Susceptibility of Fall Armyworm Field Populations to Vip3Aa/Cry Bt Maize in a Tropical Agricultural Region
Abstract
:1. Introduction
2. Material and Methods
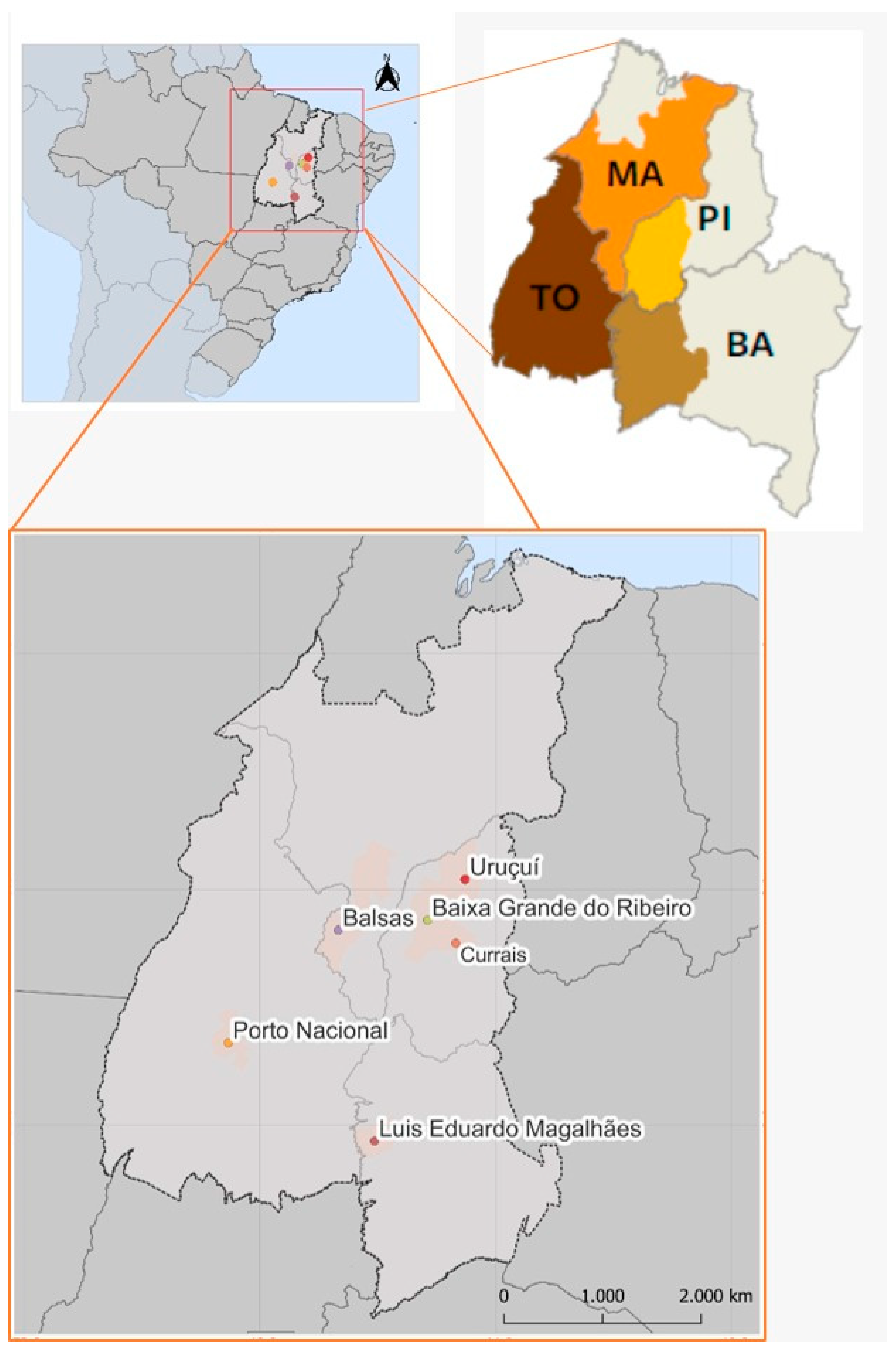
2.1. Insect Populations
2.2. Bt Maize Hybrids
2.3. Susceptibility to Vip3Aa/Cry Proteins
2.4. Data Analysis
3. Results
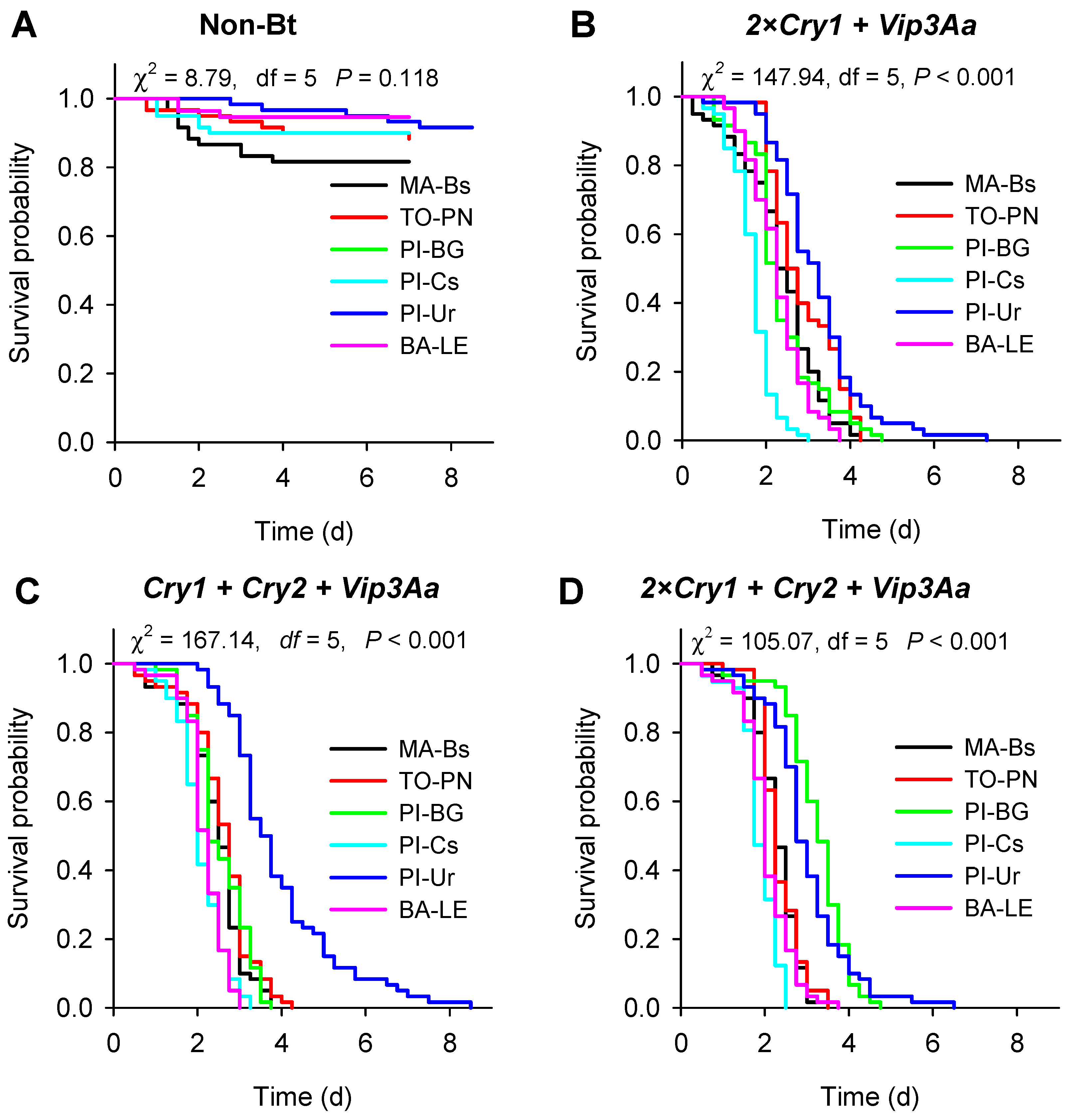
3.1. Larval Mortality after Seven Days
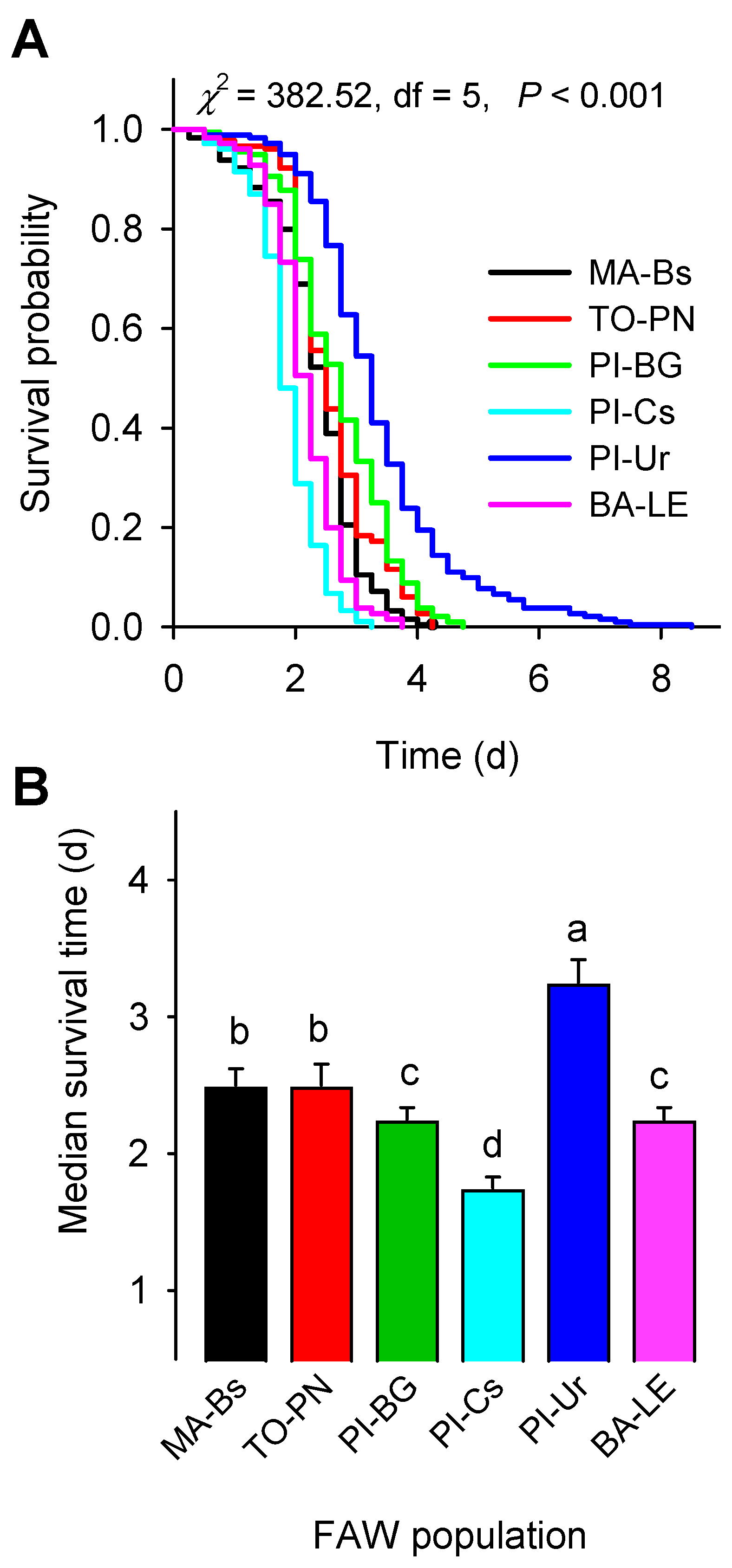
3.2. Survival Schedule as Affected by Bt Proteins and FAW Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- FAO. Manual on Integrated Fall Armyworm Management; FAO: Rome, Italy, 2020; ISBN 978-92-5-132903-0. [Google Scholar]
- Fatoretto, J.C.; Michel, A.P.; Filho, M.C.S.; Silva, N. Adaptive potential of fall armyworm (Lepidoptera: Noctuidae) limits Bt trait durability in Brazil. J. Integr. Pest Manag. 2017, 8, 17. [Google Scholar] [CrossRef]
- Mitchell, E.R.; McNeil, J.N.; Westbrook, J.K.; Silvain, J.F.; Lalanne-Cassou, B.; Chalfant, R.B.; Pair, S.D.; Waddill, V.H.; Sotomayor-Rios, A.; Proshold, F.I. Seasonal periodicity of fall armyworm, (Lepidoptera: Noctuidae) in the Caribbean basin and northward to Canada. J. Entomol. Sci. 1991, 26, 39–50. [Google Scholar] [CrossRef]
- Ministério da Agricultura e Pecuária (MAPA). 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/politica-agricola/todas-publicacoes-de-politica-agricola/projecoes-do-agronegocio/projecoes-do-agronegocio-2022-2023-a-2032-2033.pdf/ (accessed on 16 January 2024).
- Companhia Nacional de Abastecimento (CONAB). 2022. Available online: https://www.gov.br/fazenda/pt-br/central-de-conteudo/publicacoes/conjuntura-economica/agricola/2022/2022-07-07_levantamento-de-safras_conab.pdf (accessed on 16 January 2024).
- Schuster, I.; Rodrigues, R.A.O.; Linares, E. Genetically modified corn in Brazil: Historical, results and perspectives. Rev. Bras. Milho Sorgo 2022, 21, 1238. [Google Scholar] [CrossRef]
- Céleres. 2017. Available online: http://www.celeres.com.br/3o-levantamento-de-adocao-da-biotecnologia-agricola-no-brasil-safra-201617/ (accessed on 16 January 2024).
- Marques, L.H.; Santos, A.C.; Castro, B.A.; Moscardini, V.F.; Rosseto, J.; Silva, O.A.B.N.; Babcock, J.M. Assessing the efficacy of Bacillus thuringiensis (Bt) pyramided proteins Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 expressed in Bt maize against lepidopteran pests in Brazil. J. Econ. Entomol. 2019, 21, 803–811. [Google Scholar] [CrossRef]
- International Service for the Acquisition of Agri-Biotech Applications (ISAAA). GM Approval Database. 2023. Available online: http://www.isaaa.org/gmapprovaldatabase/default.asp (accessed on 31 December 2023).
- Storer, N.P.; Kubiszak, M.E.; Ed King, J.; Thompson, G.D.; Santos, A.C. Status of resistance to Bt maize in Spodoptera frugiperda: Lessons from Puerto Rico. J. Invertebr. Pathol. 2012, 110, 294–300. [Google Scholar] [CrossRef] [PubMed]
- EPA (U.S. Environmental Protection Agency). EPA Office of Pesticide Programs, Biopesticides and Pollution Prevention Division. Bacillus thuringiensis Cry1A.105 and Cry2Ab2 Insecticidal Proteins and the Genetic Material Necessary for Their Production in Corn [PC Codes 006515 (Cry2Ab2), 006514 (Cry1A.105)]. Biopesticide Registration Action. 2010. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006514_30-Sep-10.pdf (accessed on 31 December 2023).
- Carrière, Y.; Fabrick, J.A.; Tabashnik, B.E. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016, 34, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Sánchez, J.; Muñoz-Garay, C.; Matus, V.; Gill, S.S.; Soberón, M.; Bravo, A. Bacillus thuringiensis Cry1A toxins are versatile proteins with multiple modes of action: Two distinct pre-pores are involved in toxicity. Biochem. J. 2014, 459, 383–396. [Google Scholar] [CrossRef]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus thuringiensis Crystal Toxins and Mechanism of Action. In Insect Midgut and Insecticidal Proteins; Dhadialla, T.S., Gill, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 47, pp. 39–87. [Google Scholar] [CrossRef]
- Bernardi, D.; Salmeron, E.; Horikoshi, R.J.; Bernardi, O.; Dourado, P.M.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. Cross-resistance between Cry1 proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided bt maize hybrids in Brazil. PLoS ONE 2015, 10, e0140130. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, O.; Bernardi, D.; Amado, D.; Sousa, R.S.; Fatoretto, J.; Medeiros, F.C.L.; Conville, J.; Burd, T.; Omoto, C. Resistance Risk Assessment of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea Saccharalis (Lepidoptera: Crambidae) to Vip3Aa20 insecticidal protein expressed in corn. J. Econ. Entomol. 2015, 108, 2711–2719. [Google Scholar] [CrossRef]
- Santos-Amaya, O.F.; Rodrigues, J.V.C.; Souza, T.C.; Tavares, C.S.; Campos, S.O.; Guedes, R.N.C.; Pereira, E.J.G. Resistance to dual-gene Bt maize in Spodoptera frugiperda: Selection, inheritance, and cross-resistance to other transgenic events. Sci. Rep. 2015, 5, 18243. [Google Scholar] [CrossRef]
- Santos-Amaya, O.F.; Tavares, C.S.; Rodrigues, J.V.C.; Santana, I.V.; Queiroz, O.S.; Oliveira, E.E.; Guedes, R.N.C.; Pereira, E.J.G. Strong fitness costs of insect resistance to dual-gene Bt corn are magnified on less-suitable host-crop cultivars. Agronomy 2022, 12, 682. [Google Scholar] [CrossRef]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a Novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef]
- Núñez-Ramírez, R.; Huesa, J.; Bel, Y.; Ferré, J.; Casino, P.; Arias-Palomo, E. Molecular architecture and activation of the insecticidal protein Vip3Aa from Bacillus thuringiensis. Nat. Commun. 2020, 11, 3974. [Google Scholar] [CrossRef]
- Tavares, C.S.; Santos-Amaya, O.F.; Oliveira, E.E.; Paula-Moraes, S.V.; Pereira, E.J.G. Facing Bt toxins growing up: Developmental changes of susceptibility to Bt corn hybrids in fall armyworm populations and the implications for resistance management. Crop Prot. 2021, 146, 105664. [Google Scholar] [CrossRef]
- Miraldo, L.L.; Bernardi, O.; Horikoshi, R.J.; Amaral, F.S.A.; Bernardi, D.; Omoto, C. Functional dominance of different aged larvae of Bt-resistant Spodoptera frugiperda (Lepidoptera: Noctuidae) on transgenic maize expressing Vip3Aa20 protein. Crop Prot. 2016, 88, 65–71. [Google Scholar] [CrossRef]
- Wen, Z.; Conville, J.; Matthews, P.; Hootman, T.; Himes, J.; Wong, S.; Huang, F.; Ni, X.; Chen, J.S.; Bramlett, M. More than 10 years after commercialization, Vip3A-expressing MIR162 remains highly efficacious in controlling major lepidopteran maize pests: Laboratory resistance selection versus field reality. Pestic. Biochem. Physiol. 2023, 192, 105385. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Morsello, S.; Head, G.P.; Sansone, C.; Huang, F.; Gilreath, R.T.; Kerns, D.L. F2 screen, inheritance and cross-resistance of field-derived Vip3A resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) collected from Louisiana, USA. Pest Manag. Sci. 2018, 74, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, D.; Zhao, S.; Wu, K. Susceptibilities of the invasive fall armyworm (Spodoptera frugiperda) to the insecticidal proteins of Bt maize in China. Toxins 2022, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Chandrasena, D.; Difonzo, C.; Conner, J.; Head, G.; Berman, K.; Wise, J. Susceptibility of fall armyworms (Spodoptera frugiperda J.E. Smith) from Mexico and Puerto Rico to Bt proteins. Insects 2020, 11, 831. [Google Scholar] [CrossRef]
- Bernardi, O.; Bernardi, D.; Ribeiro, R.S.; Okuma, D.M.; Salmeron, E.; Fatoretto, J.; Medeiros, F.C.L.; Burd, T.; Omoto, C. Frequency of resistance to Vip3Aa20 toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) Populations in Brazil. Crop Prot. 2015, 76, 7–14. [Google Scholar] [CrossRef]
- Karsten, J.A.A.; Precetti, C.M.; Parra, J.R.P. Dados biológicos comparativos de Spodoptera frugiperda (J. E. Smith, 1797) em duas dietas artificiais e substrato natural. Rev. Agric. 1978, 53, 68–78. [Google Scholar]
- Chakroun, M.; Bel, Y.; Caccia, S.; Abdelkefi-Mesrati, L.; Escriche, B.; Ferré, J. Susceptibility of Spodoptera frugiperda and S. exigua to Bacillus thuringiensis Vip3Aa insecticidal protein. J. Invertebr. Pathol. 2012, 110, 334–339. [Google Scholar] [CrossRef]
- Waquil, M.S.; Pereira, E.J.G.; de Sousa Carvalho, S.S.; Pitta, R.M.; Waquil, J.M.; Mendes, S.M. Fitness index and lethal time of fall armyworm on Bt corn. Pesq. Agropec. Bras. 2016, 51, 563–570. [Google Scholar] [CrossRef]
- Dulmage, H.T.; Graham, H.M.; Martinez, E. Interactions between the tobacco budworm, Heliothis virescens, and the δ-endotoxin produced by the HD-1 isolate of Bacillus thuringiensis var. kurstaki: Relationship between length of exposure to the toxin and survival. J. Invertebr. Pathol. 1978, 32, 40–50. [Google Scholar] [CrossRef]
- Chambers, J.A.; Jelen, A.; Gilbert, M.P.; Jany, C.S.; Johnson, T.B.; Gawron-Burke, C. Isolation and characterization of a novel insecticidal crystal protein gene from Bacillus thuringiensis subsp. aizawai. J. Bacteriol. 1991, 173, 3966–3976. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, 112958. [Google Scholar] [CrossRef] [PubMed]
- Moscardini, V.F.; Marques, L.H.; Santos, A.C.; Rossetto, J.; Silva, O.A.B.N.; Rampazzo, P.E.; Castro, B.A. Efficacy of Bacillus thuringiensis (Bt) maize expressing Cry1F, Cry1A.105, Cry2Ab2 and Vip3Aa20 proteins to manage the fall armyworm (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2020, 137, 105269. [Google Scholar] [CrossRef]
- Lemes, A.R.N.; Davolos, C.C.; Legori, P.C.B.C.; Fernandes, O.A.; Ferré, J.; Lemos, M.V.F.; Desiderio, J.A. Synergism and antagonism between Bacillus thuringiensis Vip3A and Cry1 proteins in Heliothis virescens, Diatraea Saccharalis and Spodoptera frugiperda. PLoS ONE 2014, 9, e107196. [Google Scholar] [CrossRef] [PubMed]
- Santos-Amaya, O.F.; Tavares, C.S.; Rodrigues, J.V.C.; Souza, T.C.; Rodrigues-Silva, N.; Guedes, R.N.C.; Alves, A.P.; Pereira, E.J.G. Magnitude and allele frequency of Cry1F resistance in field populations of the fall armyworm (Lepidoptera: Noctuidae) in Brazil. J. Econ. Entomol. 2017, 110, 1770–1778. [Google Scholar] [CrossRef]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; dos Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab Maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef]
- Van Den Berg, J.; Prasanna, B.M.; Midega, C.A.O.; Ronald, P.C.; Carrière, Y.; Tabashnik, B.E. Managing fall armyworm in Africa: Can Bt maize sustainably improve control? J. Econ. Entomol. 2021, 114, 1934–1949. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Rane, R.V.; James, W.; Gordon, K.H.J.; Downes, S.; Kim, J.; Kuniata, L.; Walsh, T.K. Resistance bioassays and allele characterization inform analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) introduction pathways in Asia and Australia. J. Econ. Entomol. 2022, 115, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Niu, Y.; Silva, T.; Brown, S.; Towles, T.; Kerns, D.; Jurat-Fuentes, J.L.; Head, G.P.; Carroll, M.; Walker, W.; et al. An Extended investigation of unexpected Helicoverpa zea (Boddie) survival and ear injury on a transgenic maize hybrid expressing Cry1A/Cry2A/Vip3A toxins. Toxins 2023, 15, 474. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Z.; Kerns, D.L. Resistance of Spodoptera frugiperda to Cry1, Cry2, and Vip3Aa proteins in Bt corn and cotton in the Americas: Implications for the Rest of the World. J. Econ. Entomol. 2022, 115, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, Y.; Sun, X.; Shen, X.; Wu, Q.; Zhang, H.; Zhang, D.; Zhao, S.; Liang, G.; Wu, K. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782. [Google Scholar] [CrossRef]
- Jin, M.; Shan, Y.; Peng, Y.; Wang, W.; Zhang, H.; Liu, K.; Heckel, D.G.; Wu, K.; Tabashnik, B.E.; Xiao, Y. Downregulation of a transcription factor associated with resistance to Bt toxin Vip3Aa in the invasive fall armyworm. Proc. Natl. Acad. Sci. USA 2023, 120, e2306932120. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.T.A.; Cribb, B.W.; Downes, S.J.; Perkins, L.E.; Zalucki, M.P. Stay or move: How Bt-susceptible Helicoverpa armigera neonates behave on Bt cotton plants. Entomol. Exp. Appl. 2019, 167, 868–879. [Google Scholar] [CrossRef]
 Non-Bt;
Non-Bt;  Cry1F + Cry1Ab + Vip3Aa;
Cry1F + Cry1Ab + Vip3Aa;  Cry1A.105 + Cry2Ab + Vip3Aa;
Cry1A.105 + Cry2Ab + Vip3Aa;  Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa.
Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa.
 Non-Bt;
Non-Bt;  Cry1F + Cry1Ab + Vip3Aa;
Cry1F + Cry1Ab + Vip3Aa;  Cry1A.105 + Cry2Ab + Vip3Aa;
Cry1A.105 + Cry2Ab + Vip3Aa;  Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa.
Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa.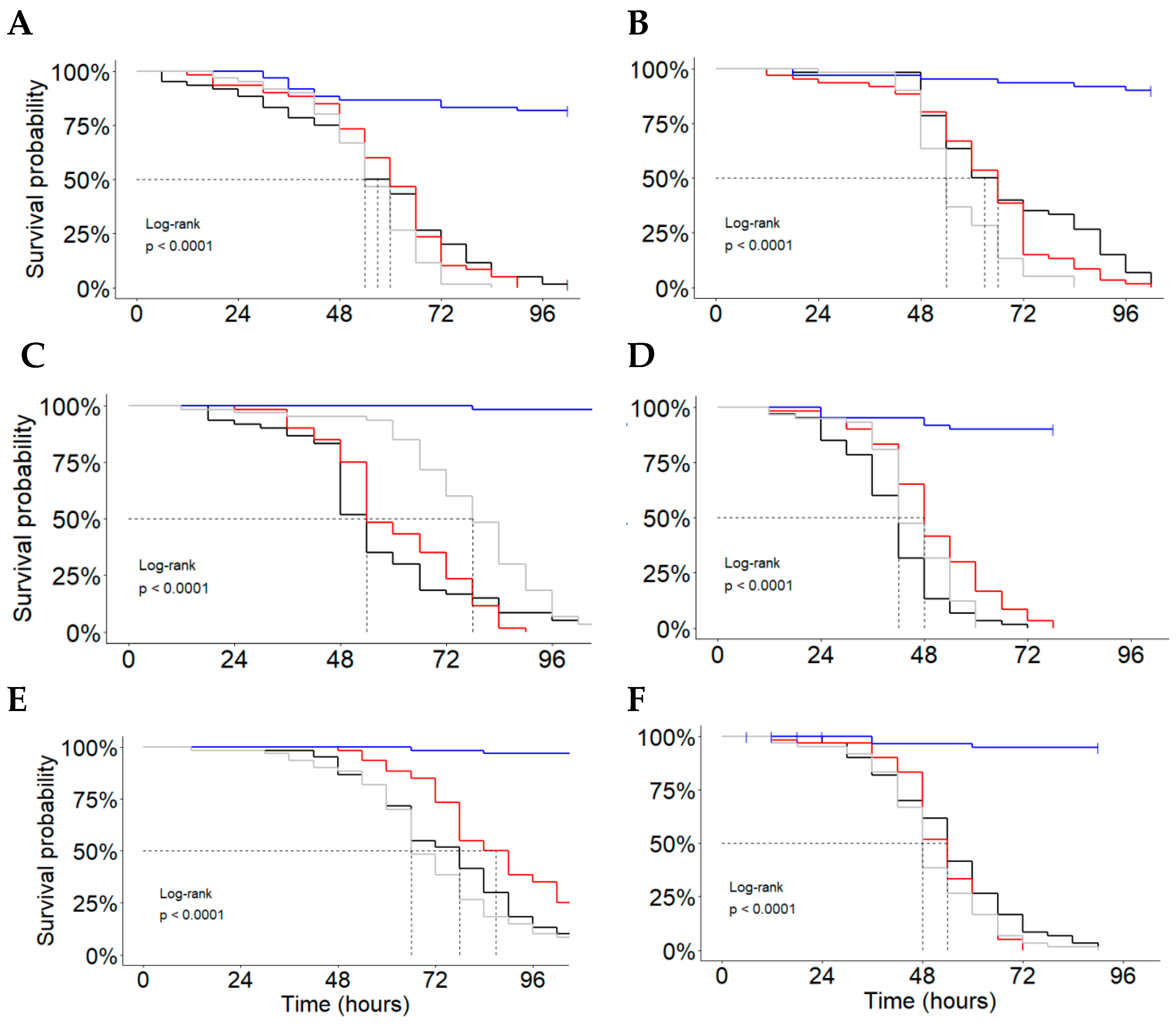

| Location, State of Brazil (Population Code) | Geographic Coordinates | Number of Larvae Collected |
|---|---|---|
| Balsas, Maranhão (MA-Bs) | 8°41′16.8″ S|46°40′9.0″ W | 1600 |
| Porto Nacional, Tocantins (TO-PN) | 10°35′46.5″ S|48°32′3.5″ W | 1350 |
| Baixa Grande, Piauí (PI-BG) | 8°31′10.1″ S|45°9′22.4″ W | 600 |
| Currais, Piauí (PI-Cs) | 8°54′26.0″ S|44°40′35.9″ W | 1000 |
| Uruçuí, Piauí (PI-Ur) | 7°49′37.8″ S|44°31′11.3″ W | 850 |
| Luis Eduardo Magalhães, Bahia (BA-LE) | 12°15′46.3″ S|46°3′19.6″ W | 1450 |
| Maize Hybrid | Lepidopteran-Active Bt Proteins | Bt Technology or Registered Trademark |
|---|---|---|
| DKB390PRO4 | Cry1A.105, Cry2Ab2, and Vip3Aa20 | VTPRO4®/YieldGard® |
| P3551PWU | Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 | PowerCore™ Ultra |
| 30F53VYHR | Cry1F, Cry1Ab, and Vip3Aa20 | Leptra®/Agrisure Viptera® |
| 30F53RR | None | None (Round Ready®) |
| Population Code | Bt Insecticidal Protein or Trait | |||
|---|---|---|---|---|
| Cry1A.105 + Cry2Ab + Vip3Aa | Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa | Cry1F + Cry1Ab + Vip3Aa | Non-Bt | |
| MA-Bs | 100 ± 0.0 a | 100 ± 0.0 a | 98.33 ± 1.7 a | 18.3 ± 5.4 b |
| TO-PN | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 12.0 ± 4.1 b |
| PI-BG | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 5.0 ± 3.0 b |
| PI-Cs | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 10.0 ± 3.9 b |
| PI-Ur | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 8.0 ± 3.6 b |
| BA-LE | 100 ± 0.0 a | 100 ± 0.0 a | 100 ± 0.0 a | 5.0 ± 3.0 b |
| Population Code | Median Survival Time in Hours, ST50 (95%CL) * | |||
|---|---|---|---|---|
| Cry1A.105 + Cry2Ab + Vip3Aa | Cry1F + Cry1A.105 + Cry2Ab + Vip3Aa | Cry1F + Cry1Ab + Vip3Aa | Non-Bt | |
| MA-Bs | 60 (54–66) BCa | 54 (50–58) Bb | 57 (54–60) Bab | NA |
| TO-PN | 66 (60–72) Ba | 54 (50–58) Bb | 63 (60–66) Ba | NA |
| PI-BG | 54 (48–60) Cb | 78 (72–84) Aa | 54 (50–58) Cb | NA |
| PI-Cs | 48 (42–54) Ca | 42 (40–44) Ca | 42 (40–44) Da | NA |
| PI-Ur | 90 (78–102) Aa | 66 (60–72) Aa | 78 (72–84) Aa | NA |
| BA-LE | 54 (48–60) Ca | 48 (42–54) BCa | 54 (50–58) Ca | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.F.T.; Silva, L.B.; Malaquias, J.B.; Salustino, A.S.; Correia Neto, D.F.; Pacheco, D.M.; Fragoso, D.B.; Pereira, E.J.G. Susceptibility of Fall Armyworm Field Populations to Vip3Aa/Cry Bt Maize in a Tropical Agricultural Region. Agronomy 2024, 14, 451. https://doi.org/10.3390/agronomy14030451
Silva AFT, Silva LB, Malaquias JB, Salustino AS, Correia Neto DF, Pacheco DM, Fragoso DB, Pereira EJG. Susceptibility of Fall Armyworm Field Populations to Vip3Aa/Cry Bt Maize in a Tropical Agricultural Region. Agronomy. 2024; 14(3):451. https://doi.org/10.3390/agronomy14030451
Chicago/Turabian StyleSilva, Alisson Franco T., Luciana B. Silva, José B. Malaquias, Angélica S. Salustino, Domingos Francisco Correia Neto, Daniel M. Pacheco, Daniel B. Fragoso, and Eliseu J. G. Pereira. 2024. "Susceptibility of Fall Armyworm Field Populations to Vip3Aa/Cry Bt Maize in a Tropical Agricultural Region" Agronomy 14, no. 3: 451. https://doi.org/10.3390/agronomy14030451










