Metabolomics Analysis of Variation in Grain Quality of High-Quality Japonica Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials and Field Design
2.2. Sample Collection
2.3. Determination of Agronomic Traits and Yield Traits
2.4. Determination of Rice Quality
2.5. Determination of Starch Extraction Content
2.6. Determination of Swelling Power and Water Solubility
Swelling power (g/g) = (m2 − m1)/(m3 − m1).
2.7. DNA Marker Analysis
2.8. Determination of 2-AP Content
2.9. Sample Metabolite Obtaining
2.10. LC-MS/MS Analysis
2.11. Metabolome Data Analysis
2.12. Statistical Analysis
3. Results
3.1. Analysis of Agronomic Traits and Yield Traits between Five Lines and Control Varieties
3.2. Analysis of Rice Quality Traits Was between Five Lines and Control Varieties
3.3. Multivariate Statistical Analysis
3.4. Metabolic Profiling
3.5. KEGG Pathway
3.6. Correlation Analysis among Rice Quality Character and Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Ma, H.; Chen, Z.; Chen, X.; Liu, G.; Hu, Q.; Xu, F.; Wei, H.; Zhang, H. Quality Characteristics of Japonica Rice in Southern and Northern China and the Effect of Environments on Its Quality. Agronomy 2022, 12, 2757. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhang, Y.D.; Zhao, C.F.; Wei, X.D.; Yao, S.; Zhou, L.H.; Zhu, Z.; Chen, T.; Zhao, Q.Y.; Zhao, L.; et al. Inheritance and breeding of japonica rice with good eating quality in Jiangsu province. Yi Chuan 2021, 43, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, L.; Sun, J.; Xu, Q.; Xu, Z.; Chen, W. Contribution and Prospect of Erect Panicle Type to japonica Super Rice. Rice Sci. 2021, 28, 431–441. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, D.; Li, Q.; Gu, M.; Liu, Q. Progresses in Research on Cloning and Functional Analysis of Key Genes Involving in Rice Grain Quality. Sci. Agric. Sin. 2016, 49, 4267–4283. [Google Scholar] [CrossRef]
- Li, S.; Ren, X.; Zhang, M.; Asimi, S.; Lv, Q.; Wang, Z.; Liang, S.; Wang, Z.; Meng, L. New perspective to guide rice breeding: Evaluating the eating quality of japonica rice. Cereal Chem. 2022, 99, 603–614. [Google Scholar] [CrossRef]
- Ch, R.; Chevallier, O.; McCarron, P.; McGrath, T.F.; Wu, D.; Nguyen Doan Duy, L.; Kapil, A.P.; McBride, M.; Elliott, C.T. Metabolomic fingerprinting of volatile organic compounds for the geographical discrimination of rice samples from China, Vietnam and India. Food Chem. 2021, 334, 127553. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Oh, Y.; Kim, T.H.; Cho, Y.H. Quantitation of 2-acetyl-1-pyrroline in aseptic-packaged cooked fragrant rice by HS-SPME/GC-MS. Food Sci. Nutr. 2019, 7, 266–272. [Google Scholar] [CrossRef]
- Okpala, N.E.; Mo, Z.; Duan, M.; Tang, X. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem. 2019, 135, 272–276. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, S.; Zhou, L.; Tao, Y.; Tian, J.; Xing, Z.; Wei, H.; Zhang, H. Characteristics of Population Quality and Rice Quality of Semi-Waxy japonica Rice Varieties with Different Grain Yields. Agriculture 2022, 12, 241. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, F.; Chen, Y.; Fan, Y.; Zhang, K.; Liu, Q.; Tu, W.; Jiang, F.; Li, G.; Zhao, H.; et al. Salicylic Acid Improves the Constitutive Freezing Tolerance of Potato as Revealed by Transcriptomics and Metabolomics Analyses. Int. J. Mol. Sci. 2022, 24, 609. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Nie, Q.; Huang, M.; Luo, C.; Sun, Y.; Ma, Y.; Yu, J.; Du, F. HPLC-based metabolomics of Dendrobium officinale revealing its antioxidant ability. Front. Plant Sci. 2023, 14, 1060242. [Google Scholar] [CrossRef]
- Qu, S.L.; Xie, J.; Wang, J.T.; Li, G.H.; Pan, X.R.; Zhao, P.J. Activities and metabolomics of Cordyceps gunnii under different culture conditions. Front. Microbiol. 2022, 13, 1076577. [Google Scholar] [CrossRef]
- Yu, X.; Qi, Q.; Li, Y.; Li, N.; Xie, Y.; Ding, A.; Shi, J.; Du, Y.; Liu, X.; Zhang, Z.; et al. Metabolomics and proteomics reveal the molecular basis of colour formation in the pericarp of Chinese wild rice (Zizania latifolia). Food Res. Int. 2022, 162, 112082. [Google Scholar] [CrossRef]
- Hu, C.; Shi, J.; Quan, S.; Cui, B.; Kleessen, S.; Nikoloski, Z.; Tohge, T.; Alexander, D.; Guo, L.; Lin, H.; et al. Metabolic variation between japonica and indica rice cultivars as revealed by non-targeted metabolomics. Sci. Rep. 2014, 4, 5067. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.C.T.; Calingacion, M.; Garson, M.J.; Fitzgerald, M.A. Lipidomics reveals associations between rice quality traits. Metabolomics 2020, 16, 54. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, L.; Shao, K.; Gu, M.; Liu, Q. Toward underlying reasons for rice starches having low viscosity and high amylose: Physiochemical and structural characteristics. J. Sci. Food Agric. 2013, 93, 1543–1551. [Google Scholar] [CrossRef]
- Konik-Rose, C.M.; Moss, R.; Rahman, S.; Appels, R.; Stoddard, F.; McMaster, G. Evaluation of the 40 mg Swelling Test for Measuring Starch Functionality. Starch Stärke 2001, 53, 14–20. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Chen, Z.; Zhong, W. Development and Application of Fragrance Gene Markers in Rice. Mol. Plant Breed. 2008, 6, 1209–1212. [Google Scholar]
- Lu, Y.; Zhang, K. A Simple Method for Isolation of Rice DNA. Chin. J. Rice Sci. 1992, 6, 47–48. [Google Scholar]
- Shi, Y.; Chen, J.; Liu, W.; Huang, Q.; Shen, B.; Leung, H.; Wu, J. Genetic analysis and gene mapping of a new rolled-leaf mutant in rice (Oryza sativa L.). Sci. China C Life Sci. 2009, 52, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, Y.; Zhang, R.; Liu, G.; Wei, H.; Zhang, H.; Zhang, H. Mid-stage nitrogen application timing regulates yield formation, quality traits and 2-acetyl-1-pyrroline biosynthesis of fragrant rice. Field Crops Res. 2022, 287, 108667. [Google Scholar] [CrossRef]
- Xiong, Q.; Sun, C.; Li, A.; Zhang, J.; Shi, Q.; Zhang, Y.; Hu, J.; Zhou, N.; Wei, H.; Liu, B.; et al. Metabolomics and biochemical analyses revealed metabolites important for the antioxidant properties of purple glutinous rice. Food Chem. 2022, 389, 133080. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Chauhan, B.S.; Khan, F.; Ihsan, M.Z.; Ullah, A.; Wu, C.; Bajwa, A.A.; et al. Responses of Rapid Viscoanalyzer Profile and Other Rice Grain Qualities to Exogenously Applied Plant Growth Regulators under High Day and High Night Temperatures. PLoS ONE 2016, 11, e0159590. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, E.; Li, C.; Cai, M.; Cheng, B.; Cao, C.; Jiang, Y. Use of Protein Content, Amylose Content, and RVA Parameters to Evaluate the Taste Quality of Rice. Front. Nutr. 2022, 8, 758547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ji, Y.; Li, E. Understanding the Influences of Rice Starch Fine Structure and Protein Content on Cooked Rice Texture. Starch Stärke 2022, 74, 2100253. [Google Scholar] [CrossRef]
- Starkenmann, C.; Niclass, Y.; Vuichoud, B.; Schweizer, S.; He, X.F. Occurrence of 2-Acetyl-1-pyrroline and Its Nonvolatile Precursors in Celtuce (Lactuca sativa L. var. augustana). J. Agric. Food Chem. 2019, 67, 11710–11717. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Lacina, O.; Hajslova, J.; Zweigenbaum, J. The use of high performance liquid chromatography-quadrupole time-of-flight mass spectrometry coupled to advanced data mining and chemometric tools for discrimination and classification of red wines according to their variety. Anal. Chim. Acta 2011, 685, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ron, G.B.; Louisa, C.L.; Bienvenido, O.J.; Jean, G.T. Cooked Rice Aroma and 2-Acetyl-1-pyrroline. Food Chem. 1983, 31, 823–826. [Google Scholar]
- Zotti, M.; De Pascali, S.A.; Del Coco, L.; Migoni, D.; Carrozzo, L.; Mancinelli, G.; Fanizzi, F.P. 1H NMR metabolomic profiling of the blue crab (Callinectes sapidus) from the Adriatic Sea (SE Italy): A comparison with warty crab (Eriphia verrucosa), and edible crab (Cancer pagurus). Food Chem. 2016, 196, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chan, P.H.; Lam, C.T.; Li, Z.; Lam, K.Y.; Yao, P.; Dong, T.T.; Lin, H.; Lam, H.; Tsim, K.W. Fruit of Ziziphus jujuba (Jujube) at two stages of maturity: Distinction by metabolic profiling and biological assessment. J. Agric. Food Chem. 2015, 63, 739–744. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Zhao, L.; Liu, J.; Shang, B.; Yang, W.; Duan, X.; Sun, H. Metabolomic Analysis Reveals Insights into Deterioration of Rice Quality during Storage. Foods 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
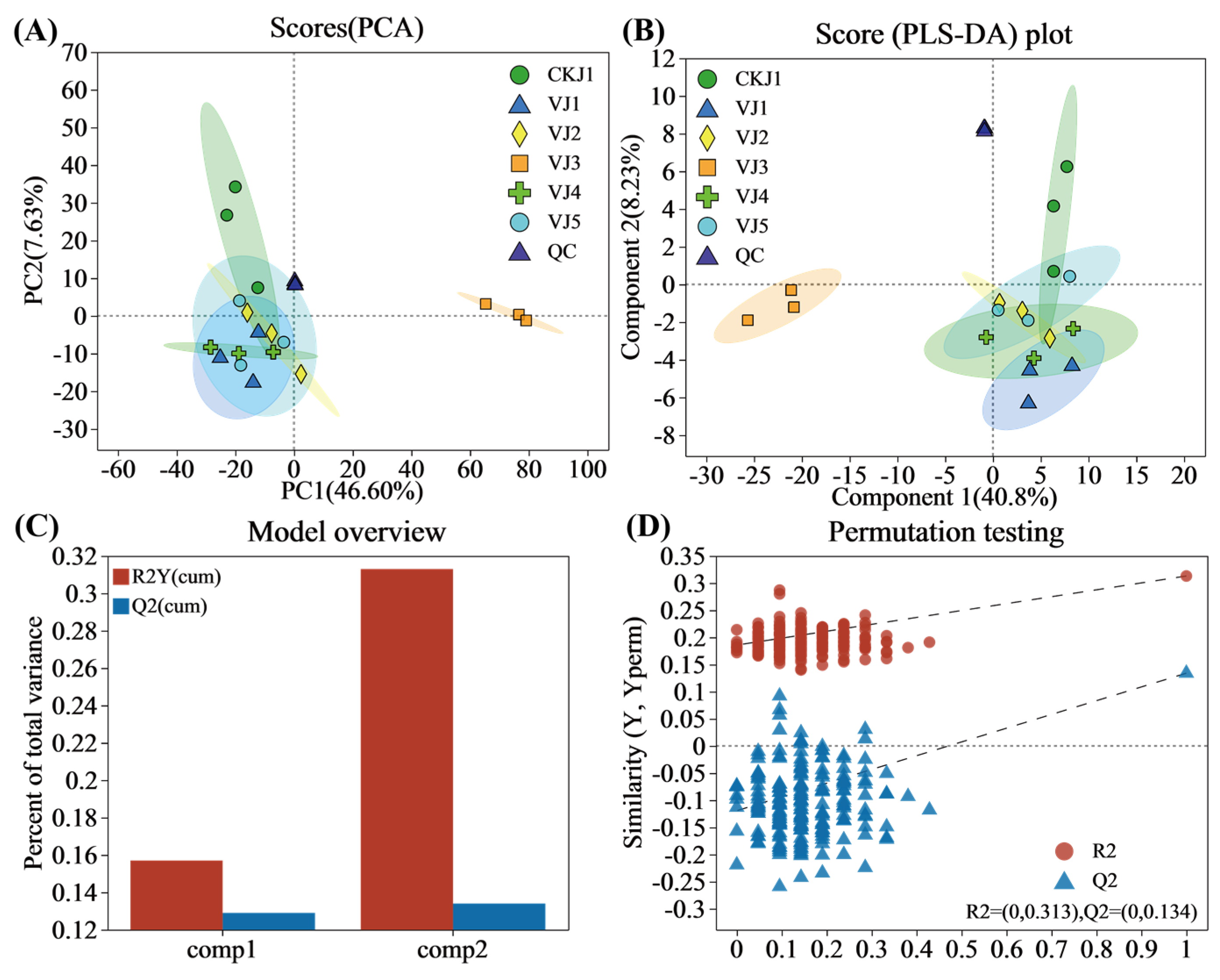

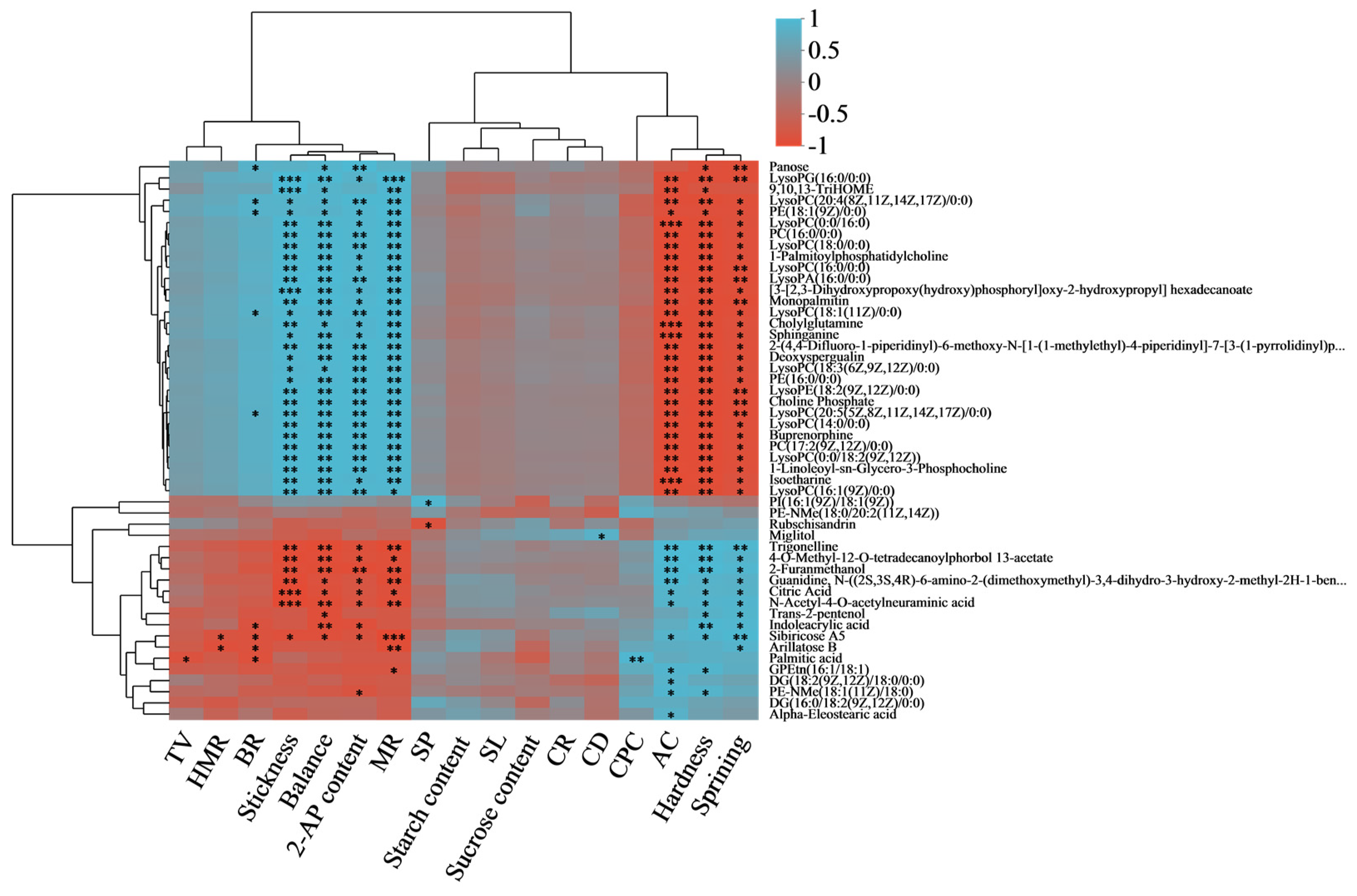
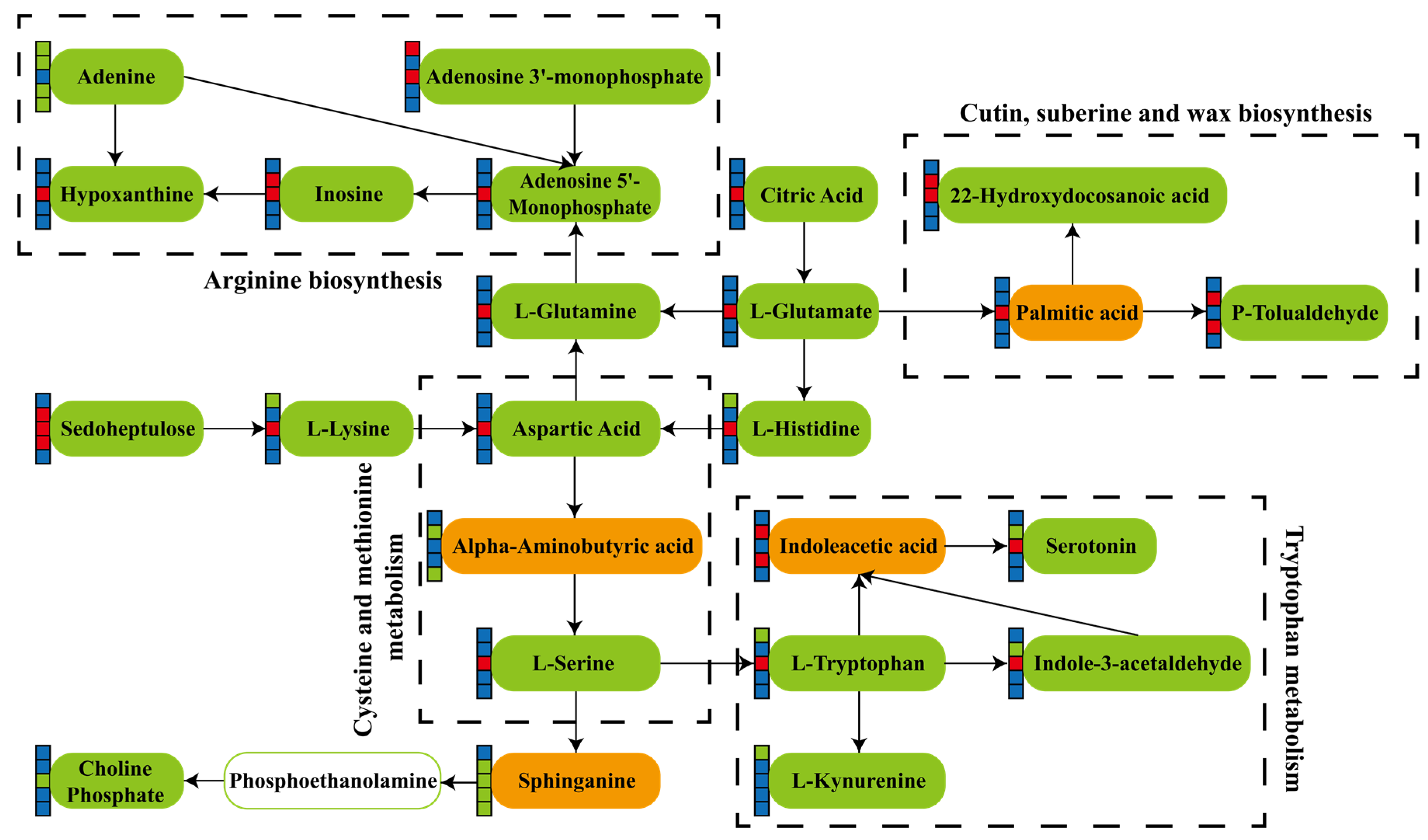
| Year | Cultivar | Heading Date | Maturation Date | Plant Height (cm) | Number of Panicles Per Hill (No.) | Number of Spikelets Per Panicle (No.) | Percentage of Filled Grains (%) | 1000-Grain Weight (g) |
|---|---|---|---|---|---|---|---|---|
| 2021 | CKJ1 | August 25 | October 12 | 96.82 ± 0.99 ab | 5.00 ± 1.41 a | 107.36 ± 1.62 a | 84.85 ± 1.09 a | 23.90 ± 0.23 a |
| VJ1 | August 26 | October 13 | 96.40 ± 1.12 b | 4.80 ± 1.10 a | 109.42 ± 3.05 a | 86.35 ± 2.05 a | 23.87 ± 0.39 a | |
| VJ2 | August 25 | October 13 | 97.16 ± 0.63 a | 5.40 ± 1.14 a | 108.72 ± 2.69 a | 85.22 ± 2.26 a | 23.81 ± 0.30 a | |
| VJ3 | August 25 | October 14 | 96.52 ± 0.83 ab | 5.20 ± 1.30 a | 106.42 ± 1.44 a | 86.86 ± 2.10 a | 24.02 ± 0.35 a | |
| VJ4 | August 24 | October 12 | 97.10 ± 0.46 ab | 5.00 ± 0.71 a | 109.60 ± 3.55 a | 86.20 ± 2.07 a | 23.98 ± 0.39 a | |
| VJ5 | August 26 | October 13 | 97.00 ± 0.71 ab | 5.40 ± 1.14 a | 107.60 ± 2.96 a | 85.58 ± 1.40 a | 23.72 ± 0.18 a | |
| 2022 | CKJ1 | August 27 | October 13 | 95.26 ± 0.66 a | 6.40 ± 1.14 a | 110.52 ± 3.21 a | 85.88 ± 1.92 a | 24.25 ± 0.33 a |
| VJ1 | August 27 | October 12 | 94.84 ± 1.38 a | 6.60 ± 0.55 a | 109.86 ± 2.64 a | 85.45 ± 1.54 a | 24.21 ± 0.21 a | |
| VJ2 | August 28 | October 13 | 95.88 ± 0.83 a | 6.00 ± 1.87 a | 109.28 ± 3.68 a | 85.84 ± 2.45 a | 24.24 ± 0.24 a | |
| VJ3 | August 28 | October 14 | 95.10 ± 1.07 a | 6.40 ± 1.14 a | 109.76 ± 4.54 a | 84.60 ± 1.27 a | 24.29 ± 0.37 a | |
| VJ4 | August 25 | October 11 | 94.78 ± 1.07 a | 7.00 ± 1.41 a | 112.26 ± 1.93 a | 85.12 ± 1.52 a | 24.17 ± 0.19 a | |
| VJ5 | August 26 | October 14 | 94.16 ± 0.90 a | 6.20 ± 1.30 a | 109.66 ± 3.36 a | 86.15 ± 1.53 a | 24.32 ± 0.37 a | |
| Analysis of variance | ||||||||
| Year | ** | ** | * | NS | ** | |||
| Cultivar | NS | NS | NS | NS | NS | |||
| Year × Cultivar | NS | NS | NS | NS | NS | |||
| Varieties | BR (%) | MR (%) | HMR (%) | CR | CD | TV | Hardness (g) | Sprining (%) | Stickiness (g) | Balance | Starch Content (mg/g) | AC (%) | CPC (g/kg) | Sucrose Content (mg/g) | 2-AP Content (ng/g) | SL | SP | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CKJ1 | 0.854 ab | 0.693 ab | 0.627 b | 87.107 ab | 44.270 a | 76.267 b | 122.096 b | 0.489 b | −913.696 b | −0.212 b | 480.527 bc | 0.107 e | 60.202 a | 12.818 a | 51.534 a | 0.06 b | 8.903 a | N |
| VJ1 | 0.858 a | 0.679 bc | 0.586 c | 86.123 bc | 37.043 cd | 83.667 a | 108.592 b | 0.464 b | −869.64 b | −0.232 b | 540.858 a | 0.139 c | 60.294 a | 12.623 a | 61.366 a | 0.078 a | 9.219 a | N |
| VJ2 | 0.855 ab | 0.702 a | 0.655 a | 76.557 d | 29.820 e | 84.433 a | 117.814 b | 0.466 b | −955.816 b | −0.240 b | 446.612 c | 0.127 d | 63.802 a | 11.974 ab | 39.218 b | 0.038 c | 8.951 a | N |
| VJ3 | 0.846 c | 0.606 d | 0.531 d | 85.420 c | 37.770 c | 75.200 b | 184.925 a | 0.560 a | −725.440 a | −0.144 a | 509.786 ab | 0.222 a | 65.358 a | 12.144 ab | 0c | 0.061 b | 9.112 a | P |
| VJ4 | 0.851 b | 0.674 c | 0.543 d | 87.400 ab | 36.517 d | 76.967 b | 122.756 b | 0.488 b | −958.134 b | −0.230 b | 521.005 ab | 0.125 d | 66.369 a | 11.076 b | 51.455 a | 0.057 b | 9.566 a | N |
| VJ5 | 0.857 a | 0.697 a | 0.619 b | 88.093 a | 39.353 b | 75.567 b | 123.107 b | 0.459 b | −938.624 b | −0.222 b | 482.712 bc | 0.146 b | 62.219 a | 12.821 a | 61.12 a | 0.057 b | 9.431 a | N |
| AC | Sucrose Content | CPC | TV | Hardness | Sprining | Stickiness | Balance | SL | SP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Starch Content | 0.259 | −0.148 | 0.021 | −0.228 | 0.093 | 0.217 | 0.386 | 0.187 | 0.875 * | 0.511 |
| AC | −0.022 | 0.426 | −0.417 | 0.904 * | 0.793 | 0.891 * | 0.872 * | 0.162 | 0.034 | |
| Sucrose Content | −0.862 * | 0.624 | −0.112 | −0.210 | 0.158 | 0.071 | 0.337 | −0.432 | ||
| CPC | −0.787 | 0.509 | 0.488 | 0.136 | 0.306 | −0.434 | 0.420 | |||
| TV | −0.640 | −0.696 | −0.299 | −0.559 | 0.098 | −0.413 | ||||
| Hardness | 0.952 ** | 0.854 * | 0.969 ** | −0.012 | −0.113 | |||||
| Sprining | 0.850 * | 0.946 ** | 0.087 | −0.168 | ||||||
| Stickiness | 0.915 * | 0.406 | −0.231 | |||||||
| Balance | 0.176 | −0.179 | ||||||||
| SL | 0.233 |
| Pathway Description | Pathway_ID | Ratio_in_Pop | p-Value | Metabolites |
|---|---|---|---|---|
| KEGG pathways between VJ1 and CKJ1 | ||||
| Cysteine and Methionine Metabolism | map00270 | 64/4834 | 0.2216 | C00041 |
| Tryptophan metabolism | map00380 | 83/4834 | 0.1005 | C00078; C00328 |
| KEGG pathways between VJ2 and CKJ1 | ||||
| Cutin, suberine, and wax biosynthesis | map00073 | 27/4834 | 0.1431 | C19623 |
| Cysteine and Methionine Metabolism | map00270 | 64/4834 | 0.1225 | C00041; C02356 |
| Tryptophan metabolism | map00380 | 83/4834 | 0.0465 | C00780; C00637; C00954 |
| Glycerophospholipid metabolism | map00564 | 56/4834 | 0.1893 | C04230 |
| KEGG pathways between VJ3 and CKJ1 | ||||
| Cutin, suberine, and wax biosynthesis | map00073 | 27/4834 | 0.1127 | C00249; C19623 |
| Tryptophan metabolism | map00380 | 83/4834 | 0.1748 | C00780; C00078; C00637 |
| Arginine Biosynthesis | map00220 | 23/4834 | 0.0231 | C00025; C00049; C00064 |
| Cysteine and Methionine Metabolism | map00270 | 64/4834 | 0.0353 | C00073; C00049; C00170; C00065 |
| Glycerophospholipid metabolism | map00564 | 56/4834 | 0.0316 | C00065; C00588; C04230; C00350 |
| KEGG pathways between VJ4 and CKJ1 | ||||
| Cutin, suberine, and wax biosynthesis | map00073 | 27/4834 | 0.1661 | C19623 |
| Tryptophan metabolism | map00380 | 83/4834 | 0.3076 | C00954 |
| Cysteine and Methionine Metabolism | map00270 | 64/4834 | 0.0979 | C00041; C00170 |
| Metabolism of Glycerophospholipid | map00564 | 56/4834 | 0.0914 | C04230; C00350 |
| KEGG pathways between VJ5 and CKJ1 | ||||
| Zeatin biosynthesis | map00908 | 39/4834 | 0.0326 | C00147; C15546 |
| Metabolism of Linoleic acid | map00591 | 28/4834 | 0.0338 | C14827; C14828 |
| Cysteine and Methionine Metabolism | map00270 | 64/4834 | 0.0567 | C00073; C02356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Wang, R.; Lu, W.; Zhu, J.; Zhang, H.; Xiong, Q.; Zhou, N. Metabolomics Analysis of Variation in Grain Quality of High-Quality Japonica Rice. Agronomy 2024, 14, 430. https://doi.org/10.3390/agronomy14030430
Shi Q, Wang R, Lu W, Zhu J, Zhang H, Xiong Q, Zhou N. Metabolomics Analysis of Variation in Grain Quality of High-Quality Japonica Rice. Agronomy. 2024; 14(3):430. https://doi.org/10.3390/agronomy14030430
Chicago/Turabian StyleShi, Qiang, Ruizhi Wang, Wenjie Lu, Jinyan Zhu, Hongcheng Zhang, Qiangqiang Xiong, and Nianbing Zhou. 2024. "Metabolomics Analysis of Variation in Grain Quality of High-Quality Japonica Rice" Agronomy 14, no. 3: 430. https://doi.org/10.3390/agronomy14030430
APA StyleShi, Q., Wang, R., Lu, W., Zhu, J., Zhang, H., Xiong, Q., & Zhou, N. (2024). Metabolomics Analysis of Variation in Grain Quality of High-Quality Japonica Rice. Agronomy, 14(3), 430. https://doi.org/10.3390/agronomy14030430





