Weed Management Methods for Herbaceous Field Crops: A Review
Abstract
:1. Introduction
2. Material and Methods
3. Physical Weed Control
3.1. Flame Weeding
3.2. Laser Weeding
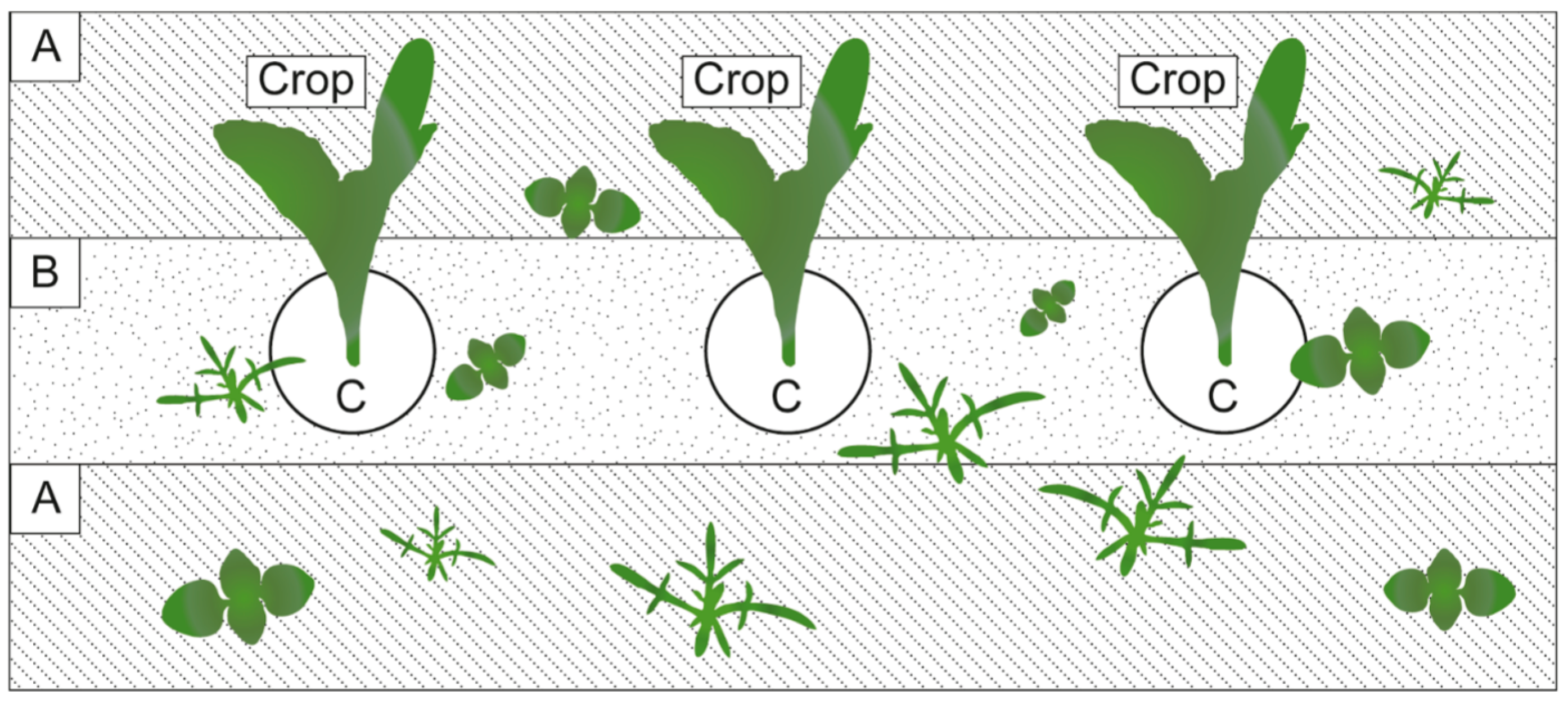
4. Mechanical Weed Control
| Listing 1. Fundamental criteria for the effectiveness of sensor-assisted mechanical weeding devices [6]. |
|
5. Biological Weed Control
6. Chemical Weed Control
7. Integrated Weed Management Strategies
8. Discussions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scavo, A.; Mauromicale, G. Integrated Weed Management in Herbaceous Field Crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Kraehmer, H.; Baur, P. Weed Anatomy; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Appleby, A. Weed Control. In Agrochemicals; Muller, F., Ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Bond, W.J.P. Non-chemical approaches to weed control in horticulture. Phytoparasitica 1992, 20, S77–S81. [Google Scholar] [CrossRef]
- Machleb, J.; Peteinatos, G.G.; Kollenda, B.L.; Andújar, D.; Gerhards, R. Sensor-based mechanical weed control: Present state and prospects. Comput. Electron. Agric. 2020, 176, 105638. [Google Scholar] [CrossRef]
- Anshelm, K. Construction and description of the new Physiological Institute. Acta Physiol. Scand Suppl. 1953, 111, 30–33. [Google Scholar]
- Kudsk, P. Advances in integrated weed management. In Advances in Integrated Weed Management; Per, K., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2022; pp. xxvi + 426. [Google Scholar] [CrossRef]
- Riemens, M.; Sønderskov, M.; Moonen, A.-C.; Storkey, J.; Kudsk, P. An Integrated Weed Management framework: A pan-European perspective. Eur. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Integrated Weed Management for Sustainable Agriculture; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2018; pp. xxii + 453. [Google Scholar]
- Dhanapal, G.N.; Nagarjun, P.; Bai, S.K.; Sindhu, K.K. Weed management in organic agriculture. Mysore J. Agric. Sci. 2019, 53, 1. [Google Scholar]
- Loddo, D.; McElroy, J.S.; Giannini, V. Problems and perspectives in weed management. Ital. J. Agron. 2021, 16, 1854. [Google Scholar] [CrossRef]
- Vasileiou, M.; Kyrgiakos, L.S.; Kleisiari, C.; Kleftodimos, G.; Vlontzos, G.; Belhouchette, H.; Pardalos, P.M. Transforming weed management in sustainable agriculture with artificial intelligence: A systematic literature review towards weed identification and deep learning. Crop Prot. 2024, 176, 106522. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Rask, A.; Kristoffersen, P. A review of non-chemical weed control on hard surfaces. Weed Res. 2007, 47, 370–380. [Google Scholar] [CrossRef]
- Jitsuyama, Y.; Ichikawa, S. Possible weed establishment control by applying cryogens to fields before snowfalls. Weed Technol. 2011, 25, 454–458. [Google Scholar] [CrossRef]
- Ascard, J. Effects of flame weeding on weed species at different developmental stages. Weed Res. 1995, 35, 397–411. [Google Scholar] [CrossRef]
- Vincent, C.; Panneton, B.; Fleurat-Lessard, F. Physical Control Methods in Plant Protection; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Cisneros, J.J.; Zandstra, B.H. Flame weeding effects on several weed species. Weed Technol. 2008, 22, 290–295. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, I.A.; Bàrberi, P. Integrating physical and cultural methods of weed control—Examples from European research. Weed Sci. 2005, 53, 369–381. [Google Scholar] [CrossRef]
- Bond, W.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Sivesind, E.C.; Leblanc, M.L.; Cloutier, D.C.; Seguin, P.; Stewart, K.A. Weed response to flame weeding at different developmental stages. Weed Technol. 2009, 23, 438–443. [Google Scholar] [CrossRef]
- Hitz, C.B.; Ewing, J.J.; Hecht, J. Introduction to Laser Technology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Witteman, W.J. The CO2 Laser; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 1987; Volume 53, pp. 1–309. [Google Scholar]
- Heisel, T.; Schou, J.; Christensen, S.; Andreasen, C. Cutting weeds with a CO2 laser. Weed Res. 2001, 41, 19–29. [Google Scholar] [CrossRef]
- Wöltjen, C.; Haferkamp, H.; Rath, T.; Herzog, D. Plant growth depression by selective irradiation of the meristem with CO2 and diode lasers. Biosyst. Eng. 2008, 101, 316–324. [Google Scholar] [CrossRef]
- Kaierle, S.; Marx, C.; Rath, T.; Hustedt, M. Find and Irradiate—Lasers Used for Weed Control: Chemical free elimination of unwanted plants. Laser Tech. J. 2013, 10, 44–47. [Google Scholar] [CrossRef]
- Coleman, G.; Betters, C.; Squires, C.; Leon-Saval, S.; Walsh, M. Low energy laser treatments control annual ryegrass (Lolium rigidum). Front. Agron. 2021, 2, 601542. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Wieliczka, D.M.; Weng, S.; Querry, M.R. Wedge shaped cell for highly absorbent liquids: Infrared optical constants of water. Appl. Opt. 1989, 28, 1714–1719. [Google Scholar] [CrossRef]
- Mathiassen, S.K.; Bak, T.; Christensen, S.; Kudsk, P. The effect of laser treatment as a weed control method. Biosyst. Eng. 2006, 95, 497–505. [Google Scholar] [CrossRef]
- Marx, C.; Barcikowski, S.; Hustedt, M.; Haferkamp, H.; Rath, T. Design and application of a weed damage model for laser-based weed control. Biosyst. Eng. 2012, 113, 148–157. [Google Scholar] [CrossRef]
- Rakhmatulin, I.; Andreasen, C.J.A. A concept of a compact and inexpensive device for controlling weeds with laser beams. Agronomy 2020, 10, 1616. [Google Scholar] [CrossRef]
- Andreasen, C.; Scholle, K.; Saberi, M. Laser weeding with small autonomous vehicles: Friends or foes? Front. Agron. 2022, 4, 841086. [Google Scholar] [CrossRef]
- Findlay, S.; Carreiro, M.; Krischik, V.; Jones, C.G. Effects of damage to living plants on leaf litter quality. Ecol. Appl. 1996, 6, 269–275. [Google Scholar] [CrossRef]
- Draycott, A.P. Sugar Beet; Blackwell Publishing Ltd.: Oxford, UK, 2008. [Google Scholar]
- Van der Linden, S.; Mouazen, A.M.; Anthonis, J.; Ramon, H.; Saeys, W. Infrared laser sensor for depth measurement to improve depth control in intra-row mechanical weeding. Biosyst. Eng. 2008, 100, 309–320. [Google Scholar] [CrossRef]
- Pedersen, S.M.; Fountas, S.; Have, H.; Blackmore, B.S. Agricultural robots—System analysis and economic feasibility. Precis. Agric. 2006, 7, 295–308. [Google Scholar] [CrossRef]
- Van Der Weide, R.; Bleeker, P.; Achten, V.; Lotz, L.; Fogelberg, F.; Melander, B. Innovation in mechanical weed control in crop rows. Weed Res. 2008, 48, 215–224. [Google Scholar] [CrossRef]
- Müter, M.; Damerow, L.; Lammers, P.S. Kameragesteuerte mechanische Unkrautbekämpfung in Pflanzenreihen. Landtechnik 2014, 69, 120–124. [Google Scholar]
- Heisel, T.; Andreasen, C.; Christensen, S. Sugarbeet yield response to competition from Sinapis arvensis or Lolium perenne growing at three different distances from the beet and removed at various times during early growth. Weed Res. 2002, 42, 406–413. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Weed-Crop Competition: A Review; Blackwell Publishing Ltd.: Ames, UK, 2007. [Google Scholar]
- Fernández-Quintanilla, C.; Peña, J.; Andújar, D.; Dorado, J.; Ribeiro, A.; López-Granados, F. Is the current state of the art of weed monitoring suitable for site-specific weed management in arable crops? Weed Res. 2018, 58, 259–272. [Google Scholar] [CrossRef]
- Tillett, N. Automatic guidance sensors for agricultural field machines: A review. J. Agric. Eng. Res. 1991, 50, 167–187. [Google Scholar] [CrossRef]
- Andújar, D.; Escolà, A.; Rosell-Polo, J.R.; Fernández-Quintanilla, C.; Dorado, J.J.C. Potential of a terrestrial LiDAR-based system to characterise weed vegetation in maize crops. Comput. Electron. Agric. 2013, 92, 11–15. [Google Scholar] [CrossRef]
- Andújar, D.; Weis, M.; Gerhards, R.J.S. An ultrasonic system for weed detection in cereal crops. Sensors 2012, 12, 17343–17357. [Google Scholar] [CrossRef]
- Guyer, D.E.; Miles, G.; Schreiber, M.; Mitchell, O.R.; Vanderbilt, V.C. Machine vision and image processing for plant identification. Trans. ASAE 1986, 29, 1500–1507. [Google Scholar] [CrossRef]
- Auernhammer, H. GPS in a basic rule for environment protection in agriculture. Proc. Autom. Agric. 21 Century 1991, 1991, 394–402. [Google Scholar]
- Hiremath, S.A.; Van Der Heijden, G.W.; Van Evert, F.K.; Stein, A.; Ter Braak, C.J.F. Laser range finder model for autonomous navigation of a robot in a maize field using a particle filter. Comput. Electron. Agric. 2014, 100, 41–50. [Google Scholar] [CrossRef]
- Keicher, R.; Seufert, H. Automatic guidance for agricultural vehicles in Europe. Comput. Electron. Agric. 2000, 25, 169–194. [Google Scholar] [CrossRef]
- Pérez-Ruiz, M.; Slaughter, D.; Gliever, C.; Upadhyaya, S. Automatic GPS-based intra-row weed knife control system for transplanted row crops. Comput. Electron. Agric. 2012, 80, 41–49. [Google Scholar] [CrossRef]
- Kurstjens, D.; Kropff, M.J. The impact of uprooting and soil-covering on the effectiveness of weed harrowing. Weed Res. 2001, 41, 211–228. [Google Scholar] [CrossRef]
- Cardina, J.; Sparrow, D.H.; McCoy, E.L. Analysis of spatial distribution of common lambsquarters (Chenopodium album) in no-till soybean (Glycine max). Weed Sci. 1995, 43, 258–268. [Google Scholar] [CrossRef]
- Clay, S.A.; Lems, G.J.; Clay, D.E.; Forcella, F.; Ellsbury, M.M.; Carlson, C.G. Sampling weed spatial variability on a fieldwide scale. Weed Sci. 1999, 47, 674–681. [Google Scholar] [CrossRef]
- Gerhards, R.; Sökefeld, M.; Timmermann, C.; Kühbauch, W.; Williams, M.J.P.A. Site-specific weed control in maize, sugar beet, winter wheat, and winter barley. Precis. Agric. 2002, 3, 25–35. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.-P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Hinz, H.L.; Winston, R.; Day, M.J.B. Biological control of weeds: An analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 2018, 63, 319–331. [Google Scholar] [CrossRef]
- Charudattan, R.J.B. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260. [Google Scholar] [CrossRef]
- Duke, D.; Romagni, R. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Mejias, F.J.; Trasobares, S.; Lopez-Haro, M.; Varela, R.M.; Molinillo, J.M.; Calvino, J.J.; Macias, F.A. In situ eco encapsulation of bioactive agrochemicals within fully organic nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 41925–41934. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chhokar, R.; Gopal, R.; Ladha, J.; Gupta, R.; Kumar, V.; Singh, M. Integrated weed management: A key to success for direct-seeded rice in the Indo-Gangetic plains. In Integrated Crop and Resource Management in the Rice–Wheat System of South Asia; International Rice Research Institute: Los Banos, Philippines, 2009; pp. 261–278. [Google Scholar]
- Hoagland, R.E.; Boyette, C.D.; Weaver, M.A.; Abbas, H.K. Bioherbicides: Research and risks. Toxin Rev. 2007, 26, 313–342. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; El-Darier, S.M. Management of the noxious weed; Medicago polymorpha L. via allelopathy of some medicinal plants from Taif region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef]
- Aliki, H.M.; Reade, J.P.; Back, M.A. Effects of concentrations of Brassica napus (L.) water extracts on the germination and growth of weed species. Allelopath. J. 2014, 34, 287. [Google Scholar]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. essential oil: A promising candidate for botanical herbicide against Echinochloa crus-galli (L.) P. Beauv. in maize cultivation. Ind. Crop. Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Hosni, K.; Hassen, I.; Sebei, H.; Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crop. Prod. 2013, 44, 263–271. [Google Scholar] [CrossRef]
- Kaab, S.; Rebey, I.; Hanafi, M.; Hammi, K.; Smaoui, A.; Fauconnier, M.-L.; De Clerck, C.; Jijakli, M.; Ksouri, R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Ootani, M.A.; dos Reis, M.R.; Cangussu, A.S.R.; Capone, A.; Fidelis, R.R.; Oliveira, W.; Barros, H.B.; Portella, A.C.F.; de Souza Aguiar, R.; dos Santos, W.F.J.B.; et al. Phytotoxic effects of essential oils in controlling weed species Digitaria horizontalis and Cenchrus echinatus. Biocatal. Agric. Biotechnol. 2017, 12, 59–65. [Google Scholar] [CrossRef]
- Koodkaew, I.; Senaphan, C.; Sengseang, N.; Suwanwong, S.J.A.; Resources, N. Characterization of phytochemical profile and phytotoxic activity of Mimosa pigra L. Agric. Nat. Resour. 2018, 52, 162–168. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.M.; Hasan, M.J.A. Bioherbicidal properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata extracts on selected crop and weed species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Amri, I.; Hanana, M.; Jamoussi, B.; Hamrouni, L. Essential oils of Pinus nigra JF Arnold subsp. laricio Maire: Chemical composition and study of their herbicidal potential. Arab. J. Chem. 2017, 10, S3877–S3882. [Google Scholar] [CrossRef]
- Morra, M.J.; Popova, I.E.; Boydston, R.A. Bioherbicidal activity of Sinapis alba seed meal extracts. Ind. Crop. Prod. 2018, 115, 174–181. [Google Scholar] [CrossRef]
- Cimmino, A.; Zonno, M.C.; Andolfi, A.; Troise, C.; Motta, A.; Vurro, M.; Evidente, A. Agropyrenol, a phytotoxic fungal metabolite, and its derivatives: A structure–activity relationship study. J. Agric. Food Chem. 2013, 61, 1779–1783. [Google Scholar] [CrossRef]
- Andolfi, A.; Boari, A.; Evidente, M.; Cimmino, A.; Vurro, M.; Ash, G.; Evidente, A. Gulypyrones A and B and Phomentrioloxins B and C Produced by Diaporthe gulyae, a Potential Mycoherbicide for Saffron Thistle (Carthamus lanatus). J. Nat. Prod. 2015, 78, 623–629. [Google Scholar] [CrossRef]
- Daniel Jr, J.J.; Zabot, G.L.; Tres, M.V.; Harakava, R.; Kuhn, R.C.; Mazutti, M.A. Fusarium fujikuroi: A novel source of metabolites with herbicidal activity. Biocatal. Agric. Biotechnol. 2018, 14, 314–320. [Google Scholar] [CrossRef]
- Kalam, S.; Khan, N.A.; Singh, J. A novel phytotoxic phenolic compound from Phoma herbarum FGCC# 54 with herbicidal potential. Chem. Nat. Compd. 2014, 50, 644–647. [Google Scholar]
- Adetunji, C.O.; Oloke, J.K.; Osemwegie, O.O. Environmental fate and effects of granular pesta formulation from strains of Pseudomonas aeruginosa C1501 and Lasiodiplodia pseudotheobromae C1136 on soil activity and weeds. Chemosphere 2018, 195, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Piyaboon, O.; Pawongrat, R.; Unartngam, J.; Chinawong, S.; Unartngam, A. Pathogenicity, host range and activities of a secondary metabolite and enzyme from Myrothecium roridum on water hyacinth from Thailand. Weed Biol. Manag. 2016, 16, 132–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhu, Y.; Li, L.; Zhang, Y.; Li, J.; Song, X.; Qiang, S. Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol. Control 2019, 139, 104093. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Technol. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Kennedy, A.C. Selective soil bacteria to manage downy brome, jointed goatgrass, and medusahead and do no harm to other biota. Biol. Control 2018, 123, 18–27. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Carlson, C.H.; Feris, K.P.; Germino, M.J.; Jandreau, C.J.; Lazarus, B.E.; Mangold, J.; Pellatz, D.W.; Ramsey, P.; Rinella, M.J.; et al. Weed-suppressive bacteria fail to control Bromus tectorum under field conditions. Rangel. Ecol. Manag. 2020, 73, 760–765. [Google Scholar] [CrossRef]
- Salama, M.; Abdelaziz, H.A.; El-Dien, M.H.Z. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014, 8, 84–89. [Google Scholar]
- Qihe, Y.; Wanhui, Y.E.; Fulin, L.; Fulin, L. Effects of allelochemicals on seed germination. Chin. J. Ecol. 2005, 24, 1459–1465. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Alsaadawi, I.S.; Baerson, S.R. Sorghum allelopathy—From ecosystem to molecule. J. Chem. Ecol. 2013, 39, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kruidhof, H.M.; Bastiaans, L.; Kropff, M.J. Cover crop residue management for optimizing weed control. Plant Soil 2009, 318, 169–184. [Google Scholar] [CrossRef]
- Tsiamis, K.; Gervasini, E.; D’amico, F.; Deriu, I.; Katsanevakis, S.; Crocetta, F.; Zenetos, A.; Arianoutsou, M.; Backeljau, T.; Bariche, M.; et al. The EASIN Editorial Board: Quality assurance, exchange and sharing of alien species information in Europe. Manag. Biol. Invasions 2016, 7, 321–328. [Google Scholar] [CrossRef]
- Ahmad Malik, M.; Khan, A.-U.-H. Weed diversity in wheat fields of Upper Indus Plains in Punjab, Pakistan. Pak. J. Weed Sci. Res. 2012, 18, 413. [Google Scholar]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and visnagin, furanochromones from Ammi visnaga (L.) Lam., as potential bioherbicides. J. Agric. Food Chem. 2016, 64, 9475–9487. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L. Essential oils: What they are and how the terms are used and defined. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–10. [Google Scholar]
- Verdeguer, M.; Sanchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A.J.F. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Boydston, R.A.; Anderson, T.; Vaughn, S.F. Mustard (Sinapis alba) seed meal suppresses weeds in container-grown ornamentals. HortScience 2008, 43, 800–803. [Google Scholar] [CrossRef]
- Liu, D.; Christians, N.E. The use of hydrolyzed corn gluten meal as a natural preemergence weed control in turf. Intl. Turfgrass Soc. Res. J. 1997, 8, 1043–1050. [Google Scholar]
- Boydston, R.A.; Morra, M.J.; Borek, V.; Clayton, L.; Vaughn, S.F. Onion and weed response to mustard (Sinapis alba) seed meal. Weed Sci. 2011, 59, 546–552. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Radhakrishnan, R.; Duc, P.A. Curtobacterium sp. MA01 generates oxidative stress to inhibit the plant growth. Biocatal. Agric. Biotechnol. 2019, 20, 101274. [Google Scholar] [CrossRef]
- Gealy, D.R.; Gurusiddaiah, S.; Ogg, A.G.; Kennedy, A.C. Metabolites fromPseudomonas fluorescensStrain D7 Inhibit Downy Brome (Bromus tectorum) Seedling Growth. Weed Technol. 2017, 10, 282–287. [Google Scholar] [CrossRef]
- Banowetz, G.M.; Azevedo, M.D.; Armstrong, D.J.; Halgren, A.B.; Mills, D.I. Germination-Arrest Factor (GAF): Biological properties of a novel, naturally-occurring herbicide produced by selected isolates of rhizosphere bacteria. Biol. Control 2008, 46, 380–390. [Google Scholar] [CrossRef]
- Halgren, A.; Maselko, M.; Azevedo, M.; Mills, D.; Armstrong, D.; Banowetz, G. Genetics of germination-arrest factor (GAF) production by Pseudomonas fluorescens WH6: Identification of a gene cluster essential for GAF biosynthesis. Microbiology 2013, 159, 36–45. [Google Scholar] [CrossRef]
- Caldwell, C.J.; Hynes, R.K.; Boyetchko, S.M.; Korber, D.R. Colonization and bioherbicidal activity on green foxtail by Pseudomonas fluorescens BRG100 in a pesta formulation. Can. J. Microbiol. 2012, 58, 1–9. [Google Scholar] [CrossRef]
- Quail, J.W.; Ismail, N.; Pedras, M.S.; Boyetchko, S.M. Pseudophomins A and B, a class of cyclic lipodepsipeptides isolated from a Pseudomonas species. Acta Crystallogr. C 2002, 58, o268–o271. [Google Scholar] [CrossRef]
- Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S.; Trognitz, F. Comparative genome analysis of the vineyard weed endophyte Pseudomonas viridiflava CDRTc14 showing selective herbicidal activity. Sci. Rep. 2017, 7, 17336. [Google Scholar] [CrossRef]
- Kremer, R.J. Bioherbicides and nanotechnology: Current status and future trends. Nano-Biopestic. Today Future Perspect. 2019, 353–366. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E. Bioherbicidal potential ofXanthomonas campestrisfor controlling Conyza canadensis. Biocontrol Sci. Technol. 2014, 25, 229–237. [Google Scholar] [CrossRef]
- Bailey, K.L.; Boyetchko, S.M.; Längle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. [Google Scholar] [CrossRef]
- Charudattan, R. Evaluation of Alternaria Cassiae as a Mycoherbicide for Sicklepod (Cassia obtusifolia) in Regional Field Tests; Auburn University, Alabama Agricultural Station, Department of Research Information: Auburn, AL, USA, 1986. [Google Scholar]
- Dumas, M.; Wood, J.; Mitchell, E.; Boyonoski, N. Control of Stump Sprouting ofPopulus tremuloidesandP. grandidentataby Inoculation withChondrostereum purpureum. Biol. Control 1997, 10, 37–41. [Google Scholar] [CrossRef]
- Boyette, C.; Hoagland, R.; Stetina, K. Extending the host range of the bioherbicidal fungus Colletotrichum gloeosporioides f. sp. aeschynomene. Biocontrol Sci. Technol. 2019, 29, 720–726. [Google Scholar] [CrossRef]
- Nandhini, C.; Ganesh, P.; Yoganathan, K.; Kumar, D. Efficacy of Colletotrichum gloeosporioides, potential fungi for bio control of Echinochloa crus-galli (Barnyard grass). J. Drug Deliv. Ther. 2019, 9, 72–75. [Google Scholar] [CrossRef]
- Galea, V.J. Use of stem implanted bioherbicide capsules to manage an infestation of Parkinsonia aculeata in northern Australia. Plants 2021, 10, 1909. [Google Scholar] [CrossRef]
- Morris, M.; Wood, A.; Den Breeÿen, A. Plant pathogens and biological control of weeds in South Africa: A review of projects and progress during the last decade. Afr. Entomol. Mem. 1999, 1, 129–137. [Google Scholar]
- Butt, T.M.; Copping, L.G. Fungal biological control agents. Pestic. Outlook 2000, 11, 186–191. [Google Scholar] [CrossRef]
- Green, S. A review of the potential for the use of bioherbicides to control forest weeds in the UK. Forestry 2003, 76, 285–298. [Google Scholar] [CrossRef]
- Andersen, R.N.; Walker, H.L. Colletotrichum coccodes: A pathogen of eastern black nightshade (Solanum ptycanthum). Weed Sci. 1985, 33, 902–905. [Google Scholar] [CrossRef]
- Bowers, R. Commercialization of Collego™–An Industrialist’s View. Weed Sci. 1986, 34, 24–25. [Google Scholar] [CrossRef]
- Mortensen, K. The potential of an endemic fungus, Colletotrichum gloeosporioides, for biological control of round-leaved mallow (Malva pusilla) and velvetleaf (Abutilon theophrasti). Weed Sci. 1988, 36, 473–478. [Google Scholar] [CrossRef]
- Vieira, B.; Dias, L.; Langoni, V.; Lopes, E. Liquid fermentation of Colletotrichum truncatum UFU 280, a potential mycoherbicide for beggartick. Australas. Plant Pathol. 2018, 47, 277–283. [Google Scholar] [CrossRef]
- Fernando, W.; Watson, A.; Paulitz, T. A simple technique to observe conidial germination on leaf surfaces. Mycologist 1993, 7, 188–189. [Google Scholar] [CrossRef]
- Fernando, W.; Watson, A.; Paulitz, T. Phylloplane Pseudomonas spp. enhance disease caused by Colletotrichum coccodes on velvetleaf. Biol. Control 1994, 4, 125–131. [Google Scholar] [CrossRef]
- Fernando, W.; Watson, A.; Paulitz, T. The role of Pseudomonas spp. and competition for carbon, nitrogen and iron in the enhancement of appressorium formation by Colletotrichum coccodes on velvetleaf. Eur. J. Plant Pathol. 1996, 102, 1–7. [Google Scholar] [CrossRef]
- Boyetchko, S.M.; Bailey, K.L.; Hynes, R.K.; Peng, G. 30 Development of the Mycoherbicide, BioMal. In A Global Perspective; CABI Publishing: Wallingford, UK, 2007; p. 274. [Google Scholar]
- Roberts, J.; Florentine, S.; Fernando, W.D.; Tennakoon, K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Osadebe, V.; Dauda, N.; Ede, A.; Chimdi, G.; Echezona, B. The use of bioherbicides in weed control: Constraints and prospects. Afr. J. Agric. Tech. 2021, 21, 37–54. [Google Scholar]
- Ridings, W. Biological control of stranglervine in citrus–a researcher’s view. Weed Sci. 1986, 34, 31–32. [Google Scholar] [CrossRef]
- Phatak, S.C.; Sumner, D.R.; Wells, H.D.; Bell, D.K.; Glaze, N.C. Biological control of yellow nutsedge with the indigenous rust fungus Puccinia canaliculata. Science 1983, 219, 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dieyeh, M.H.; Watson, A.K. Grass overseeding and a fungus combine to control Taraxacum officinale. J. Appl. Ecol. 2007, 44, 115–124. [Google Scholar] [CrossRef]
- Aneja, K.; Kumar, V.; Jiloha, P.; Kaur, M.; Sharma, C.; Surain, P.; Dhiman, R.; Aneja, A. Potential bioherbicides: Indian perspectives. Biotechnol. Prospect. Appl. 2013, 197–215. [Google Scholar]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 245–266. [Google Scholar]
- Sekhar, J.; Sandhya, S.; Vinod, K.; Banji, D.; Sudhakar, K.; Chaitanya, R.S.N.A.K.K. Plant toxins-useful and harmful effects. Hygeia-J. Drugs 2012, 4, 79–90. [Google Scholar]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Grayson, M.; Cosgrove, S.; Crowe, S.; Hope, W.; McCarthy, J.; Mills, J.; Mouton, J.; Paterson, D.L. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, Antiviral Drugs. CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zimdahl, R.L. Fundamentals of Weed Science; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Llewellyn, R.; Ronning, D.; Clarke, M.; Mayfield, A.; Walker, S.; Ouzman, J. Impact of Weeds in Australian Grain Production; Grains Research and Development Corporation: Canberra, ACT, Australia, 2016. [Google Scholar]
- Eslami, S. Weed management in conservation agriculture systems. In Recent Advances in Weed Management; Springer: New York, NY, USA, 2014; pp. 87–124. [Google Scholar]
- Engel, R.; Ilnicki, R.D. Turf weeds and their control. Turfgrass Sci. 1969, 14, 240–287. [Google Scholar]
- Heap, I.J.A.M. The International Survey of Herbicide Resistant Weeds. Available online: https://weedscience.org/Home.aspx (accessed on 2 February 2024).
- Dodge, A.J.E.A. Physiology of Herbicide Action. By M. Devine, SO Duke and C. Fedtke. Englewood Cliffs, New Jersey: PTR Prentice Hall (1992), pp. 441,£ 84.55. ISBN 0-13-679663-X. Exp. Agric. 1993, 29, 524. [Google Scholar] [CrossRef]
- Holm, F.; Kirkland, K.J.; Stevenson, F.C.J.W.T. Defining optimum herbicide rates and timing for wild oat (Avena fatua) control in spring wheat (Triticum aestivum). Weed Technol. 2000, 14, 167–175. [Google Scholar] [CrossRef]
- Kim, D.; Brain, P.; Marshall, E.; Caseley, J.C. Modelling herbicide dose and weed density effects on crop: Weed competition. Weed Res. 2002, 42, 1–13. [Google Scholar] [CrossRef]
- Curran, W.S. Persistence of herbicides in soil. Crop. Soils 2016, 49, 16–21. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Rohan, M.; Stodart, B.J.; Chen, C.; Wu, H.; Doran, G.S. Persistence of atrazine and trifluralin in a clay loam soil undergoing different temperature and moisture conditions. Environ. Pollut. 2021, 276, 116687. [Google Scholar] [CrossRef]
- Moretto, J.A.S.; Altarugio, L.M.; Andrade, P.A.; Fachin, A.L.; Andreote, F.D.; Stehling, E.G. Changes in bacterial community after application of three different herbicides. FEMS Microbiol. Lett. 2017, 364, fnx113. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Yang, Q.; Bai, Y.; Cui, P.; Wen, C.; Liu, C.; Chen, Z.; Tang, J.; Che, J.; et al. Herbicide Selection Promotes Antibiotic Resistance in Soil Microbiomes. Mol. Biol. Evol. 2021, 38, 2337–2350. [Google Scholar] [CrossRef]
- Gaw, S.; Close, M.E.; Flintoft, M.J. Fifth national survey of pesticides in groundwater in New Zealand. N. Z. J. Mar. Freshw. Res. 2008, 42, 397–407. [Google Scholar] [CrossRef]
- Hollaway, K.; Kookana, R.S.; Noy, D.; Smith, J.; Wilhelm, N. Crop damage caused by residual acetolactate synthase herbicides in the soils of south-eastern Australia. Aust. J. Exp. Agric. 2006, 46, 1323–1331. [Google Scholar] [CrossRef]
- Florencia, F.M.; Carolina, T.; Enzo, B.; Leonardo, G. Effects of the herbicide glyphosate on non-target plant native species from Chaco forest (Argentina). Ecotoxicol. Environ. Saf. 2017, 144, 360–368. [Google Scholar] [CrossRef]
- Felsot, A.S.; Unsworth, J.B.; Linders, J.B.; Roberts, G.; Rautman, D.; Harris, C.; Carazo, E. Agrochemical spray drift; assessment and mitigation—A review. J. Environ. Sci. Health Part B 2010, 46, 1–23. [Google Scholar] [CrossRef]
- Freydier, L.; Lundgren, J.G. Unintended effects of the herbicides 2,4-D and dicamba on lady beetles. Ecotoxicology 2016, 25, 1270–1277. [Google Scholar] [CrossRef]
- Correia, F.V.; Moreira, J.C. Effects of glyphosate and 2,4-D on earthworms (Eisenia foetida) in laboratory tests. Bull. Environ. Contam. Toxicol. 2010, 85, 264–268. [Google Scholar] [CrossRef]
- Maillot, T.; Vioix, J.B.; Colbach, N. Site-specific herbicide spraying can control weeds as well as full spraying in the long-term. A simulation study. Comput. Electron. Agric. 2023, 214, 108338. [Google Scholar] [CrossRef]
- Nazarkov, M.; Gerasimova, I. Dynamics of weeds and integrated weed control in crop rotation with cereals. Rasteniev’dni Nauk./Bulg. J. Crop Sci. 2023, 60, 17–25. [Google Scholar]
- Mauromicale, G.; Monaco, A.L.; Longo, A.M.; Restuccia, A.J.W.s. Soil solarization, a nonchemical method to control branched broomrape (Orobanche ramosa) and improve the yield of greenhouse tomato. Weed Sci. 2005, 53, 877–883. [Google Scholar] [CrossRef]
- Lombardo, S.; Longo, A.; Monaco, A.L.; Mauromicale, G. The effect of soil solarization and fumigation on pests and yields in greenhouse tomatoes. Crop Prot. 2012, 37, 59–64. [Google Scholar] [CrossRef]
- Mauro, R.P.; Monaco, A.L.; Lombardo, S.; Restuccia, A.; Mauromicale, G. Eradication of Orobanche/Phelipanche spp. seedbank by soil solarization and organic supplementation. Sci. Hortic. 2015, 193, 62–68. [Google Scholar] [CrossRef]
- Mauromicale, G.; Restuccia, G.; Marchese, M.J.A. Soil solarization, a non-chemical technique for controlling Orobanche crenata and improving yield of faba bean. Agronomie 2001, 21, 757–765. [Google Scholar] [CrossRef]
- Horowitz, M.; Regev, Y.; Herzlinger, G. Solarization for weed control. Weed Sci. 1983, 31, 170–179. [Google Scholar] [CrossRef]
- Berti, A.; Zanin, G.; Onofri, A.; Sattin, M. Sistema integrato di gestione delle malerbe (IWMS). In Malerbologia; Patron Editore: Granarolo Dell’emilia, Italy, 2001; pp. 659–716. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Bàrberi, P.; Mazzoncini, M. Changes in weed community composition as influenced by cover crop and management system in continuous corn. Weed Sci. 2001, 49, 491–499. [Google Scholar] [CrossRef]
- Rudell, E.C.; Zanrosso, B.A.; Frandaloso, D.; Giacomini, A.J.; Spadotto, D.V.; Vargas, L.; Nunes, A.L.; Santos, F.M. Integrated weed management strategies in a long-term crop rotation system. Adv. Weed Sci. 2023, 41, e020220053. [Google Scholar] [CrossRef]
- Weisberger, D.; Nichols, V.; Liebman, M. Does diversifying crop rotations suppress weeds? A meta-analysis. PLoS ONE 2019, 14, e0219847. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Bajwa, A.; Chauhan, B. Weed management using crop competition in Australia. Crop Prot. 2017, 95, 8–13. [Google Scholar] [CrossRef]
- Sheng, Q.; Yu, X.; Zhou, X.; Tian, G.; Wu, H.; Geng, G.; Yan, J.; Li, J.; Ren, T.; Lu, J. Response of biomass and nutrient competition between oilseed rape and weed to the rate of N, P and K fertilizer. Sci. Agric. Sin. 2023, 56, 481–489. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Weed management in New Zealand pastures. Agronomy 2019, 9, 448. [Google Scholar] [CrossRef]
- Wardle, D.; Nicholson, K.; Ahmed, M.; Rahman, A. Influence of pasture forage species on seedling emergence, growth and development of Carduus nutans. J. Appl. Ecol. 1995, 32, 225–233. [Google Scholar] [CrossRef]
- Eerens, J.; Rahman, A.; James, T.K. Optimising pasture production to minimise weed growth. Proc. N. Z. Grassl. Assoc. 2002, 64, 143–146. [Google Scholar] [CrossRef]
- Hu, L.; Liu, H.; He, J.; Chen, G.; Wang, Z.; Wang, C. Research progress and prospect of intelligent weeding robot. J. S. China Agric. Univ. 2023, 44, 34–42. [Google Scholar]
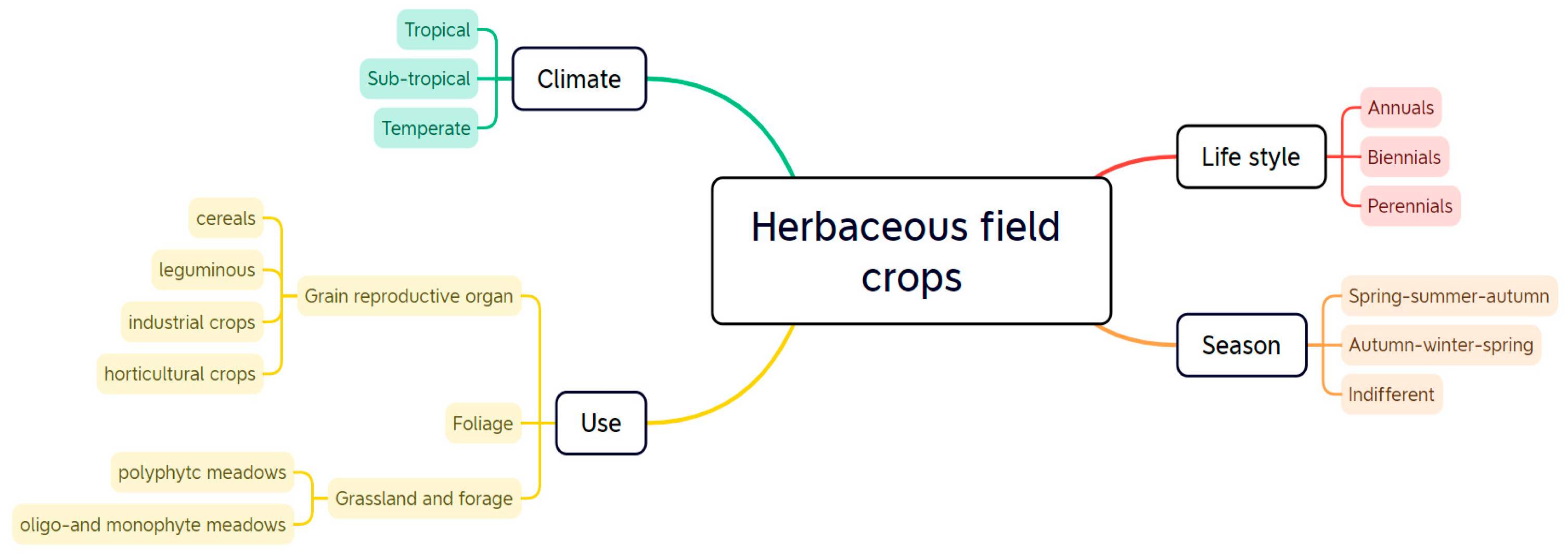
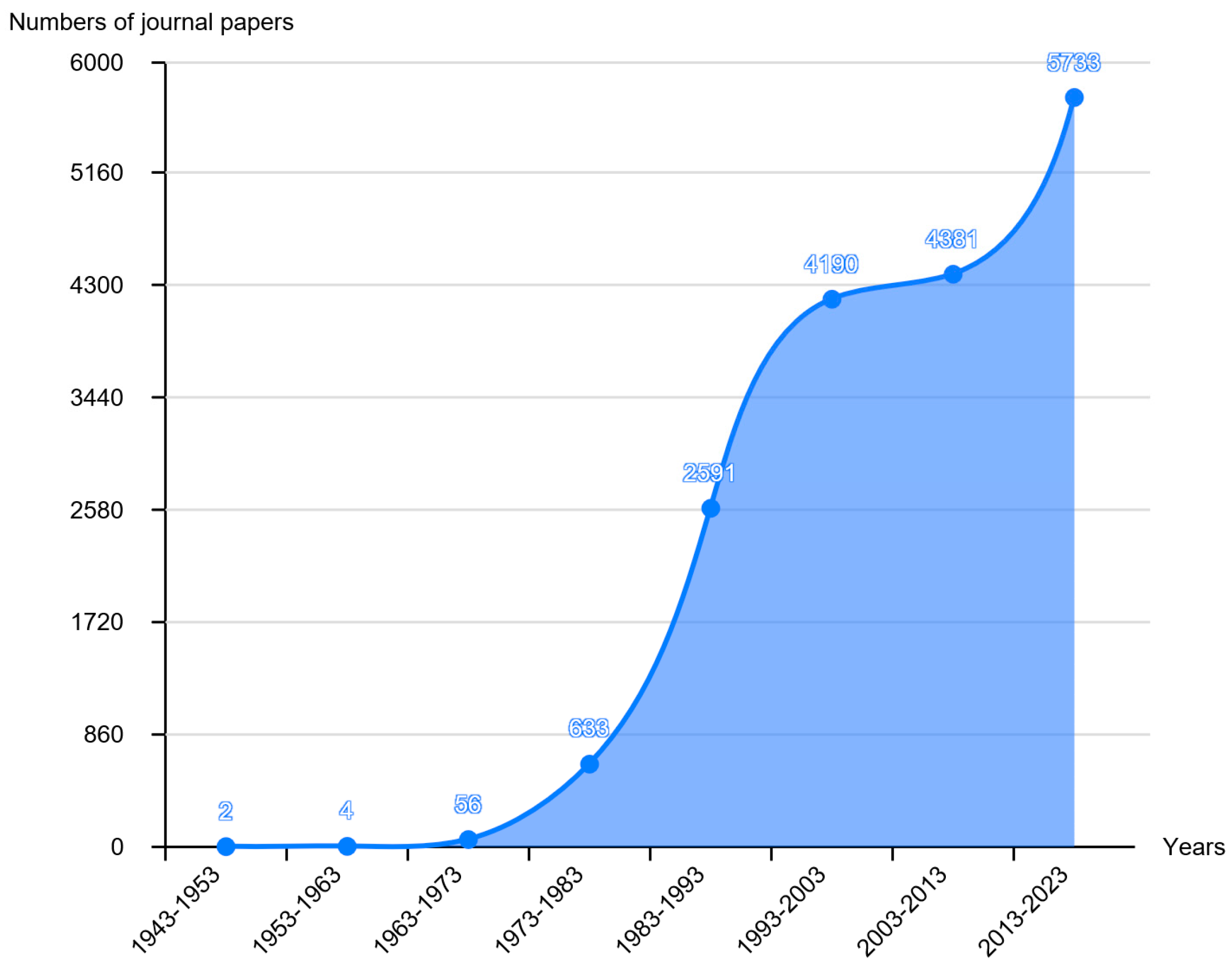
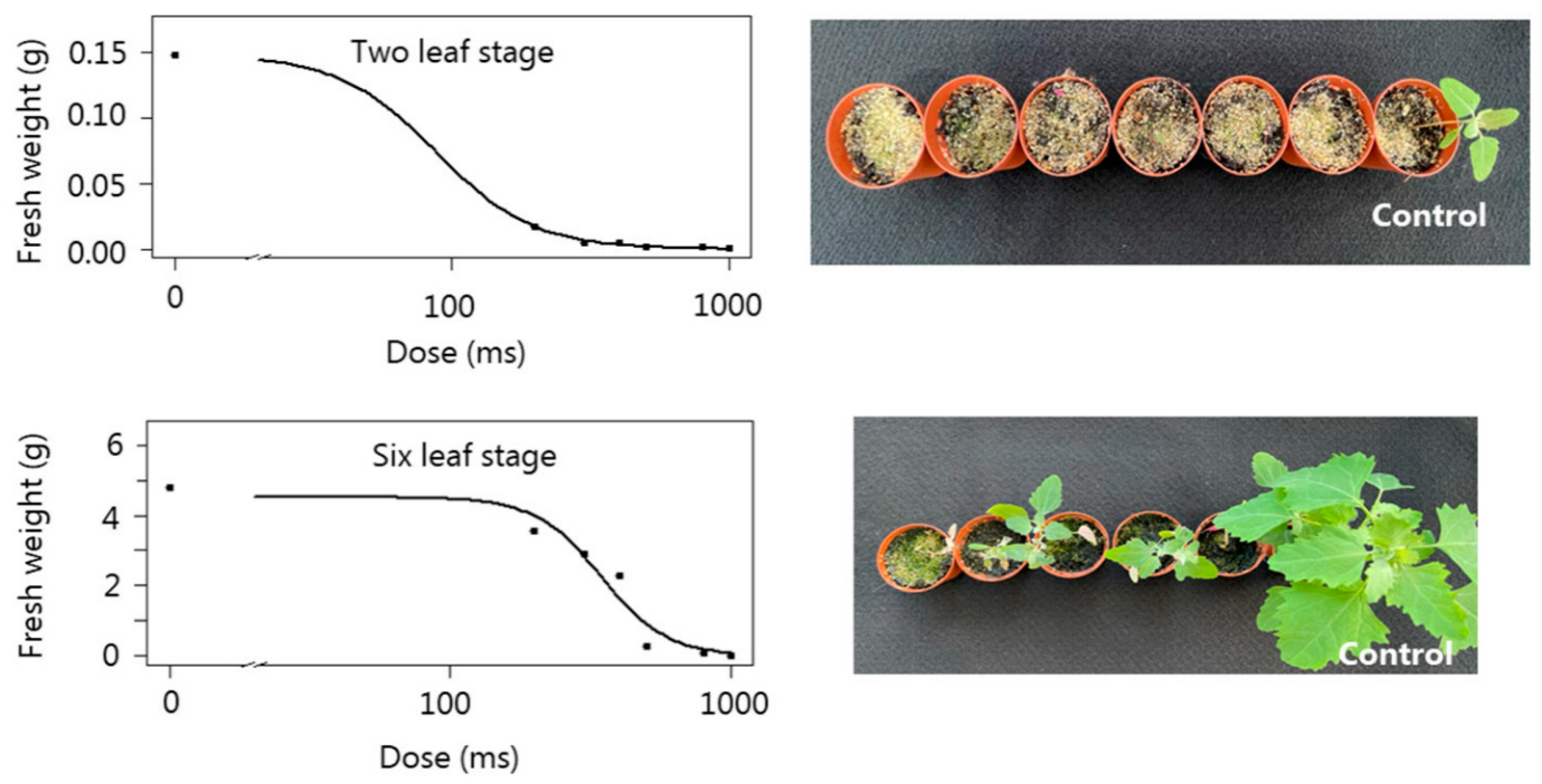
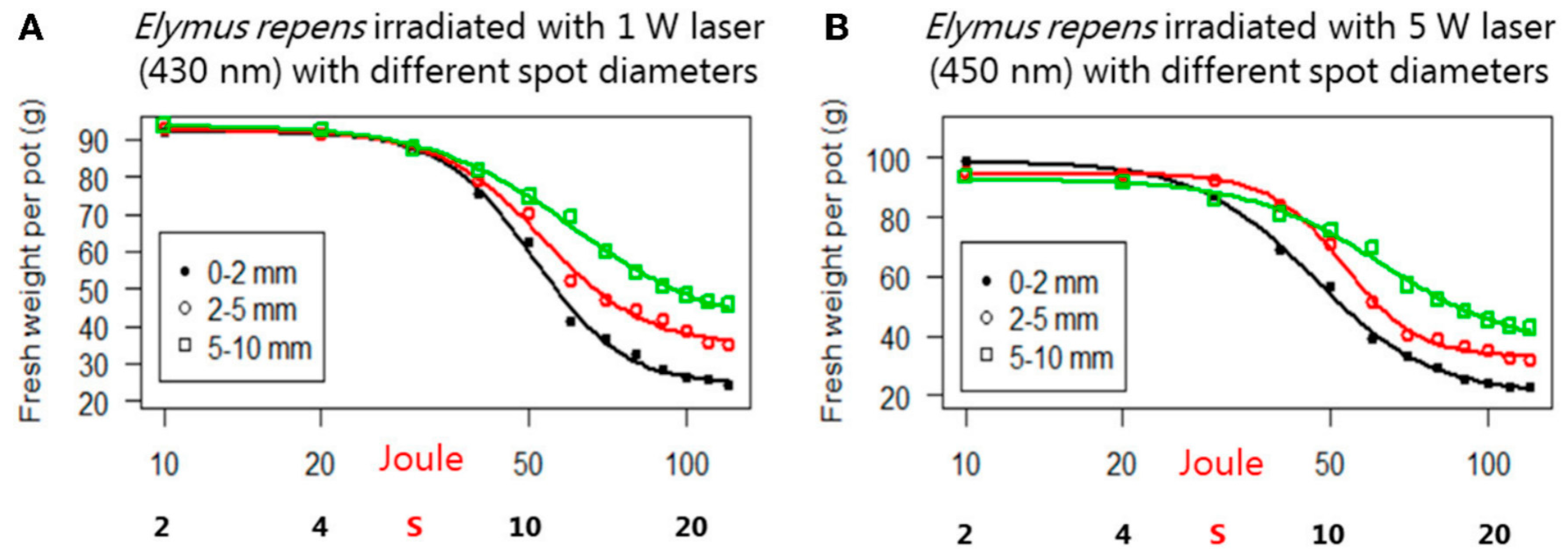

| Article Number | Contribution of the Article | References |
|---|---|---|
| Article 1 | Explore the application of artificial intelligence in weed management, focusing on weed recognition and deep learning. | [13] |
| Article 2 | Describe traditional and non-traditional weed control strategies from a sustainability perspective, highlighting the value of applying precision weed control strategies. | [14] |
| Article 3 | Present a four-step process for developing an integrated weed management strategy. | [1] |
| Weed Management Methods | Number of References |
|---|---|
| Physical weed control | 20 |
| Mechanical weed control | 21 |
| Biological weed control | 63 |
| Chemical weed control | 34 |
| Source | Phytotoxic Effects | Target Weeds | References |
|---|---|---|---|
| Achillea santolina L. | Hinders growth and modifies metabolic activities | Medicago polymorpha L. | [65] |
| Brassica napus L. | Suppress germination and root length | Phalaris minor Retz., Convolvulus arvensis L., Sorghum halepense (L.) Pers. | [66] |
| Carum carvi L. | Damage to leaves and biochemical alterations in plant tissues | Echinochloa crus-galli (L.) P. Beauv. | [67] |
| Chrysanthemum coronarium L. | Inhibition of sprouting and development | Sinapis arvensis L., Phalaris canariensis L. | [68] |
| Cynara cardunculus L. | Inhibit the sprouting and development, leading to necrosis or chlorosis | Trifolium incarnatum L., Silybum marianum (L.) Gaertn., P. minor | [69] |
| Cymbopogon nardus (L.) Rendle. | Suppress the sprouting and growth of plants while lowering the levels of chlorophyll and proteins | Digitaria horizontalis Willd., Cenchrus echinatus L. | [70] |
| Mimosa pigra L. | Deceleration in root development | Lactuca sativa L., Ruellia tuberosa L. | [71] |
| Parthenium hysterophorus L. | The process of seed sprouting and growth | Oryza sativa f. Spontanea Roshev., Echinochloa colona (L.) Link., Euphorbia hirta L., Ageratum conyzoides L. | [72] |
| Pinus densiflora Siebold and Zucc. | Suppressed development of shoots and roots | Lolium multiflorum Lam., Digitaria sanguinalis (L.) Scop. | [73] |
| Pinus nigra J.F. Arnold | Suppressed development of shoots and roots | P. canariensis, Trifolium campestre Schreb., S. arvensis | [74] |
| Sinapis alba L. Fungi | Reduced dry biomass | Amaranthus powellii S. Watson, Setaria viridis (L.) P. Beauv. | [75] |
| Ascochyta agropyrina | Reduced root growth | Chenopodium album L., Cirsium arvense (L.) Scop., Mercurialis annua L., Sonchus oleraceus L., Setariavirdis (L.) P. Beauv. | [76] |
| Diaporthe gulyae | Necrosis | Papaver rhoes L., Ecballium elaterium (L.) A. Rich., Urtica dioica L., Hedysarum coronarium L. | [77] |
| Fusarium fujikuroi | Chlorosis, necrosis, and reduced height and root length | Cucumis sativus L., Sorghum bicolor (L.) Moench. | [78] |
| Phoma herbarum | Maximum toxicity | P. hysterophorus, Lantana camara L., Hyptis suaveolens (L.) Piot., Sida acuta Burm.f. | [79] |
| Lasiodiplodiapseudotheobromae | Suppression of germ activity | Solanum lycopersicum L., Amaranthus hybridus L., E. crus-galli | [80] |
| Myrothecium roridum | Gangrene | Eichhornia crassipes (Mart.) Solms | [81] |
| Bacteria | Reduced weed density | Solidago canadensis L. | [82] |
| Pseudomonas aeruginosa | Hindered the sprouting, growth, and germination of seeds | A. hybridus, S. lycopersicum, E. crus-galli, Pennisetum purpureum Schumach. | [78,83] |
| Pseudomonas fluorescens | Inhibit the sprouting and development of roots | Bromus tectorum L., Aegilops cylindrical Host, Taeniatherum caput-medusae (L.) Nevski | [84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.-T.; Su, W.-H. Weed Management Methods for Herbaceous Field Crops: A Review. Agronomy 2024, 14, 486. https://doi.org/10.3390/agronomy14030486
Gao W-T, Su W-H. Weed Management Methods for Herbaceous Field Crops: A Review. Agronomy. 2024; 14(3):486. https://doi.org/10.3390/agronomy14030486
Chicago/Turabian StyleGao, Wen-Tao, and Wen-Hao Su. 2024. "Weed Management Methods for Herbaceous Field Crops: A Review" Agronomy 14, no. 3: 486. https://doi.org/10.3390/agronomy14030486
APA StyleGao, W.-T., & Su, W.-H. (2024). Weed Management Methods for Herbaceous Field Crops: A Review. Agronomy, 14(3), 486. https://doi.org/10.3390/agronomy14030486







