Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
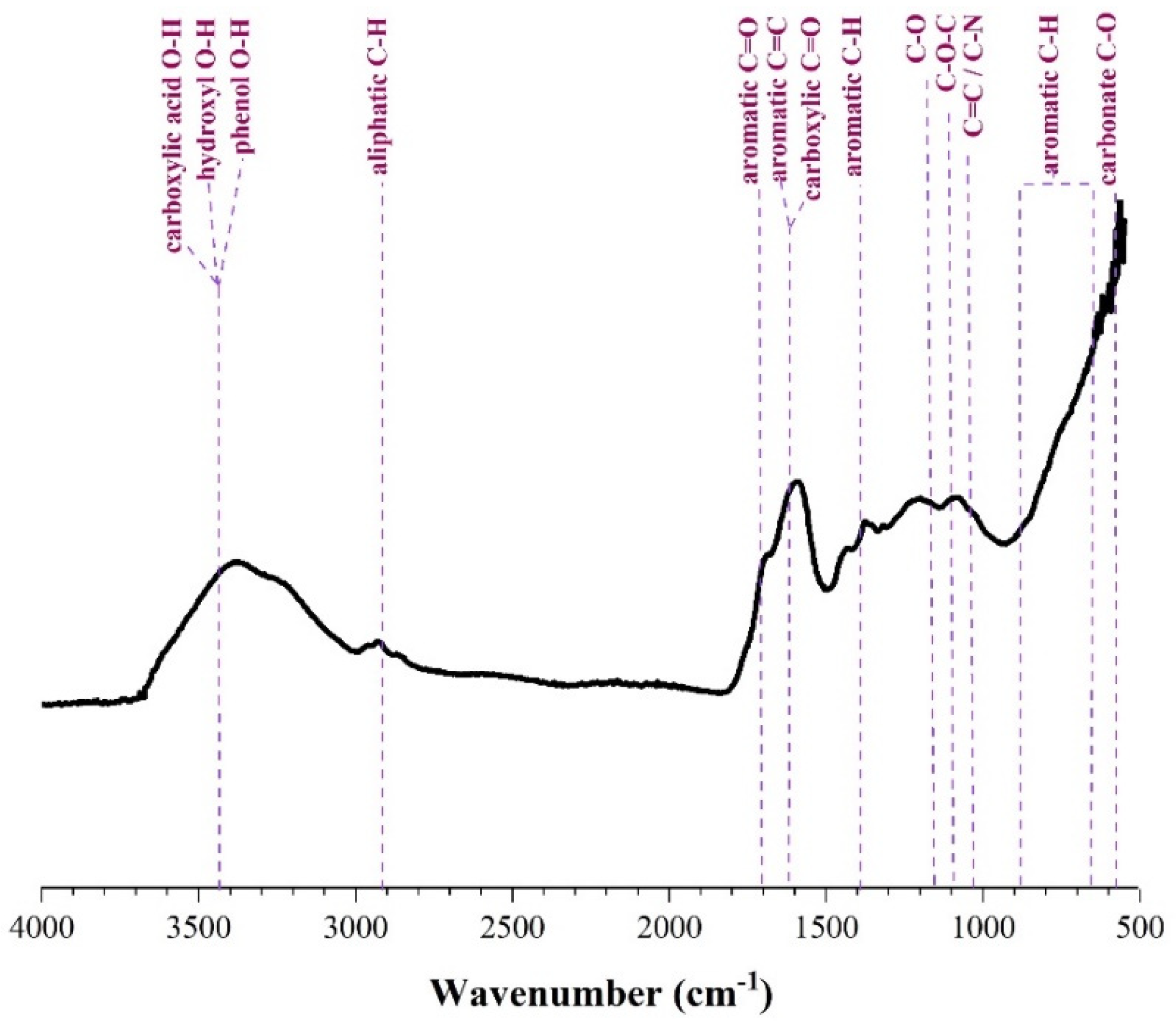
2.2. Biochar Preparation and Characterization
2.3. Experimental Design
2.4. Soil Characteristics
2.5. Data Analysis
3. Results
3.1. Changes in Soil pH and Cations
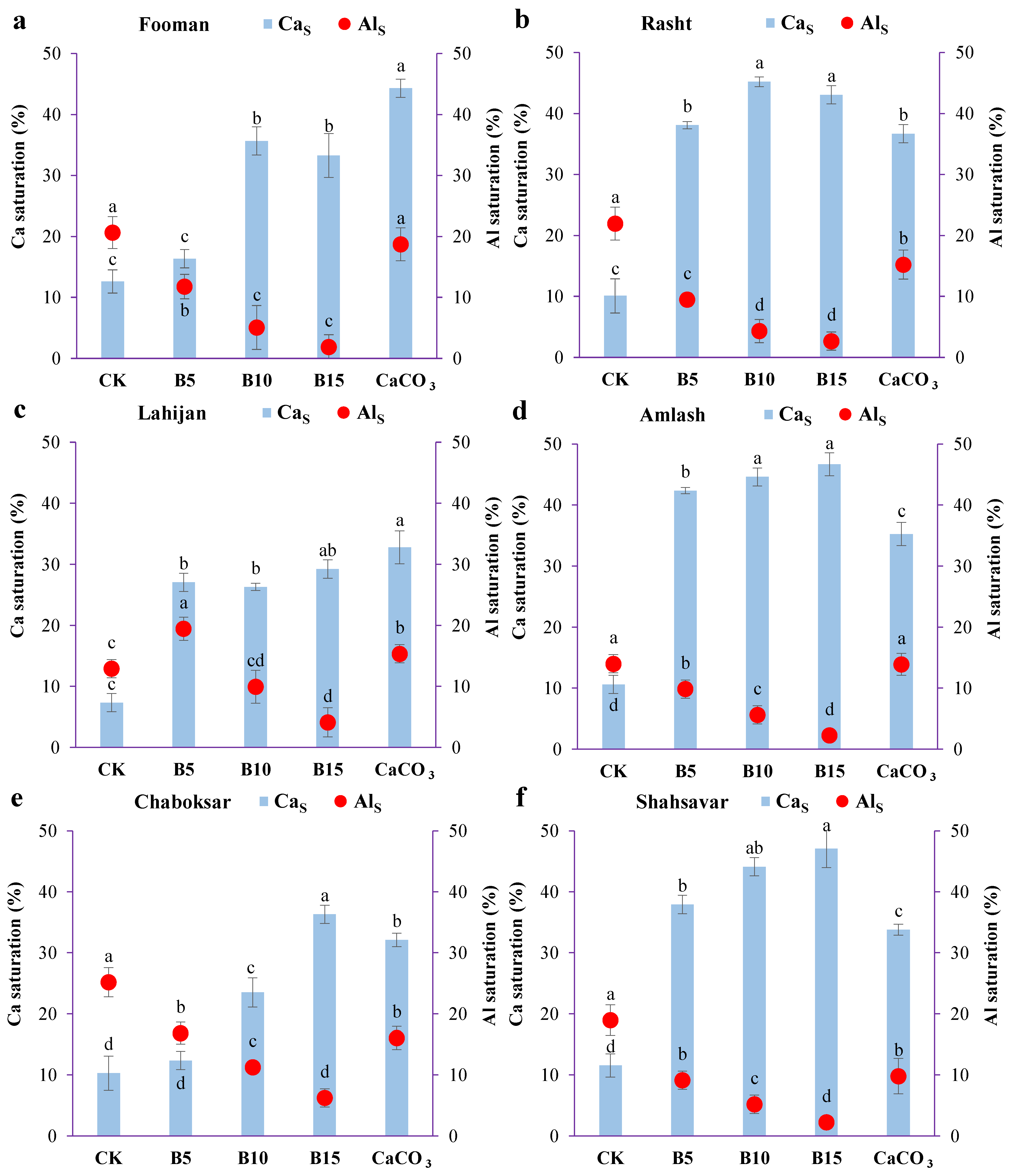
3.2. Soil Al and Ca Saturation
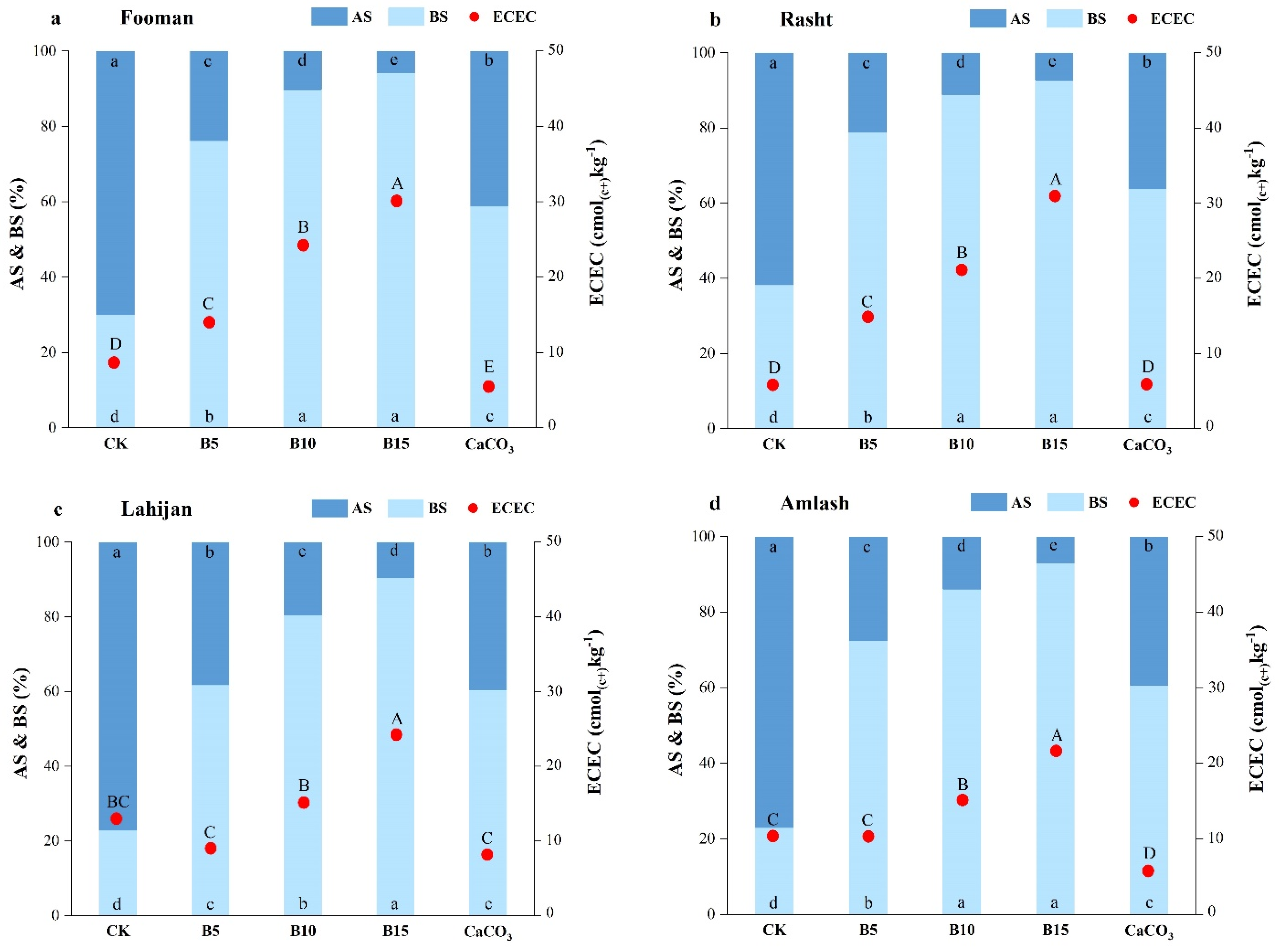
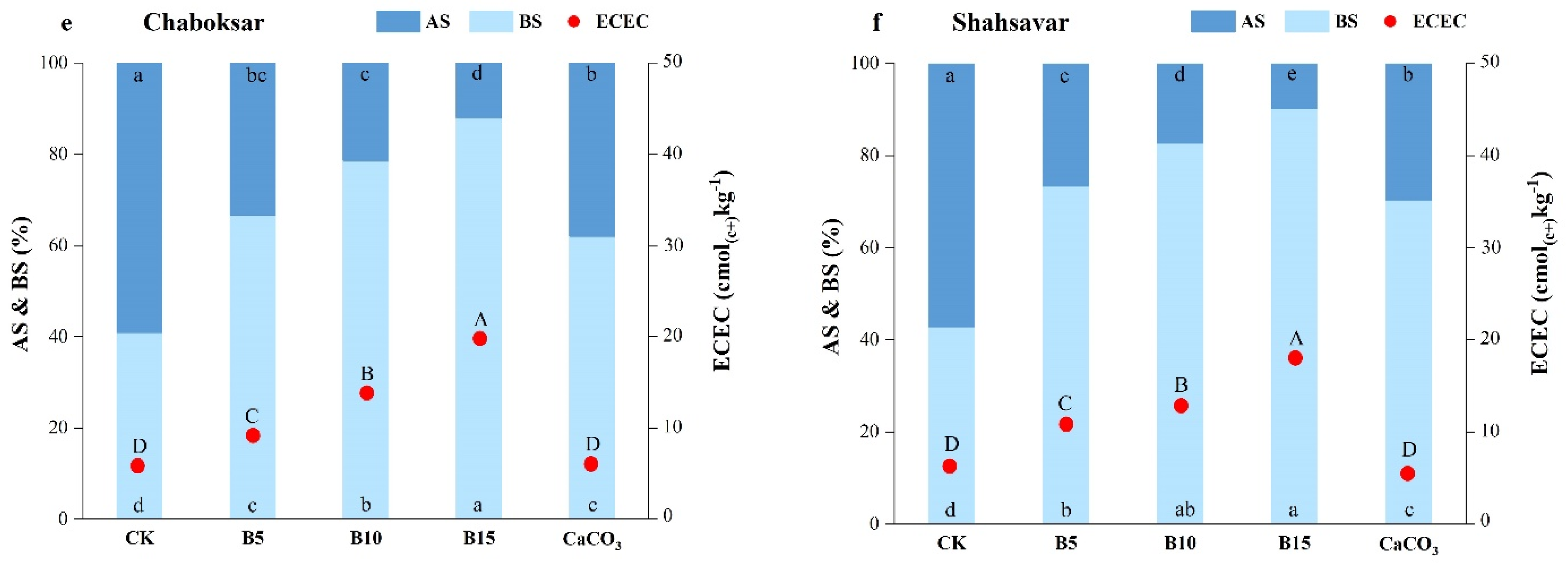
3.3. Soil Effective Cation Exchange Capacity (ECEC)
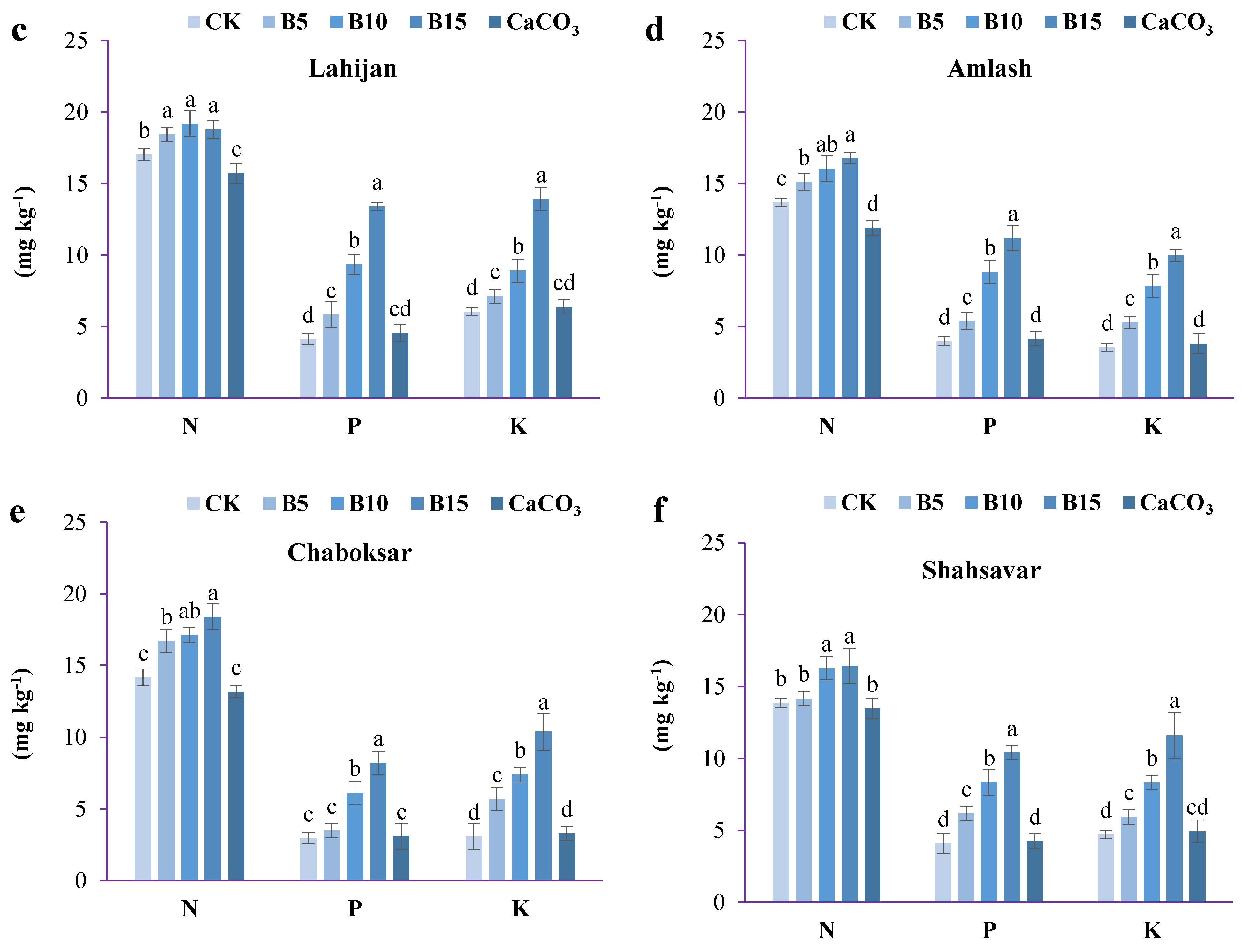
3.4. Changes in Available NPK
3.5. Pearson Correlation between Soil Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, S.; Feng, H.; Yang, F.; Zhao, Z.; Hu, X.; Wei, C.; Liang, T.; Li, H.; Geng, Y. Does Dual Reduction in Chemical Fertilizer and Pesticides Improve Nutrient Loss and Tea Yield and Quality? A Pilot Study in a Green Tea Garden in Shaoxing, Zhejiang Province, China. Environ. Sci. Pollut. Res. 2019, 26, 2464–2476. [Google Scholar] [CrossRef]
- Warner, J.M.; Mann, M.L.; Chamberlin, J.; Tizale, C.Y. Estimating Acid Soil Effects on Selected Cereal Crop Productivities in Ethiopia: Comparing Economic Cost-Effectiveness of Lime and Fertilizer Applications. PLoS ONE 2023, 18, e0280230. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular Approaches Unravel the Mechanism of Acid Soil Tolerance in Plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, R.; Zhang, C. Does the Adoption of Complex Fertilizers Contribute to Fertilizer Overuse? Evidence from Rice Production in China. J. Clean. Prod. 2019, 219, 677–685. [Google Scholar] [CrossRef]
- Le, V.S.; Herrmann, L.; Hudek, L.; Nguyen, T.B.; Bräu, L.; Lesueur, D. How Application of Agricultural Waste Can Enhance Soil Health in Soils Acidified by Tea Cultivation: A Review. Environ. Chem. Lett. 2022, 20, 813–839. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Banik, A. Exploring Tea (Camellia sinensis) Microbiome: Insights into the Functional Characteristics and Their Impact on Tea Growth Promotion. Microbiol. Res. 2022, 254, 126890. [Google Scholar] [CrossRef]
- Xia, W.; Li, C.; Nie, J.; Shao, S.; Rogers, K.M.; Zhang, Y.; Li, Z.; Yuan, Y. Stable Isotope and Photosynthetic Response of Tea Grown under Different Temperature and Light Conditions. Food Chem. 2022, 368, 130771. [Google Scholar] [CrossRef]
- Da Silva, C.P.; De Almeida, B.G.; Romero, R.E.; De Alencar, T.L.; Lobato, M.G.R.; Oliveira, L.D.S.; Souza, L.D.S.; Costa, M.C.G.; Mota, J.C.A. Cohesive Character in Alfisols, Ultisol and Oxisols in Northeast of Brazil: Relationship with Tensile Strength and Particle Size. Geoderma Reg. 2020, 23, e00341. [Google Scholar] [CrossRef]
- Haby, V.A.; Russelle, M.P.; Skogley, E.O. Testing Soils for Potassium, Calcium, and Magnesium. In SSSA Book Series; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 181–227. ISBN 978-0-89118-862-9. [Google Scholar]
- Xu, Q.; Hu, K.; Wang, X.; Wang, D.; Knudsen, M.T. Carbon Footprint and Primary Energy Demand of Organic Tea in China Using a Life Cycle Assessment Approach. J. Clean. Prod. 2019, 233, 782–792. [Google Scholar] [CrossRef]
- Ray, P.; Chattaraj, S.; Bandyopadhyay, S.; Jena, R.K.; Singh, S.K.; Ray, S.K. Shifting Cultivation, Soil Degradation, and Agricultural Land-use Planning in the Northeastern Hill Region of India Using Geo-spatial Techniques. Land Degrad. Dev. 2021, 32, 3870–3892. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F.; De Vries, W. Modeling Soil Acidification in Typical Chinese Cropping Systems. Sci. Total Environ. 2018, 613–614, 1339–1348. [Google Scholar] [CrossRef]
- Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J.; Dovciak, M.; Bailey, S.W.; Antidormi, M.R.; Zarfos, M.R. Soil Base Saturation Combines with Beech Bark Disease to Influence Composition and Structure of Sugar Maple-Beech Forests in an Acid Rain-Impacted Region. Ecosystems 2018, 21, 795–810. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil Acidification in Chinese Tea Plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.; Kabir, A.; Jones, C.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, M. Aluminum Uptake and Migration from the Soil Compartment into Betula Pendula for Two Different Environments: A Polluted and Environmentally Protected Area of Poland. Environ. Sci. Pollut. Res. 2016, 23, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Liao, H. Organic Acid Anions: An Effective Defensive Weapon for Plants against Aluminum Toxicity and Phosphorus Deficiency in Acidic Soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium Is Essential for Root Growth and Development of Tea Plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Xia, E.-H.; Tong, W.; Wu, Q.; Wei, S.; Zhao, J.; Zhang, Z.-Z.; Wei, C.-L.; Wan, X.-C. Tea Plant Genomics: Achievements, Challenges and Perspectives. Hortic. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Gebrewold, A.Z. Review on Integrated Nutrient Management of Tea (Camellia sinensis L.). Cogent Food Agric. 2018, 4, 1543536. [Google Scholar] [CrossRef]
- Barcelos, J.P.Q.; Mariano, E.; Jones, D.L.; Rosolem, C.A. Topsoil and Subsoil C and N Turnover Are Affected by Superficial Lime and Gypsum Application in the Short-Term. Soil Biol. Biochem. 2021, 163, 108456. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of Biochar Application on Crop Productivity, Soil Carbon Sequestration, and Global Warming Potential Controlled by Biochar C:N Ratio and Soil pH: A Global Meta-Analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, L.; Chen, B. Driving Forces Linking Microbial Community Structure and Functions to Enhanced Carbon Stability in Biochar-Amended Soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef]
- Masud, M.M.; Baquy, M.A.-A.; Akhter, S.; Sen, R.; Barman, A.; Khatun, M.R. Liming Effects of Poultry Litter Derived Biochar on Soil Acidity Amelioration and Maize Growth. Ecotoxicol. Environ. Saf. 2020, 202, 110865. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, J.; Ni, N.; Xu, R. Understanding the Biochar’s Role in Ameliorating Soil Acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How Do Different Feedstocks and Pyrolysis Conditions Effectively Change Biochar Modification Scenarios? A Critical Analysis of Engineered Biochars under H2O2 Oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Zamanian, K. In-situ Biochar Production Associated with Paddies: Direct Involvement of Farmers in Greenhouse Gases Reduction Policies besides Increasing Nutrients Availability and Rice Production. Land Degrad. Dev. 2021, 32, 3893–3904. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum Toxicity in Plants and Its Possible Mitigation in Acid Soils by Biochar: A Review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Moudrý, J.; Menšík, L. Preliminary Findings on Cadmium Bioaccumulation and Photosynthesis in Rice (Oryza sativa L.) and Maize (Zea mays L.) Using Biochar Made from C3- and C4-Originated Straw. Plants 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The Effect of Rice Husk Biochar on Soil Nutrient Status, Microbial Biomass and Paddy Productivity of Nutrient Poor Agriculture Soils. Catena 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of Rice Husk Biochar on Selected Soil Properties and Nitrate Leaching in Loamy Sand and Clay Soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Asadi, H.; Ghorbani, M.; Rezaei-Rashti, M.; Abrishamkesh, S.; Amirahmadi, E.; Chengrong, C.; Gorji, M. Application of Rice Husk Biochar for Achieving Sustainable Agriculture and Environment. Rice Sci. 2021, 28, 325–343. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E. Effect of Rice Husk Biochar (RHB) on Some of Chemical Properties of an Acidic Soil and the Absorption of Some Nutrients. J. Appl. Sci. Environ. Manag. 2018, 22, 313. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E. Effect of Rice Husk Biochar on Some Physical Characteristics of Soil and Corn Growth in a Loamy Soil. Iran. J. Soil Res. 2018, 32, 305–318. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effect of Rice Husk Biochar on Nitrate Leaching in a Clayey Soil. Iran. J. Soil Res. 2016, 29, 127–434. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and Quantification of Biochar Alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation Exchange Capacity of Biochar: An Urgent Method Modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Mingorance, M.D.; Barahona, E.; Fernández-Gálvez, J. Guidelines for Improving Organic Carbon Recovery by the Wet Oxidation Method. Chemosphere 2007, 68, 409–413. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, J.; Li, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y. Effects of Biochar Amendment on Nitrogen Mineralization in Black Soil with Different Moisture Contents under Freeze-Thaw Cycles. Geoderma 2019, 353, 459–467. [Google Scholar] [CrossRef]
- Horta, M.D.C.; Torrent, J. The Olsen P Method as an Agronomic and Environmental Test for Predicting Phosphate Release from Acid Soils. Nutr. Cycl. Agroecosyst. 2007, 77, 283–292. [Google Scholar] [CrossRef]
- Lu, D.; Li, C.; Sokolwski, E.; Magen, H.; Chen, X.; Wang, H.; Zhou, J. Crop Yield and Soil Available Potassium Changes as Affected by Potassium Rate in Rice–Wheat Systems. Field Crops Res. 2017, 214, 38–44. [Google Scholar] [CrossRef]
- Tournassat, C.; Greneche, J.-M.; Tisserand, D.; Charlet, L. The Titration of Clay Minerals. J. Colloid Interface Sci. 2004, 273, 224–233. [Google Scholar] [CrossRef]
- Kostin, A.S.; Krechetov, P.P.; Chernitsova, O.V.; Terskaya, E.V. Geochemical Transformations in Liquid and Solid Phases of Sod–Podzols in Technogenically Disturbed Landscapes of the Moscow Brown Coal Basin. Mosc. Univ. Soil Sci. Bull. 2021, 76, 283–290. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Li, W.; Johnson, C.E. Relationships among pH, Aluminum Solubility and Aluminum Complexation with Organic Matter in Acid Forest Soils of the Northeastern United States. Geoderma 2016, 271, 234–242. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Lv, W.-X.; Zhao, Z.-Q.; Yang, Y.-P.; Zhang, L.-X.; Wang, L.-Y.; Jing, C.-Y.; Duan, G.-L.; Zhu, Y.-G. Aluminum Adsorption and Antimonite Oxidation Dominantly Regulate Antimony Solubility in Soils. Chemosphere 2022, 309, 136651. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Jalali, M. Effect of Heavy Metals on pH Buffering Capacity and Solubility of Ca, Mg, K, and P in Non-Spiked and Heavy Metal-Spiked Soils. Environ. Monit. Assess. 2016, 188, 342. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.-L.; Zhao, X.-R.; Yang, S.-H.; Mulder, J.; Dörsch, P.; Peng, X.-H.; Zhang, G.-L. Soil Acidification and Loss of Base Cations in a Subtropical Agricultural Watershed. Sci. Total Environ. 2022, 827, 154338. [Google Scholar] [CrossRef]
- Duan, Y.; Lee, J.Y.; Xi, S.; Sun, Y.; Ge, J.; Ong, S.J.H.; Chen, Y.; Dou, S.; Meng, F.; Diao, C.; et al. Anodic Oxidation Enabled Cation Leaching for Promoting Surface Reconstruction in Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 7418–7425. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of Biochar Surface Characteristics in the Adsorption of Aromatic Compounds: Pore Structure and Functional Groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of Biochar on Chemical Properties of Acidic Soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Wan, Q.; Yuan, J.-H.; Xu, R.-K.; Li, X.-H. Pyrolysis Temperature Influences Ameliorating Effects of Biochars on Acidic Soil. Environ. Sci. Pollut. Res. 2014, 21, 2486–2495. [Google Scholar] [CrossRef]
- He, L.; Zhao, J.; Wang, M.; Liu, Y.; Wang, Y.; Yang, S.; Wang, S.; Zhao, X.; Lyu, H. Long-Term Successive Seasonal Application of Rice Straw-Derived Biochar Improves the Acidity and Fertility of Red Soil in Southern China. Agronomy 2023, 13, 505. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Interactions of Aluminum with Biochars and Oxidized Biochars: Implications for the Biochar Aging Process. J. Agric. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of Chemical Oxidation on Surface Oxygen-Containing Functional Groups and Adsorption Behavior of Biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A Critical Review on the Application and Recent Developments of Post-Modified Biochar in Supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecký, M.; Kolář, L. A Meta-analysis on the Impacts of Different Oxidation Methods on the Surface Area Properties of Biochar. Land Degrad. Dev. 2023, 34, 299–312. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and Characterization of Biochar Produced from Slow Pyrolysis of Pigeon Pea Stalk and Bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W.; Shen, Z.; Hou, D. Biochar Aging: Mechanisms, Physicochemical Changes, Assessment, And Implications for Field Applications. Environ. Sci. Technol. 2020, 54, 14797–14814. [Google Scholar] [CrossRef]
- Li, J.; Liang, N.; Jin, X.; Zhou, D.; Li, H.; Wu, M.; Pan, B. The Role of Ash Content on Bisphenol A Sorption to Biochars Derived from Different Agricultural Wastes. Chemosphere 2017, 171, 66–73. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined Effects of Biochar Properties and Soil Conditions on Plant Growth: A Meta-Analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhou, X.; Liu, Q.; Wan, Y.; Cai, J.; Chen, W.; Chen, F.; Ji, L.; Cheng, L.; Luo, H. Properties of Eupatorium adenophora Spreng (Crofton Weed) Biochar Produced at Different Pyrolysis Temperatures. Environ. Eng. Sci. 2019, 36, 937–946. [Google Scholar] [CrossRef]
- Wei, L.; Xu, G.; Shao, H.; Sun, J.; Chang, S.X. Regulating Environmental Factors of Nutrients Release from Wheat Straw Biochar for Sustainable Agriculture. CLEAN-Soil Air Water 2013, 41, 697–701. [Google Scholar] [CrossRef]
- Joseph, U.E.; Toluwase, A.O.; Kehinde, E.O.; Omasan, E.E.; Tolulope, A.Y.; George, O.O.; Zhao, C.; Hongyan, W. Effect of Biochar on Soil Structure and Storage of Soil Organic Carbon and Nitrogen in the Aggregate Fractions of an Albic Soil. Arch. Agron. Soil Sci. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Palansooriya, K.N.; Sang, M.K.; Oh, D.X.; Park, J.; Hwang, S.Y.; Igalavithana, A.D.; Gu, C.; Ok, Y.S. Combined Effect of Biochar and Soil Moisture on Soil Chemical Properties and Microbial Community Composition in Microplastic-contaminated Agricultural Soil. Soil Use Manag. 2022, 38, 1446–1458. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Ko, J.; Wu, H.; Xu, Q. Structure Characteristics of Bio-Char Generated from Co-Pyrolysis of Wooden Waste and Wet Municipal Sewage Sludge. Fuel Process. Technol. 2019, 183, 48–54. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar Application to Low Fertility Soils: A Review of Current Status, and Future Prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of Different Straw Biochars on Soil Organic Carbon, Nitrogen, Available Phosphorus, and Enzyme Activity in Paddy Soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced Nitrification and Abundance of Ammonia-Oxidizing Bacteria in Acidic Soil Amended with Biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef]
- Yoo, G.; Lee, Y.O.; Won, T.J.; Hyun, J.G.; Ding, W. Variable Effects of Biochar Application to Soils on Nitrification-Mediated N2O Emissions. Sci. Total Environ. 2018, 626, 603–611. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yan, W.; Shangguan, Z. Effect of Biochar Application Method on Nitrogen Leaching and Hydraulic Conductivity in a Silty Clay Soil. Soil Tillage Res. 2018, 183, 100–108. [Google Scholar] [CrossRef]



| Property | Fooman | Rasht | Lahijan | Amlash | Chaboksar | Shahsavar |
|---|---|---|---|---|---|---|
| General climate | Humid subtropical | Humid subtropical | Humid subtropical | Humid subtropical | Mediterranean | Mediterranean |
| Average annual temperature (°C) | 19.82 ± 1.34 * | 19.41 ± 1.47 | 18.46 ± 2.45 | 18.91 ± 0.97 | 19.33 ± 0.23 | 20.41 ± 1.23 |
| Average annual precipitation (mm) | 110.2 ± 5.64 | 107.6 ± 2.75 | 102.4 ± 6.89 | 89.95 ± 4.53 | 51.71 ± 9.54 | 54.58 ± 3.45 |
| Rainy days per year | 130.1 ± 1.42 | 127.1 ± 1.28 | 120.9 ± 1.37 | 116.8 ± 1.93 | 79.89 ± 1.81 | 84.31 ± 1.21 |
| Relative humidity (%) | 74.83 ± 2.43 | 73.33 ± 3.73 | 69.82 ± 1.21 | 68.43 ± 1.75 | 62.41 ± 3.85 | 65.85 ± 6.15 |
| Monthly sunshine (h−1) | 11.02 ± 1.2 | 10.75 ± 0.68 | 10.23 ± 0.94 | 10.61 ± 0.83 | 10.43 ± 0.82 | 11.01 ± 1.34 |
| Parent material | Granite | Granite | Granite | Granite | Phyllite | Phyllite |
| Clay minerals | Mica, Montmorillonite | Quartz, Mica | Mica, Feldspar | Feldspar, Montmorillonite | Quartz, Chlorite | Chlorite, Sericite mica |
| Soil taxonomy | Typic Hapludalfs | Typic Hapludalfs | Ultic Hapludalfs | Inceptic Hapludalfs | Typic Dystrudepts | Typic Udorthents |
| Sand:Silt:Clay (%) | 78.3:14.2:7.5 | 57.1:38.2:4.7 | 61.4:10.3:28.3 | 50.1:39.6:10.3 | 55.7:10.1:34.2 | 44.6:24.2:31.2 |
| Soil texture | Loamy sand | Sandy clay | Sandy loam | Sandy clay | Sandy loam | Loam |
| Property | Value | Property | Value |
|---|---|---|---|
| pH | 8.91 | O/C | 0.15 |
| EC (dS m−1) | 0.28 | H/C | 0.61 |
| CEC (cmol(c+) kg−1) | 68.2 | BY (%) | 40.9 |
| Ca2+Ex (cmol(c+) kg−1) | 5.24 | MC (%) | 0.22 |
| Mg2+Ex (cmol(c+) kg−1) | 7.15 | VM (%) | 30.7 |
| K+Ex (cmol(c+) kg−1) | 4.18 | AC (%) | 17.8 |
| Na+Ex (cmol(c+) kg−1) | 1.15 | FC (%) | 51.3 |
| PAv (mg g−1) | 3.76 | SSA (m2 g−1) | 151.8 |
| C (%) | 63.4 | BD (g cm−3) | 0.23 |
| H (%) | 3.24 | Vt (cm3 g−1) | 0.118 |
| N (%) | 3.15 | Vmicro (cm3 g−1) | 0.067 |
| O (%) | 12.4 | Vmeso & macro (cm3 g−1) | 0.051 |
| Sites | Treatments | pH | EC (dS m−1) | OC (%) | Ex-Base Cations (cmol(c+) kg−1) | Ex-Acid Cations (cmol(c+) kg−1) | Av-N Content (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | Na+ | H+ | Al3+ | NH4+ | NO3− | |||||
| Fooman | CK | 3.52 d | 0.35 b | 2.49 d | 1.11 d | 0.57 d | 0.23 d | 0.75 c | 3.020 a | 1.82 a | 5.32 a | 8.54 d |
| B5 | 4.39 c | 0.37 b | 4.76 b | 2.29 c | 4.98 c | 2.65 c | 0.77 c | 0.407 b | 1.65 a | 4.27 b | 12.85 c | |
| B10 | 5.39 b | 0.41 a | 6.57 ab | 8.87 b | 7.58 b | 4.96 b | 0.92 b | 0.041 c | 1.26 b | 3.24 c | 13.45 b | |
| B15 | 6.28 a | 0.42 a | 7.33 a | 10.2 a | 12.3 a | 5.24 a | 1.12 a | 0.005 d | 0.57 c | 2.19 d | 16.42 a | |
| CaCO3 | 4.49 c | 0.39 a | 2.99 cd | 2.58 c | 0.42 d | 0.25 d | 0.18 d | 0.324 b | 1.09 b | 5.38 a | 8.34 d | |
| Rasht | CK | 4.01 c | 0.22 b | 1.85 e | 0.67 e | 0.69 d | 0.36 d | 0.83 b | 0.977 a | 1.46 a | 6.36 a | 7.15 c |
| B5 | 4.72 b | 0.28 ab | 3.65 c | 5.72 c | 3.83 c | 1.41 c | 0.91 b | 0.191 b | 1.42 a | 5.54 b | 10.72 b | |
| B10 | 5.64 a | 0.29 ab | 5.35 b | 10.3 b | 6.23 b | 2.87 b | 0.87 b | 0.023 c | 0.98 c | 5.11 b | 11.14 b | |
| B15 | 6.22 a | 0.32 a | 7.21 a | 13.6 a | 9.72 a | 4.92 a | 1.03 a | 0.006 d | 0.84 c | 3.47 c | 15.53 a | |
| CaCO3 | 4.71 b | 0.31 a | 2.62 d | 2.46 d | 0.59 d | 0.39 d | 0.84 b | 0.195 b | 1.02 b | 6.21 a | 6.72 c | |
| Lahijan | CK | 3.16 c | 0.49 b | 1.44 e | 1.01 d | 1.12 d | 0.68 d | 0.35 c | 6.918 a | 1.78 a | 7.95 a | 9.09 d |
| B5 | 5.01 b | 0.51 b | 3.01 c | 2.42 c | 1.56 c | 1.13 c | 0.42 b | 0.098 c | 1.74 a | 6.98 ab | 11.45 c | |
| B10 | 5.41 a | 0.53 ab | 4.85 b | 4.13 b | 4.83 b | 3.22 b | 0.46 b | 0.039 d | 1.56 b | 5.47 b | 13.73 b | |
| B15 | 5.97 a | 0.56 a | 6.92 a | 7.29 a | 9.21 a | 5.58 a | 0.51 a | 0.011 e | 1.02 c | 3.32 c | 15.47 a | |
| CaCO3 | 4.47 bc | 0.43 c | 2.81 d | 2.63 c | 1.07 d | 0.76 d | 0.39 bc | 0.339 b | 1.39 b | 7.54 a | 8.17 d | |
| Amlash | CK | 3.29 d | 0.36 b | 2.35 e | 1.12 e | 0.42 d | 0.28 c | 0.62 b | 5.129 a | 1.48 a | 6.25 a | 7.44 d |
| B5 | 4.35 c | 0.39 ab | 4.55 c | 4.35 c | 1.84 c | 0.31 c | 0.95 a | 0.447 b | 1.01 b | 5.37 b | 9.75 c | |
| B10 | 5.42 b | 0.41 a | 6.28 b | 6.84 b | 3.53 b | 1.94 b | 0.91 a | 0.038 d | 0.86 c | 3.62 c | 12.42 b | |
| B15 | 6.38 a | 0.43 a | 7.79 a | 10.7 a | 6.35 a | 3.34 a | 0.96 a | 0.004 e | 0.51 d | 2.06 d | 14.71 a | |
| CaCO3 | 4.81 c | 0.42 a | 3.55 d | 2.31 d | 0.62 d | 0.29 c | 0.76 b | 0.155 c | 0.91 b | 5.89 a | 6.02 d | |
| Chaboksar | CK | 4.21 c | 0.69 b | 2.19 d | 0.69 e | 1.29 d | 0.56 c | 0.21 c | 0.617 a | 1.69 a | 6.69 a | 7.46 d |
| B5 | 5.59 b | 0.71 ab | 3.01 c | 1.16 d | 4.15 c | 0.64 b | 0.32 b | 0.026 c | 1.58 b | 6.28 a | 10.43 c | |
| B10 | 6.24 a | 0.73 a | 5.44 b | 3.23 b | 6.29 b | 0.94 a | 0.36 ab | 0.006 d | 1.55 b | 4.37 b | 13.76 b | |
| B15 | 6.66 a | 0.76 a | 6.28 a | 7.16 a | 8.82 a | 0.99 a | 0.41 a | 0.002 d | 1.23 bc | 2.12 c | 16.28 a | |
| CaCO3 | 5.32 b | 0.71 ab | 2.35 cd | 2.24 c | 1.32 d | 0.54 b | 0.23 c | 0.048 b | 1.12 c | 6.05 a | 7.11 d | |
| Shahsavar | CK | 3.97 c | 0.37 b | 2.53 d | 0.86 e | 1.02 c | 0.87 c | 0.43 c | 1.072 a | 1.41 a | 6.78 a | 7.08 a |
| B5 | 4.68 b | 0.39 b | 3.89 c | 5.35 c | 1.52 b | 0.89 c | 0.44 c | 0.209 b | 1.02 b | 5.44 b | 8.74 b | |
| B10 | 5.49 a | 0.42 a | 5.31 b | 7.84 b | 1.97 ab | 0.95 b | 0.59 b | 0.032 d | 0.71 c | 3.63 c | 12.63 c | |
| B15 | 6.13 a | 0.43 a | 7.21 a | 11.5 a | 2.19 a | 1.26 a | 0.73 a | 0.007 e | 0.39 d | 2.12 d | 14.32 d | |
| CaCO3 | 4.73 b | 0.38 b | 3.35 cd | 2.24 d | 1.09 c | 0.92 b | 0.42 c | 0.186 c | 0.65 c | 6.33 a | 7.13 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy 2024, 14, 533. https://doi.org/10.3390/agronomy14030533
Ghorbani M, Amirahmadi E, Bernas J, Konvalina P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy. 2024; 14(3):533. https://doi.org/10.3390/agronomy14030533
Chicago/Turabian StyleGhorbani, Mohammad, Elnaz Amirahmadi, Jaroslav Bernas, and Petr Konvalina. 2024. "Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas" Agronomy 14, no. 3: 533. https://doi.org/10.3390/agronomy14030533
APA StyleGhorbani, M., Amirahmadi, E., Bernas, J., & Konvalina, P. (2024). Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy, 14(3), 533. https://doi.org/10.3390/agronomy14030533







