Selenium Application Provides Nutritional and Metabolic Benefits to Wheat Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Physiological and Agronomic Analyses
2.2.1. Grain Yield
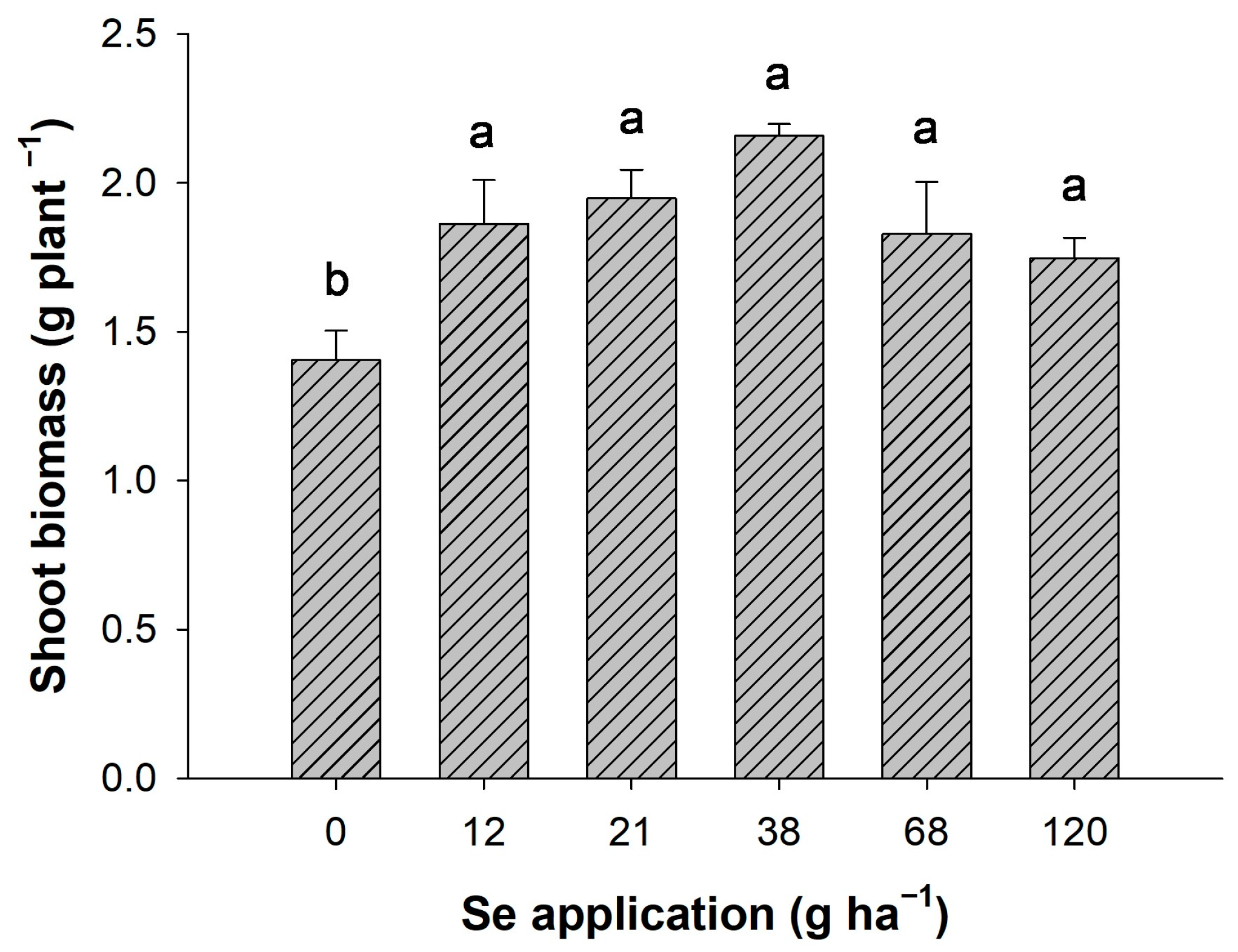
2.2.2. Quantification of Shoot Biomass and Contents of Carbohydrates, Proteins, and Total Amino Acids
2.2.3. Contents of Selenium, Sulfur, Phosphorus, and Nitrogen
2.2.4. Antioxidant Enzymes
2.2.5. Hydrogen Peroxide (H2O2) and Lipid Peroxidation (MDA)
2.2.6. Chlorophyll Index
2.3. Statistical Analysis
3. Results
3.1. Agronomic Wheat Yield
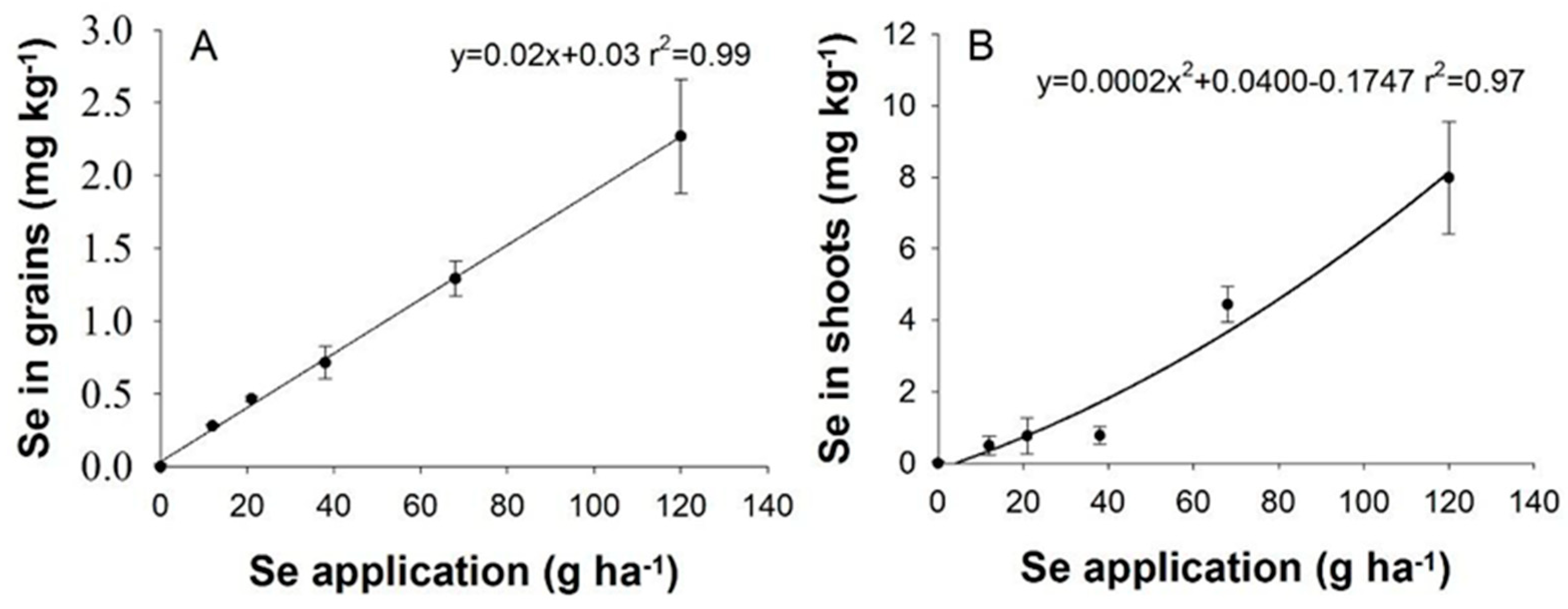
3.2. Selenium Contents of Leaves and Grains
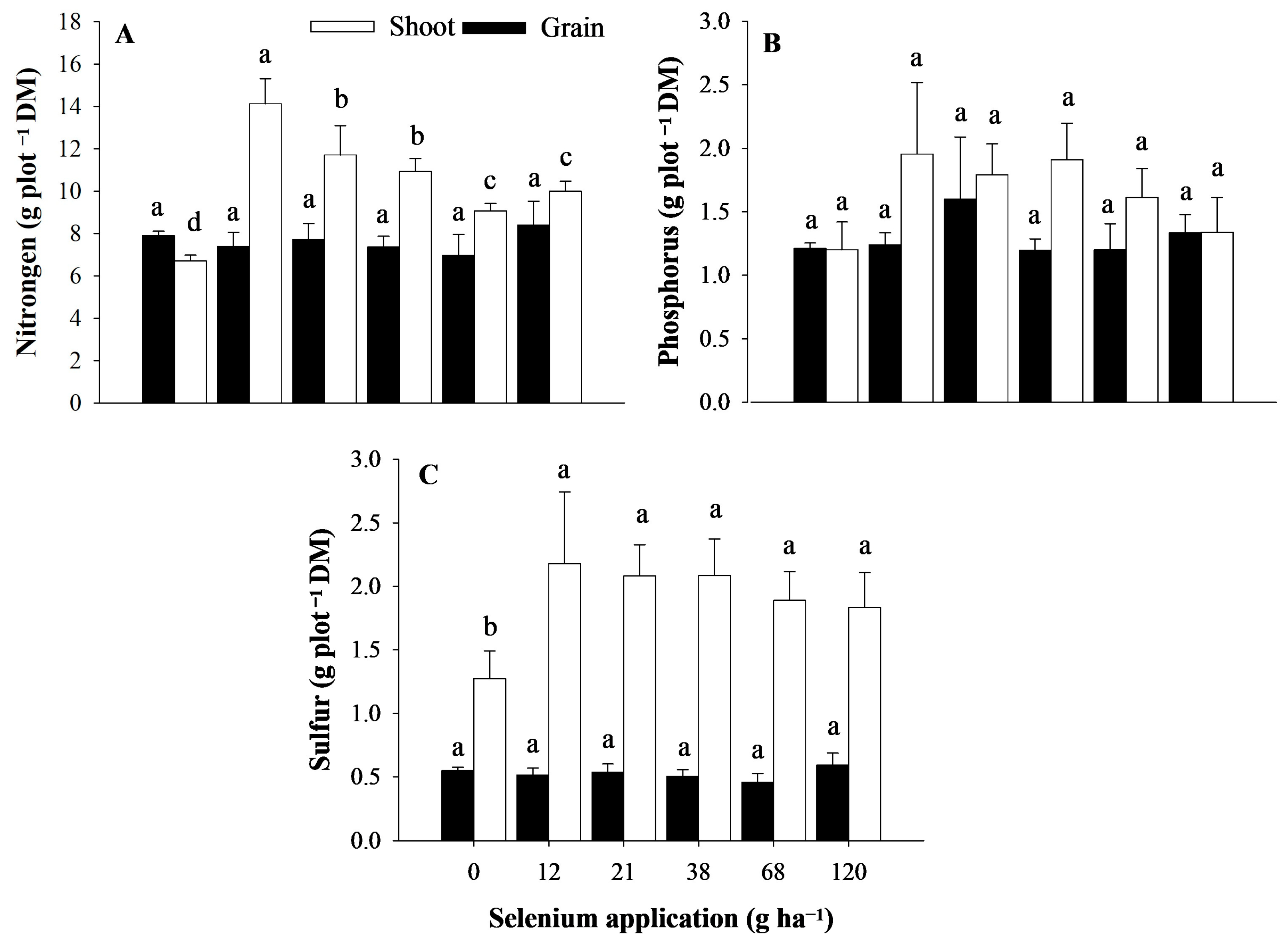
3.3. Contents of Sulfur, Phosphorus, and Nitrogen
3.4. Antioxidant Metabolism
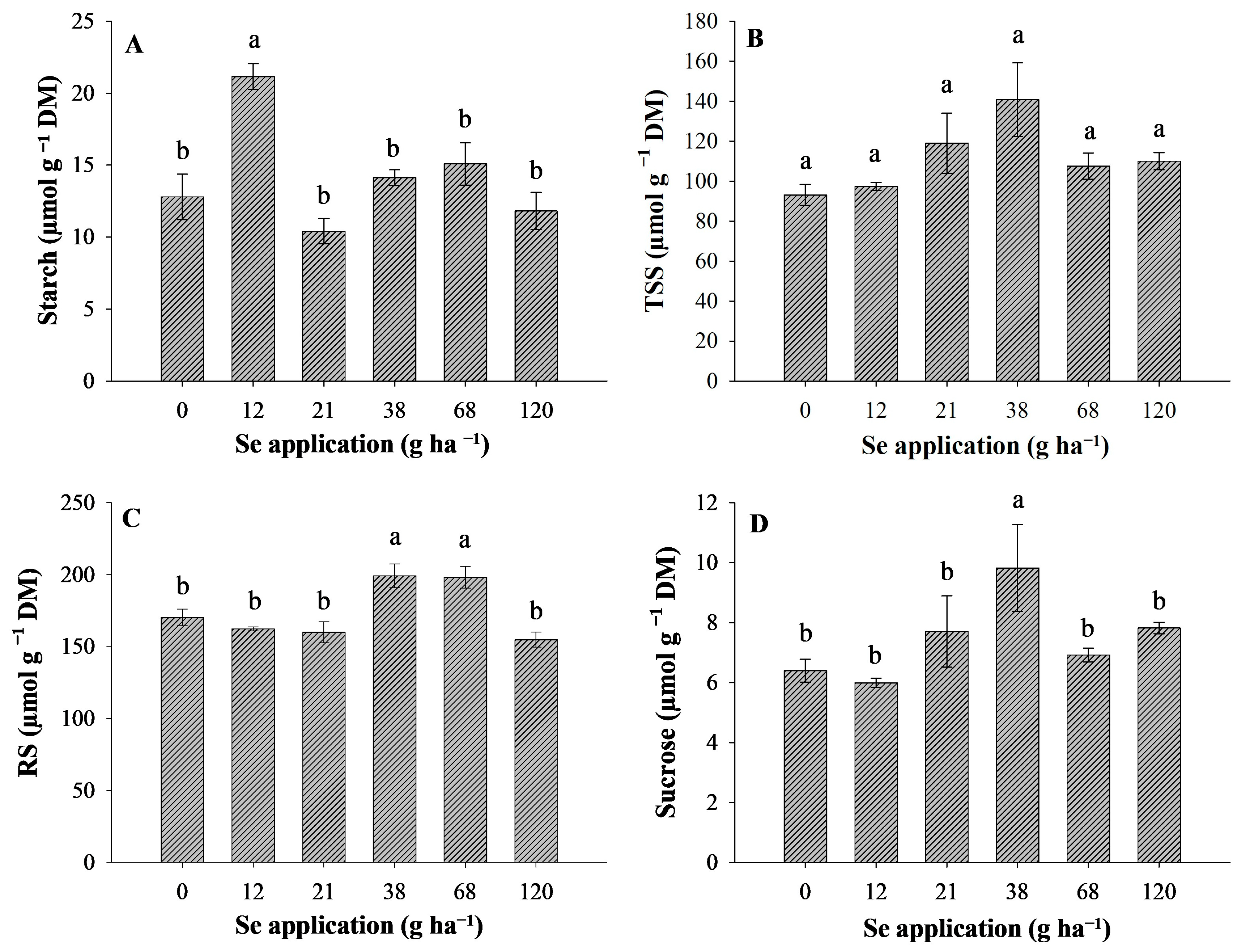
3.5. Contents of Major Carbohydrates
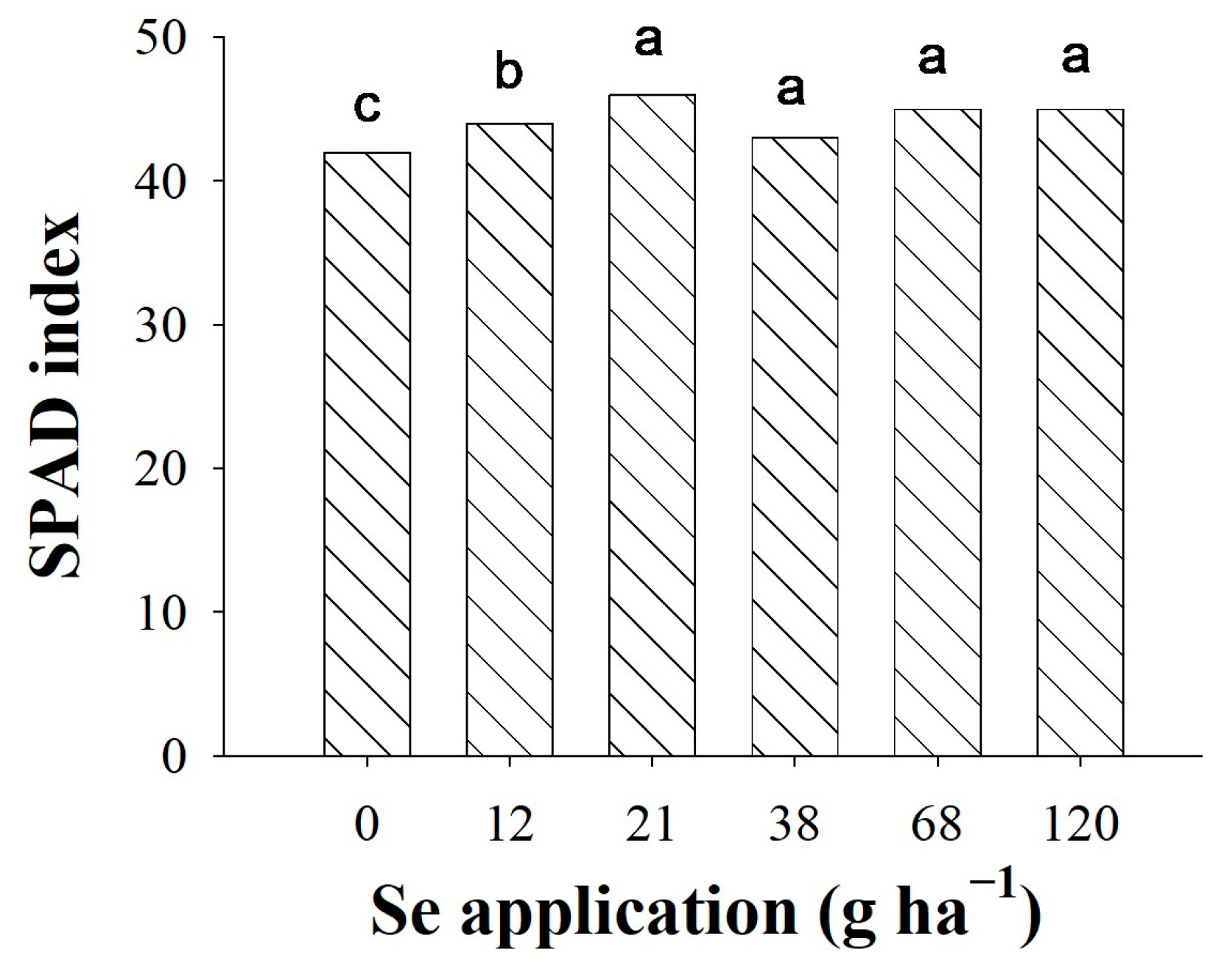
3.6. SPAD Reading
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2021, 200, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; Mcgrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium Biofortification and Phytoremediation Phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium behavior in the soil environment and its implication for human health. Ciênc. Agrotecnol. 2017, 41, 605–615. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento: Trigo. 2018. Available online: http://www.agricultura.gov.br (accessed on 30 June 2023).
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Delaqua, D.; Carnier, R.; Berton, R.S.; Corbi, F.C.A.; Coscione, A.R. Increase of selenium concentration in wheat grains through foliar application of sodium selenate. J. Food Compos. Anal. 2021, 99, 103886. [Google Scholar] [CrossRef]
- Ravello, R.A.V.; de Oliveira, C.; Lessa, J.; Boas, L.V.V.; de Castro, E.M.; Guilherme, L.R.G.; Lopes, G. Selenium application influenced selenium biofortification and physiological traits in water-deficit common bean plants. Crop Pasture Sci. 2021, 73, 44–55. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta (Bba)-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Lara, T.S.; Lessa, J.H.d.L.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of Growth in Mungbean (Phaseolus aureus Roxb.) by Selenium is Associated with Stimulation of Carbohydrate Metabolism. Biol. Trace Elem. Res. 2010, 143, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; U.S. Department of Agriculture Handbook; Natural Resources Conservation Service: Washington, DC, USA, 1999; p. 436.
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- EMBRAPA. Empresa Brasileira de Pesquisa Agropecuária, Manual de Métodos de Análise de Solo; Embrapa Solos: Brasília, Brazil, 2017. [Google Scholar]
- de Lima Lessa, J.H.; Araujo, A.M.; Ferreira, L.A.; da Silva Júnior, E.C.; de Oliveira, C.; Corguinha, A.P.; Martins, F.A.; de Carvalho, H.W.; Guilherme, L.R.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Mapa/ACS: Brasília, Brazil, 2009.
- Dische, Z. General color reactions. In Carbohydrate Chemistry; Whistler, R.L., Wolfram, M.L., Eds.; Academic: New York, NY, USA, 1962; pp. 477–520. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Cocking, E.C.; Yemm, E.W. Estimation of amino acids by ninhydrin. Biochem. J. 1954, 58, 7–8. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. Avaliação do Estado Nutricional das Plantas: Princípios e Perspectivas, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-Aeration following Hypoxia or Anoxia Leads to Activation of the Antioxidative Defense System in Roots of Wheat Seedlings1. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; Mchale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs: Sisvar. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of selenium (Se) uptake on plant growth and yield in common buckwheat (Fagopyrum esculentum Moench). Field Crops Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual effects of different selenium species on wheat. Plant Physiol. Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, M.I.; Asgher, M.; Fatma, M.; Masood, A.; Syeed, S. Salinity Tolerance in Plants: Revisiting the role of sulfur metabolites. J. Plant Biochem. Physiol. 2014, 2, 1000120. [Google Scholar]
- Keskinen, R.; Turakainen, M.; Hartikainen, H. Plant availability of soil selenate additions and selenium distribution within wheat and ryegrass. Plant Soil 2010, 333, 301–313. [Google Scholar] [CrossRef]
- Galinha, C.; Sánchez-Martínez, M.; Pacheco, A.M.; Freitas, M.D.; Coutinho, J.; Maçãs, B.; Almeida, A.S.; Pérez-Corona, M.T.; Madrid, Y.; Wolterbeek, H.T. Characterization of selenium-enriched wheat by agronomic biofortification. J. Food Sci. Technol. 2014, 52, 4236–4245. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Y.; Liu, D.; Cheng, J.; Zhao, G.; Fahima, T.; Yan, J. Effects of Different Selenium Application Methods on Wheat (Triticum aestivum L.) Biofortification and Nutritional Quality. Phyton 2020, 89, 423–435. [Google Scholar] [CrossRef]
- Dzomba, E.; Djikic, M.; Gadzo, D.; Cengic-Dzomba, S.; Loncaric, Z.; Singh, B.R. Effect of different doses and application methods of sodium selenate on selenium status in maize for silage. Agric. Food Sci. 2018, 27, 255–263. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Shaheen, N.; Islam, M.S.; Habibullah-Al-Mamun, M.; Islam, S.; Banu, C.P. Trace elements in two staple cereals (rice and wheat) and associated health risk implications in Bangladesh. Environ. Monit. Assess. 2015, 187, 326. [Google Scholar] [CrossRef]
- Fernandes, K.F.; Berton, R.S.; Coscione, A.R. Selenium biofortification of rice and radish: Effect of soil texture and efficiency of two extractants. Plant Soil Environ. 2014, 60, 105–110. [Google Scholar] [CrossRef]
- Vymazal, J. Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci. Total Environ. 2016, 544, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, P.; Zhang, W.; Hui, F.; Chen, X. Effects of selenium application on nutrient uptake and nutritional quality of Codonopsis lanceolata. Sci. Hortic. 2017, 225, 574–580. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Clemente, A.d.C.S.; de Andrade, T.; Guilherme, L.R.G. Genotypic Variation and Biofortification with Selenium in Brazilian Wheat Cultivars. J. Environ. Qual. 2018, 47, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Blasco, B.; Cervilla, L.M.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Sanchez-Rodriguez, E.; Romero, L.; Ruiz, J.M. Nitrogen-Use Efficiency in Relation to Different Forms and Application Rates of Se in Lettuce Plants. J. Plant Growth Regul. 2009, 29, 164–170. [Google Scholar] [CrossRef]
- Nazir, F.; Kumari, S.; Mahajan, M.; Khan, M.I.R. An explicit story of plant abiotic stress resilience: Overtone of selenium, plant hormones and other signaling molecules. Plant Soil 2022, 486, 135–163. [Google Scholar] [CrossRef]
- Stulen, I.; Kok, L.J. Foreword: Exploring interactions between sulfate and nitrate uptake at a whole plant level. In Sulfur Metabolism in Plants: Mechanisms and Applications to Food Security and Responses to Climate Change; Springer: Dordrecht, The Netherlands, 2012; pp. 1–8. [Google Scholar]
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Effects on the accumulation of calcium, magnesium, iron, manganese, copper and zinc of adding the two inorganic forms of selenium to solution cultures of Zea mays. Plant Physiol. Biochem. 2016, 98, 128–137. [Google Scholar] [CrossRef]
- Li, H.-F.; McGrath, S.P.; Zhao, F.-J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Z.; Li, J.; Zhao, P.; Qin, S.; Nie, Z. The Impact of Phosphorus Supply on Selenium Uptake During Hydroponics Experiment of Winter Wheat (Triticum aestivum) in China. Front. Plant Sci. 2018, 9, 373. [Google Scholar] [CrossRef]
- dos Santos, M.J.; de Lima Lessa, J.H.; de Assis, M.B.; Raymundo, J.F.; Ribeiro, B.T.; Guilherme, L.R.; Lopes, G. Selenium desorption in tropical soils by sulfate and phosphate, and selenium biofortification of Mombaça grass under increasing rates of phosphate fertilisation. Crop Pasture Sci. 2021, 73, 56–66. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, T.; Ye, J.; Hou, Z. Selenium accumulation in wheat (Triticum aestivum L.) as affected by coapplication of either selenite or selenate with phosphorus. Soil Sci. Plant Nutr. 2017, 63, 37–44. [Google Scholar] [CrossRef]
- Praus, L.; Száková, J.; Steiner, O.; Goessler, W. Rapeseed (Brassica napus L.) biofortification with selenium: How do sulphate and phosphate influence the efficiency of selenate application into soil? Arch. Agron. Soil Sci. 2019, 65, 2059–2072. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; de Almeida, H.J.; Ávila, F.W.; Guilherme, L.R.G.; Bastos, C.E.A.; Ávila, P.A. Selenato e selenito na produção, nutrição mineral e biofortificação com selênio em cultivares de alface1. Rev. Bras. Ciênc. Solo 2011, 35, 1347–1355. [Google Scholar] [CrossRef][Green Version]
- Ramos, S.; Faquin, V.; Guilherme, L.; Castro, E.; Ávila, F.; Carvalho, G.; Bastos, C.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Feng, R.W.; Wei, C.Y. Antioxidative mechanisms on selenium accumulation in Pteris vittata a potential selenium phytoremediation plant. Plant Soil Environ. 2012, 58, 105–110. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Yasar, F.; Uzal, O. Oxidativer Stress und antioxidative Enzymaktivitäten bei Tomatenpflanzen (Solanum lycopersicum), die bei zwei verschiedenen Lichtintensitäten angebaut wurden. Gesunde Pflanz. 2022, 75, 479–485. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Yue, Z.; Li, J.; Zhao, J. The Effects of Selenium on Physiological Traits, Grain Selenium Content and Yield of Winter Wheat at Different Development Stages. Biol. Trace Elem. Res. 2012, 151, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Sekyere, A.; Kontturi, J.; Hajiboland, R.; Rahmat, S.; Aliasgharzad, N.; Hartikainen, H.; Seppänen, M.M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 2013, 373, 541–552. [Google Scholar] [CrossRef]
- Sucu, S.; Yağcı, A. Effects of Selenium Treatments on Physical and Chemical Traits of Some Grape Cultivars. Erwerbs-Obstbau 2023, 65, 2055–2062. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.P.; Xue, N.W.; Dai, X.J.; Zhang, X.; Gao, Z.Q. Effect of Foliar Application of Selenium on Nutrient Concentration and Yield of Coloredgrain Wheat in China. Appl. Ecol. Environ. Res. 2019, 17, 2187–2202. [Google Scholar] [CrossRef]
- Zhu, Y.; Siegwolf, R.T.W.; Durka, W.; Körner, C. Phylogenetically balanced evidence for structural and carbon isotope responses in plants along elevational gradients. Oecologia 2009, 162, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Sharma, S.; Singh, D. Influence of selenium on carbohydrate accumulation in developing wheat grains. Commun. Soil Sci. Plant Anal. 2018, 49, 1650–1659. [Google Scholar] [CrossRef]
- Bocchini, M.; D’amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil Selenium (Se) Biofortification Changes the Physiological, Biochemical and Epigenetic Responses to Water Stress in Zea mays L. by Inducing a Higher Drought Tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef]
- Wood, C.W.; Reeves, D.W.; Duffield, R.R.; Edmisten, K.L. Field chlorophyll measurements for evaluation of corn nitrogen status1. J. Plant Nutr. 1992, 15, 487–500. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]

| pH | K | P | Ca | Mg | Al | H+Al | SB | T | V |
|---|---|---|---|---|---|---|---|---|---|
| mg dm−3 | cmolc dm−3 | % | |||||||
| 5.1 | 62.0 | 17.5 | 1.3 | 0.3 | 0.8 | 7.8 | 1.7 | 9.6 | 18.2 |
| SOM | S | Fe | Mn | Cu | B | Zn | Clay | Silt | Sand |
| g kg−1 | mg dm−3 | g kg−1 | |||||||
| 38 | 22.4 | 28.2 | 8.4 | 1.2 | 0.10 | 2.3 | 420 | 190 | 390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, T.S.; Correia, T.S.; de Oliveira, C.; Lessa, J.H.d.L.; de Souza, K.R.D.; Corguinha, A.P.B.; da Silva, E.C., Jr.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Application Provides Nutritional and Metabolic Benefits to Wheat Plants. Agronomy 2024, 14, 462. https://doi.org/10.3390/agronomy14030462
Lara TS, Correia TS, de Oliveira C, Lessa JHdL, de Souza KRD, Corguinha APB, da Silva EC Jr., Martins FAD, Lopes G, Guilherme LRG. Selenium Application Provides Nutritional and Metabolic Benefits to Wheat Plants. Agronomy. 2024; 14(3):462. https://doi.org/10.3390/agronomy14030462
Chicago/Turabian StyleLara, Túlio Silva, Tatiane Santos Correia, Cynthia de Oliveira, Josimar Henrique de Lima Lessa, Kamila Rezende Dázio de Souza, Ana Paula Branco Corguinha, Ediu Carlos da Silva, Jr., Fábio Aurélio Dias Martins, Guilherme Lopes, and Luiz Roberto Guimarães Guilherme. 2024. "Selenium Application Provides Nutritional and Metabolic Benefits to Wheat Plants" Agronomy 14, no. 3: 462. https://doi.org/10.3390/agronomy14030462
APA StyleLara, T. S., Correia, T. S., de Oliveira, C., Lessa, J. H. d. L., de Souza, K. R. D., Corguinha, A. P. B., da Silva, E. C., Jr., Martins, F. A. D., Lopes, G., & Guilherme, L. R. G. (2024). Selenium Application Provides Nutritional and Metabolic Benefits to Wheat Plants. Agronomy, 14(3), 462. https://doi.org/10.3390/agronomy14030462







