Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Assessment of Gas Exchange and Chlorophyll-a Fluorescence
2.3. Evaluation of Water Status of Plants
2.4. Determination of Damage to Cell Membranes
2.5. Determination of Chloroplast Pigment Concentration
2.6. Statistical Analysis
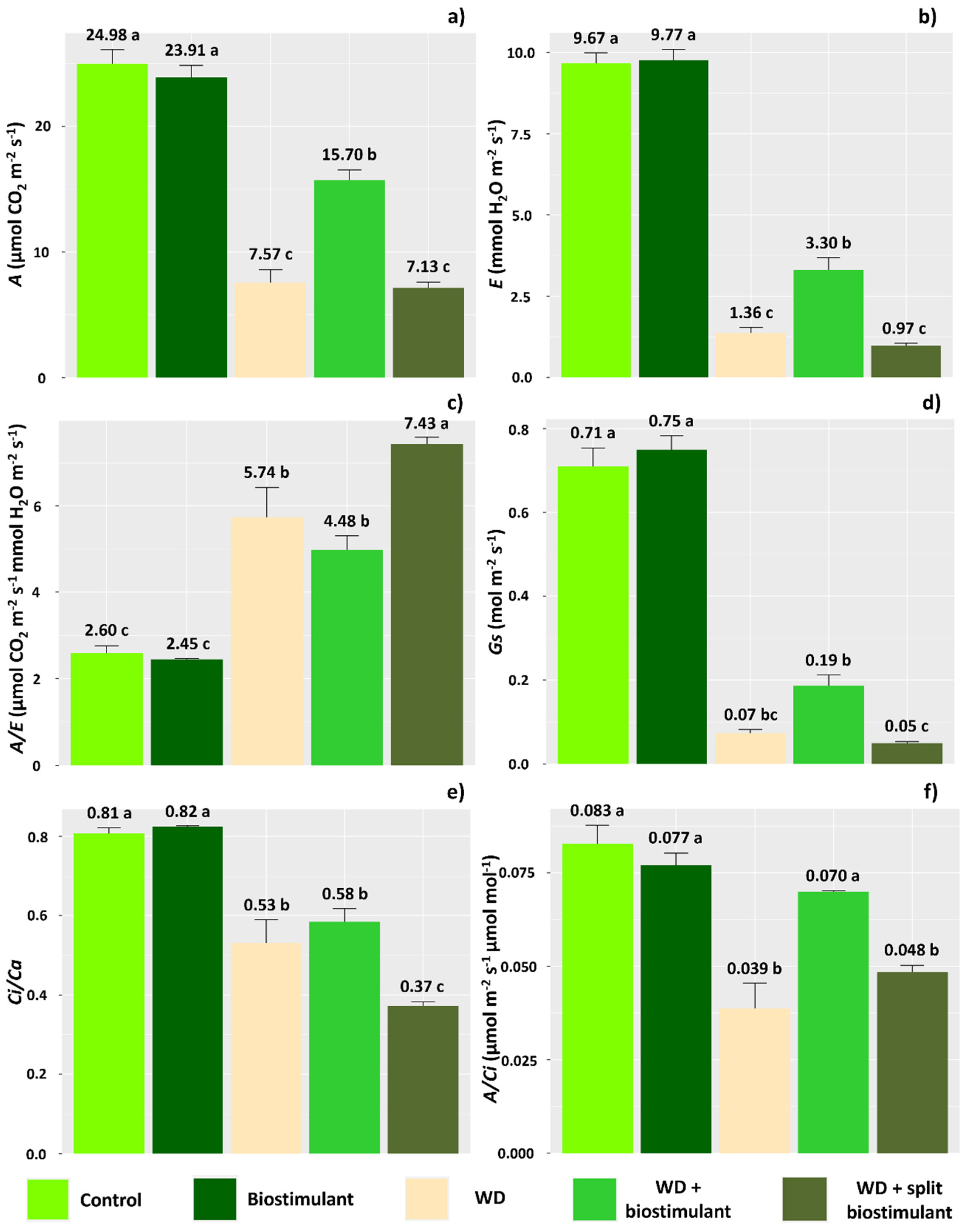
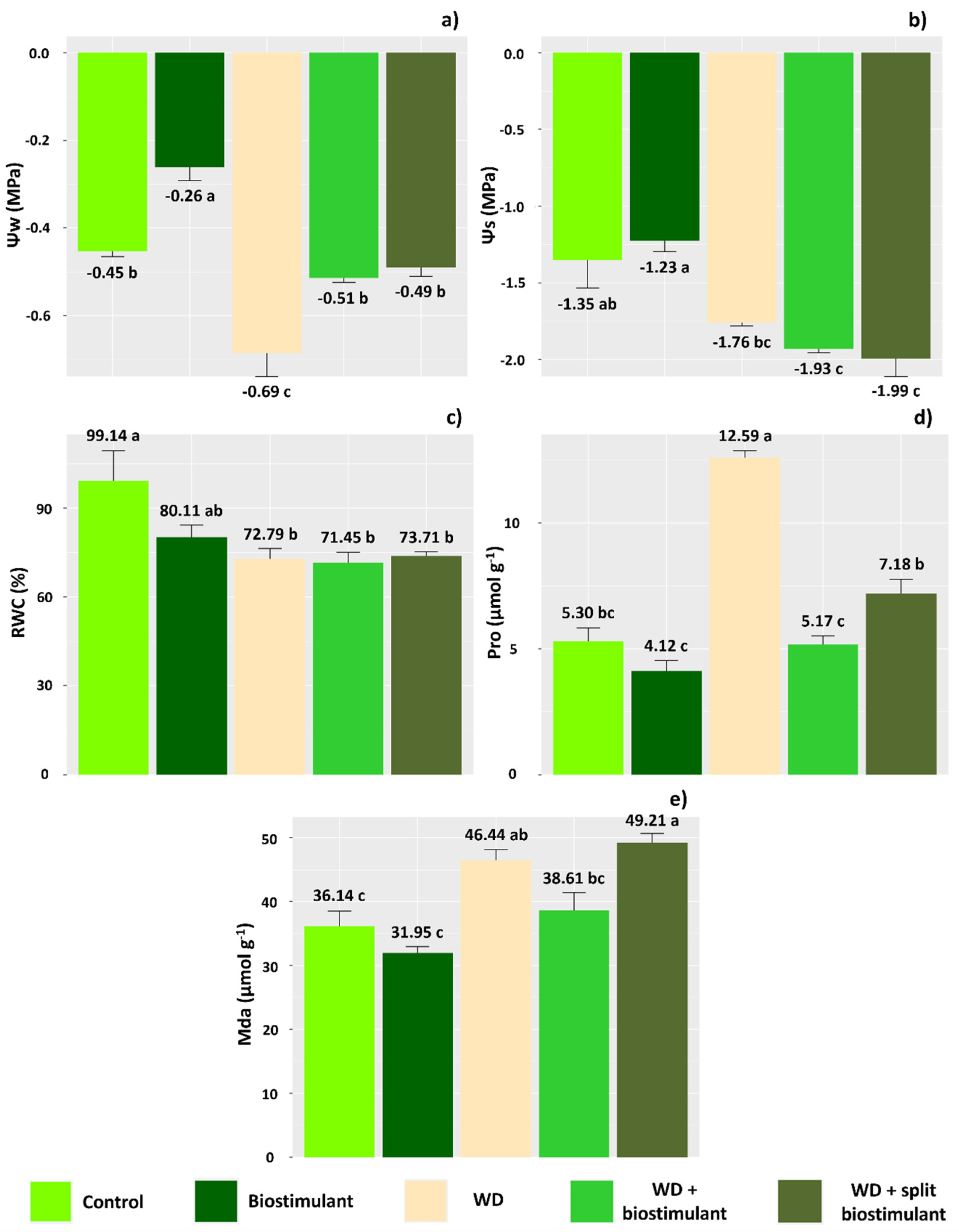
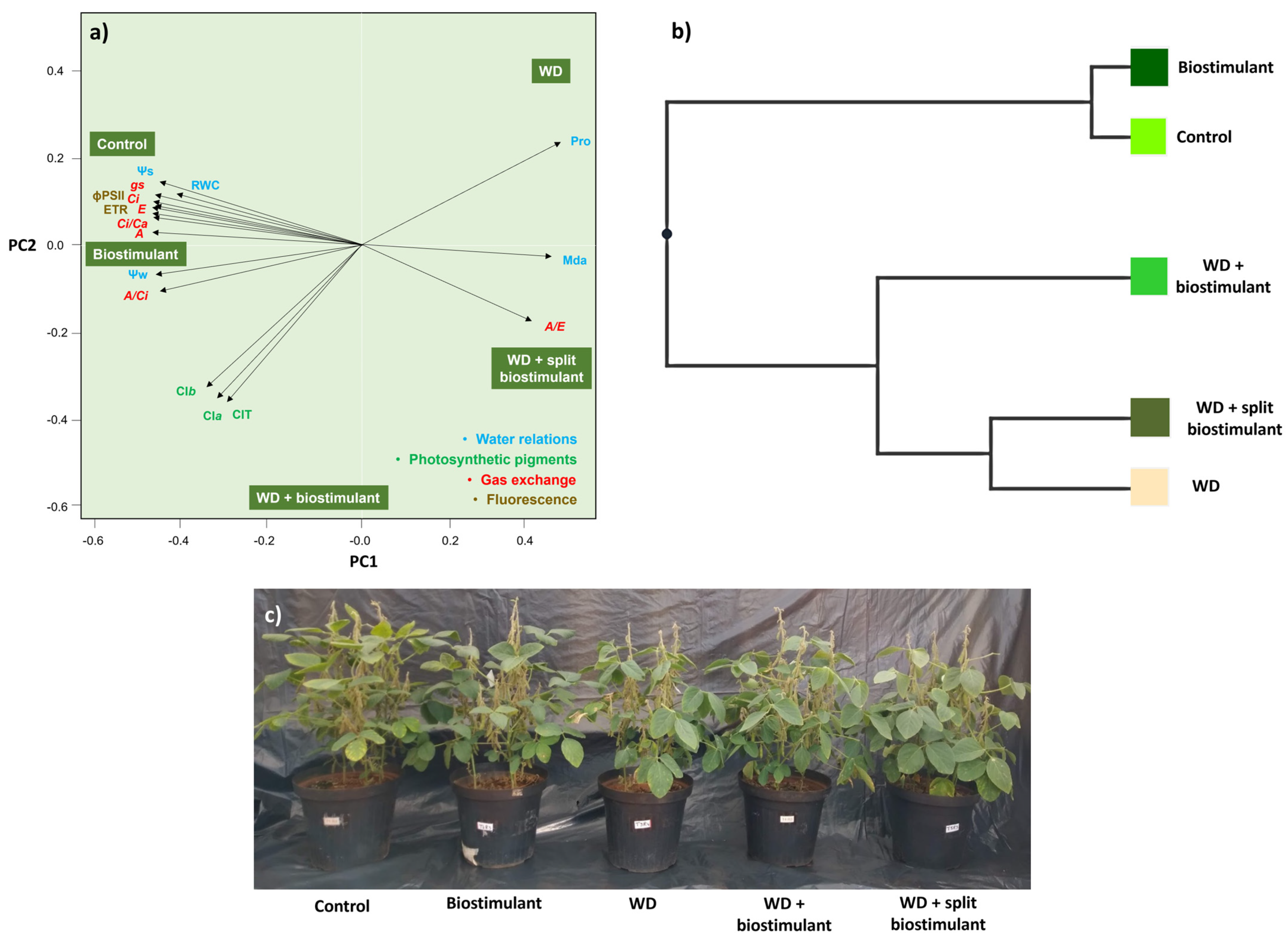
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.O.N.J.A.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Vanloocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yue, L.; Wang, C.; Zhu, X.; Wang, Z.; Xing, B. Photosynthetic response mechanisms in typical C3 and C4 plants upon La2O3 nanoparticle exposure. Environ. Sci. Nano 2020, 7, 81–92. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef] [PubMed]
- Berlato, M.A.; Matzenauer, R.; Bergamaschi, H. Evapotranspiração máxima da soja, relações com a evapotranspiração calculada pela equação de Penman, evaporação de tanque “classe A” e radiação solar global. Agron. Sulriograndense 1986, 22, 243–259. [Google Scholar]
- Farias, J.R.B.; Nepomuceno, A.L.; Neumaier, N. Ecofisiologia da Soja; Embrapa Soja: Londrina, Brazil, 2007; p. 10. [Google Scholar]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Biostimulant application, under reduced nutrient supply, enhances quality and sustainability of ornamental containerized transplants. Agronomy 2023, 13, 765. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M.A. Biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; Mckeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; De Camargo, P.R.; Gaziola, S.A.; Azevedo, R.A. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comun. Sci. 2018, 9, 292–297. [Google Scholar] [CrossRef]
- Do Rosário Rosa, V.; Dos Santos, A.L.F.; Da Silva, A.A.; Sab, M.P.V.; Germino, G.H.; Cardoso, F.B.; De Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; VanderWeide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Marques, P.A.A.; Rezende, R.; Wenneck, G.S.; Terassi, D.D.S.; Andrean, A.F.B.A.; Matumoto-Pintro, P.T. Deficit irrigation with Ascophyllum nodosum extract application as a strategy to increase tomato yield and quality. Agronomy 2023, 13, 1853. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Verdeguer, M. Effect of two biostimulants, based on Ascophyllum nodosum extracts, on strawberry performance under mild drought stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L. Modelling the impacts of climate change and crop management measures on soybean phenology in China. J. Clean. Prod. 2020, 262, 121271. [Google Scholar] [CrossRef]
- Ramteke, R.; Gupta, G.K.; Singh, D.V. Growth and yield responses of soybean to climate change. Agric. Res. 2015, 4, 319–323. [Google Scholar] [CrossRef]
- Grassini, P.; La Menza, N.C.; Edreira, J.I.R.; Monzón, J.P.; Tenorio, F.A.; Specht, J.E. Soybean. In Crop Physiology Case Histories for Major Crops; Sadras, V., Calderini, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 282–319. [Google Scholar]
- de Andrade, C.L.L.; da Silva, A.G.; Melo, G.B.; Ferreira, R.V.; Moura, I.C.S.; Siqueira, G.G. Biostimulants derived from Ascophyllum nodosum associated of glyphosate in agronomic characteristics of soybean RR®. Rev. Bras. Herbic. 2018, 17. [Google Scholar] [CrossRef]
- Esfahani, Z.; Barzegar, T.; Ghahremani, Z.; Nikbakht, J. Effects of foliar application of Megafol on yield, fruit quality and water use efficiency of tomato Cv. Rio Grande under water deficit stress. J. Crop Improv. 2018, 19, 995–1009. [Google Scholar] [CrossRef]
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação, 2nd ed.; Embrapa: Brasília, Brazil, 2004; p. 416. [Google Scholar]
- Valagro. Bioestimulantes. Available online: www.valagro.com/brazil/pt/produtos/farm/bioestimulante/megafol/ (accessed on 28 February 2019).
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Catsky, J. Water saturation deficit (relative water content). In Methods of Studying Plant Water Relations; Slavik, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 136–154. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.R.; Freire, M.B.G.S.; Cunha, K.P.V.; Nascimento, C.W.A.; Ruiz, H.A.; Lins, C.M.T. Biomass, anatomical change sand osmotic potential in Atriplex numularia L. cultivated in sodic saline soil under water stress. Environ. Exp. Bot. 2012, 82, 20–27. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco friendlier protocol for quantifying proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 27 March 2023).
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Palau, J.; Romeu, A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol. Biol. Evol. 1999, 16, 1125–1134. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef]
- Abdalla, M.; Ahmed, M.A.; Cai, G.; Wankmüller, F.; Schwartz, N.; Litig, O.; Carminati, A. Stomatal closure during water deficit is controlled by below-ground hydraulics. Ann. Bot. 2022, 129, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Gu, L.; Shi, Y.; Chen, H.; Liu, Y.; Lu, F.; Zhou, G. Plant hydraulic conductivity determines photosynthesis in rice under PEG-induced drought stress. Pak. J. Bot. 2021, 53, 409–417. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.; Safdar, M.E.; Brestic, M.; Liu, J. Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme system under a split-root system. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef]
- Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem. Soc. Trans. 2021, 49, 2007–2019. [Google Scholar] [CrossRef]
- Wijewardene, I.; Shen, G.; Zhang, H. Enhancing crop yield by using Rubisco activase to improve photosynthesis under elevated temperatures. Stress Biol. 2021, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, G.H. The carboxylation and oxygenation of ribulose 1, 5-bisphosphate: The primary events in photosynthesis and photorespiration. Ann. Rev. Plant Physiol. 1981, 32, 349–382. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy 2019, 10, 6. [Google Scholar] [CrossRef]
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a seaweed extract strongly improves drought tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Santos, C.C.; Silva, A.A.S.; Carvalho de Oliveira, C.H.; Silverio, J.M.; dos Santos Dias, A.; Linné, J.A.; Scalon, S.P.Q.; Alovisi, A.M.T. Ascophyllum nodosum seaweed extract in Inga edulis seedlings under drought and the potential of phenotypic plasticity. J. Appl. Phycol. 2023, 35, 3123–3135. [Google Scholar] [CrossRef]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as biofertilizer in modern agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Biostimulants Market by Active Ingredient (Humic Substances, Amino Acids, Seaweed Extracts, Microbial Amendments), Crop Type (Fruits & Vegetables, Cereals, Turf & Ornamentals), Application Method, Form, and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html?gclid=CjwKCAjw4_H6BRALEiwAvgfzq1LVX47L4C4O0v0leN5GfYGuk0xW2oF25JDZhWGs03E3I2rL1kEwGxoCnsAQAvD_BwE (accessed on 11 October 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, G.B.; da Silva, A.G.; da Costa, A.C.; Alves da Silva, A.; Rosa, M.; Bessa, L.A.; Rodrigues, C.R.; Castoldi, G.; Vitorino, L.C. Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean. Agronomy 2024, 14, 414. https://doi.org/10.3390/agronomy14030414
Melo GB, da Silva AG, da Costa AC, Alves da Silva A, Rosa M, Bessa LA, Rodrigues CR, Castoldi G, Vitorino LC. Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean. Agronomy. 2024; 14(3):414. https://doi.org/10.3390/agronomy14030414
Chicago/Turabian StyleMelo, Gabriel Bressiane, Alessandro Guerra da Silva, Alan Carlos da Costa, Adnan Alves da Silva, Márcio Rosa, Layara Alexandre Bessa, Carlos Ribeiro Rodrigues, Gustavo Castoldi, and Luciana Cristina Vitorino. 2024. "Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean" Agronomy 14, no. 3: 414. https://doi.org/10.3390/agronomy14030414
APA StyleMelo, G. B., da Silva, A. G., da Costa, A. C., Alves da Silva, A., Rosa, M., Bessa, L. A., Rodrigues, C. R., Castoldi, G., & Vitorino, L. C. (2024). Foliar Application of Biostimulant Mitigates Water Stress Effects on Soybean. Agronomy, 14(3), 414. https://doi.org/10.3390/agronomy14030414






