; Wang, Y.; Wang, L.; Yao, S.; Cong, C.; Ren, L.; Zhang, B. Impacts of soil compaction and historical soybean variety growth on soil macropore structure. Soil Tillage Res. 2021, 214, 105166. [Google Scholar] [CrossRef]
- Ramos, M.C.; Sánchez, E.P.; Bonilla, D.P.; Martínez, C.C.; Lampurlanés, J. Soil sealing and soil water content under no-tillage and conventional tillage in irrigated corn: Effects on grain yield. Hydrol. Process. 2019, 33, 2095–2109. [Google Scholar] [CrossRef]
- Reichert, J.M.; Fontanela, E.; Awe, G.O.; Fasinmirin, J.T. Is cassava yield affected by inverting tillage, chiseling or additional compaction of no-till sandy-loam soil? Rev. Bras. Cienc. Solo 2021, 45, e0200134. [Google Scholar] [CrossRef]
- Viana, J.L.; de Souza, J.L.M.; Auler, A.C.; Oliveira, R.A.; Araújo, R.M.; Hoshide, A.K.; de Abreu, D.C.; da Silva, W.M. Water Dynamics and Hydraulic Functions in Sandy Soils: Limitations to Sugarcane Cultivation in Southern Brazil. Sustainability 2023, 15, 7456. [Google Scholar] [CrossRef]
- Meurer, K.; Barron, J.; Chenu, C.; Coucheney, E.; Fielding, M.; Hallett, P.; Herrmann, A.M.; Keller, T.; Koestel, J.; Larsbo, M.; et al. A framework for modelling soil structure dynamics induced by biological activity. Glob. Chang Biol. 2020, 26, 5382–5403. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.P.; Schossler, T.R.; Santos, I.L.; Melo, N.B.; Nóbrega, J.C.A.; Santos, G.G. Physical-hydric attributes in Latossolo Amarelo under systems of use in the Cerrado/Caatinga ecotone areas in Piauí State, Brazil. An. Acad. Bras. Cienc. 2021, 93, 20190667. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chakraborty, D. Global meta-analysis suggests that no-tillage favourably changes soil structure and porosity. Geoderma 2022, 405, 115443. [Google Scholar] [CrossRef]
- Hu, R.; Liu, Y.; Chen, T.; Zheng, Z.; Peng, G.; Zou, Y.; Tang, C.; Schan, X.; Zhou, Q.; Li, J. Responses of soil aggregates, organic carbon, and crop yield to short-term intermittent deep tillage in Southern China. J. Clean. Prod. 2021, 298, 126767. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Ayoubi, S.; Mirbagheri, Z.; Mosaddeghi, M.R.; Xu, M. Spatial prediction of soil aggregate stability and soil organic carbon in aggregate fractions using machine learning algorithms and environmental variables. Geoderma Reg. 2021, 27, e00440. [Google Scholar] [CrossRef]
- Castioni, G.A.F.; Cherubin, M.R.; Bordonall, R.d.O.C.; Barbosa, L.C.; Menandro, L.M.S.; Carvalho, J.L.N. Straw removal affects soil physical quality and sugarcane yield in Brazil. Bioenergy Res. 2019, 12, 789–800. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Sun, Y.; Han, J.; Hu, F.; Li, J.; Li, X. Effects of the changes of particle surface electric field and interaction force on the reclaimed soil aggregate structural stability under the application of different soil conditioners. Agronomy 2023, 13, 1866. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, P.; Sharma, S. Changes in Soil Organic Pool and Carbon Preservation Capacity of Macro- and Micro-aggregates in Response to Land-Use Change in North-Western India. J Soil Sci. Plant Nutr. 2023, 23, 2849–2867. [Google Scholar] [CrossRef]
- Mustafa, A.; Minggang, X.; Shah, S.A.A.; Abrar, M.M.; Nan, S.; Baoren, W.; Zejiang, C.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nouri, A.; Singh, S.; Anapalli, S.; Lee, J.; Arelli, P.; Jagadamma, S. Soil organic carbon and aggregation in response to thirty-nine years of tillage management in the southeastern US. Soil Tillage Res. 2020, 197, 104523. [Google Scholar] [CrossRef]
- Martíni, A.F.; Valani, G.P.; da Silva, L.F.S.; Bolonhezi, D.; Prima, S.D.; Cooper, M. Long-term trial of tillage systems for sugarcane: Effect on topsoil hydrophysical attributes. Sustainability 2021, 13, 3448. [Google Scholar] [CrossRef]
- Kumar, A.; Naresh, R.K.; Singh, S.; Mahajan, N.C.; Singh, O. Soil aggregation and organic carbon fractions and indices in conventional and conservation agriculture under vertisol soils of sub-tropical ecosystems: A review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2236–2253. [Google Scholar] [CrossRef]
- Zhong, W.; Shuai, Q.; Zeng, P.; Guo, Z.; Hu, K.; Wang, X.; Zeng, F.; Zhu, J.; Feng, X.; Lin, S.; et al. Effect of Ecologically Restored Vegetation Roots on the Stability of Shallow Aggregates in Ionic Rare Earth Tailings Piles. Agronomy 2023, 13, 993. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Moorman, T.B. Tillage intensity effects on soil structure indicators-A US meta-analysis. Sustainability 2020, 12, 2071. [Google Scholar] [CrossRef]
- Shoumik, B.A.A.; Islam, S. Vertical distribution of soil aggregates and associated organic carbon fractions under conventional vegetable-and rice-based tillage operations. Soil Res. 2022, 61, 83–93. [Google Scholar] [CrossRef]
- Jin, H.; Huang, S.; Shi, D.; Li, J.; Li, J.; Li, Y.; Zhu, H. Effects of Different Tillage Practices on Soil Stability and Erodibility for Red Soil Sloping Farmland in Southern China. Agronomy 2023, 13, 1310. [Google Scholar] [CrossRef]
- Knabner, I.K.; Amelung, W. Soil organic matter in major pedogenic soil groups. Geoderma 2021, 384, 114785. [Google Scholar] [CrossRef]
- Długosz, A.P.; Długosz, J.; Frąc, M.; Gryta, A.; Boruta, B.B. Enzymatic activity and functional diversity of soil microorganisms along the soil profile–A matter of soil depth and soil-forming processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Sauzet, O.; Cammas, C.; Gilliot, J.M.; Montagne, D. Long-term quantification of the intensity of clay-sized particles transfers due to earthworm bioturbation and eluviation/illuviation in a cultivated Luvisol. Geoderma 2023, 4299, 116251. [Google Scholar] [CrossRef]


| Irrigation Treatment Water | Soil Depth Layers | |||
|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | |
| Root volume (mm3) | ||||
| TSE(20 cm) | 193.99 ± 129.09 * Aa | 100.64 ± 64.36 B | 55.48 ± 40.10 BC | 37.60 ± 24.86 C |
| TSE(40 cm) | 135.14 ± 53.30 Aab | 105.10 ± 89.80 AB | 54.27 ± 28.01 BC | 29.73 ± 17.39 C |
| SRW(20 cm) | 200.44 ± 84.40 Aa | 120.45 ± 74.34 B | 59.36 ± 29.35 C | 23.15 ± 12.36 C |
| SRW(40 cm) | 74.83 ± 50.30 Bb | 85.78 ± 80.15 A | 56.70 ± 47.65 AB | 21.71 ± 20.45 B |
| NI | 165.98 ± 124.65 Aa | 125.28 ± 62.71 A | 58.48 ± 38.68 B | 44.13 ± 37.21 B |
| Root area (mm2) | ||||
| TSE(20 cm) | 1842.46 ± 1018.40 Aa | 879.18 ± 536.63 B | 512.88 ± 337.18 BC | 363.24 ± 172.04 C |
| TSE(40 cm) | 1245.77 ± 471.19 Aab | 840.35 ± 650.80 AB | 500.36 ± 167.42 BC | 308.91 ± 173.60 C |
| SRW(20 cm) | 1773.36 ± 663.35 Aa | 977.94 ± 484.56 B | 510.58 ± 170.11 BC | 233.42 ± 84.73 C |
| SRW(40 cm) | 772.18 ± 498.72 Ab | 704.20 ± 597.28 A | 455.53 ± 363.78 AB | 190.30 ± 137.23 B |
| NI | 1602.06 ± 1047.34 Aa | 1041.88 ± 403.19 B | 501.95 ± 260.11 C | 369.97 ± 230.16 C |
| Root length (mm) | ||||
| TSE(20 cm) | 2226.39 ± 1145.07 Aa | 994.98 ± 659.46 B | 620.75 ± 411.87 B | 439.98 ± 197.84 B |
| TSE(40 cm) | 1386.85 ± 577.65 Abc | 876.12 ± 657.91 AB | 613.05 ± 200.95 B | 405.46 ± 237.62 B |
| SRW(20 cm) | 1938.48 ± 757.73 Aab | 1052.65 ± 421.39 B | 577.84 ± 162.11 BC | 302.98 ± 105.19 C |
| SRW(40 cm) | 952.09 ± 567.00 Ac | 767.63 ± 578.28 AB | 498.72 ± 395.25 AB | 222.94 ± 118.72 B |
| NI | 1888.55 ± 1167.21 Aab | 1097.96 ± 303.44 B | 558.10 ± 272.46 BC | 418.46 ± 211.90 C |
| Soil density (g cm3) | ||||
| TSE(20 cm) | 1.23 ± 0.09 BC | 1.39 ± 0.15 Aa | 1.31 ± 0.05 ABa | 1.21 ± 0.03 C |
| TSE(40 cm) | 1.28 ± 0.09 AB | 1.29 ± 0.12 Aab | 1.19 ± 0.06 BCb | 1.11 ± 0.03 C |
| SRW(20 cm) | 1.27 ± 0.09 A | 1.25 ± 0.12 ABb | 1.27 ± 0.06 Aab | 1.16 ± 0.06 B |
| SRW(40 cm) | 1.29 ± 0.08 A | 1.29 ± 0.16 Aab | 1.18 ± 0.08 Bb | 1.16 ± 0.07 B |
| NI | 1.27 ± 0.09 AB | 1.30 ± 0.12 Aab | 1.18 ± 0.08 BCb | 1.16 ± 0.06 C |
| Variables | Soil Depth Layers | |||
|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | |
| PD (g cm3) | 2.62 ± 0.13 * | 2.63 ± 0.15 | 2.59 ± 0.21 | 2.62 ± 0.16 |
| MACRO-P (cm3 cm3) | 0.13 ± 0.04 a | 0.10 ± 0.04 b | 0.09 ± 0.03 b | 0.12 ± 0.04 a |
| MICRO-P (cm3 cm3) | 0.41 ± 0.03 | 0.41 ± 0.02 | 0.43 ± 0.03 | 0.41 ± 0.04 |
| TP (cm3 cm3) | 0.54 ± 0.03 a | 0.51 ± 0.04 c | 0.52 ± 0.04 bc | 0.53 ± 0.03 ab |
| WAD (mm) | 1.68 ± 0.40 a | 1.64 ± 0.51 ab | 1.43 ± 0.62 b | 1.18 ± 0.45 c |
| SA (%) | 26.80 ± 11.12 a | 26.05 ± 13.84 a | 23.14 ± 15.96 ab | 17.97 ± 10.98 b |
| A > 1 (%) | 47.89 ± 9.84 a | 46.87 ± 13.77 a | 37.66 ± 17.28 b | 29.77 ± 12.73 c |
| ASI (%) | 93.45 ± 2.73 a | 92.80 ± 3.76 a | 89.06 ± 4.81 b | 84.88 ± 5.64 c |
| DC (g kg−1) | 382.38 ± 27.34 | 417.23 ± 25.39 | 416.83 ± 49.52 | 376.27 ± 110.86 |
| TC (g kg−1) | 558.82 ± 29.26 c | 587.46 ± 25.81 b | 630.20 ± 56.71 a | 622.13 ± 26.22 a |
| DF (%) | 31.47 ± 5.00 b | 28.91 ± 6.33 b | 33.64 ± 7.90 ab | 39.56 ± 17.74 a |
| DD (%) | 68.53 ± 5.00 a | 71.09 ± 6.30 a | 66.36 ± 7.90 ab | 60.44 ± 17.73 b |
| Variables | Irrigation Treatment Water | ||||
|---|---|---|---|---|---|
| TSE 20 cm | TSE 40 cm | SRW 20 cm | SRW 40 cm | NI | |
| PD (g cm3) | 2.56 ± 0.18 * | 2.65 ± 0.21 | 2.61 ± 0.10 | 2.63 ± 0.18 | 2.64 ± 0.13 |
| MACRO-P (cm3 cm3) | 0.11 ± 0.03 | 0.11 ± 0.03 | 0.12 ± 0.04 | 0.10 ± 0.04 | 0.13 ± 0.04 |
| MICRO-P (cm3 cm3) | 0.42 ± 0.03 | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.42 ± 0.04 | 0.41 ± 0.05 |
| TP (cm3 cm3) | 0.52 ± 0.04 | 0.52 ± 0.03 | 0.54 ± 0.03 | 0.52 ± 0.05 | 0.54 ± 0.03 |
| WAD (mm) | 1.55 ± 0.61 | 1.26 ± 0.25 | 1.49 ± 0.55 | 1.52 ± 0.56 | 1.58 ± 0.61 |
| A > 1 (%) | 43.05 ± 17.19 | 35.03 ± 9.41 | 41.06 ± 15.80 | 40.36 ± 15.94 | 43.24 ± 16.85 |
| SA (%) | 24.84 ± 15.42 | 17.45 ± 5.24 | 23.72 ± 13.61 | 25.16 ± 13.70 | 26.29 ± 15.48 |
| ASI (%) | 91.21 ± 5.37 | 89.31 ± 4.19 | 90.14 ± 5.89 | 89.27 ± 6.00 | 90.29 ± 6.00 |
| DC (g kg−1) | 406.57 ± 44.04 | 404.25 ± 90.03 | 413.56 ± 42.92 | 381.44 ± 76.34 | 385.04 ± 67.27 |
| TC (g kg−1) | 597.97 ± 44.80 | 603.94 ± 32.91 | 594.81 ± 69.14 | 601.35 ± 41.85 | 600.19 ± 35.93 |
| DF (%) | 31.91 ± 6.48 | 32.89 ± 14.93 | 30.04 ± 7.48 | 36.18 ± 13.12 | 35.95 ± 10.62 |
| DD (%) | 68.09 ± 6.47 | 67.11 ± 14.93 | 69.96 ± 7.48 | 63.82 ± 13.12 | 64.05 ± 10.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Sobrinho, O.P.; Santos, L.N.S.d.; Teixeira, M.B.; Soares, F.A.L.; Gonçalves, I.Z.; Barbosa, E.A.A.; Nazário, A.A.; Matsura, E.E.; Vitorino, L.C.; Reis, M.N.O.; et al. How Does Irrigation with Wastewater Affect the Physical Soil Properties and the Root Growth of Sugarcane under Subsurface Drip? Agronomy 2024, 14, 788. https://doi.org/10.3390/agronomy14040788
Lopes Sobrinho OP, Santos LNSd, Teixeira MB, Soares FAL, Gonçalves IZ, Barbosa EAA, Nazário AA, Matsura EE, Vitorino LC, Reis MNO, et al. How Does Irrigation with Wastewater Affect the Physical Soil Properties and the Root Growth of Sugarcane under Subsurface Drip? Agronomy. 2024; 14(4):788. https://doi.org/10.3390/agronomy14040788
Chicago/Turabian StyleLopes Sobrinho, Oswaldo Palma, Leonardo Nazário Silva dos Santos, Marconi Batista Teixeira, Frederico Antônio Loureiro Soares, Ivo Zution Gonçalves, Eduardo Augusto Agnellos Barbosa, Aline Azevedo Nazário, Edson Eiji Matsura, Luciana Cristina Vitorino, Mateus Neri Oliveira Reis, and et al. 2024. "How Does Irrigation with Wastewater Affect the Physical Soil Properties and the Root Growth of Sugarcane under Subsurface Drip?" Agronomy 14, no. 4: 788. https://doi.org/10.3390/agronomy14040788






