Occurrence of Diseases and Seed Yield of Early Maturing Soybean Cultivars Grown under the Conditions of Central Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Assessment of the Health Status of Soybean Plants
2.4. Assessment of Meteorological Conditions
| Quantile (%) | >0.95 | 0.90–0.95 | 0.80–0.90 | 0.70–0.80 | 0.60–0.70 | 0.40–070 | 0.30–0.40 | 0.20–0.30 | 0.10–0.20 | 0.05–0.10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature characterization of month | Extremely warm | Anomalously warm | Very warm | Warm | Slightly warm | Normal | Slightly cold | Cold | Very cold | Extremely cold |
2.5. Statistical Analysis
3. Results
3.1. Weather Conditions during the Research Period
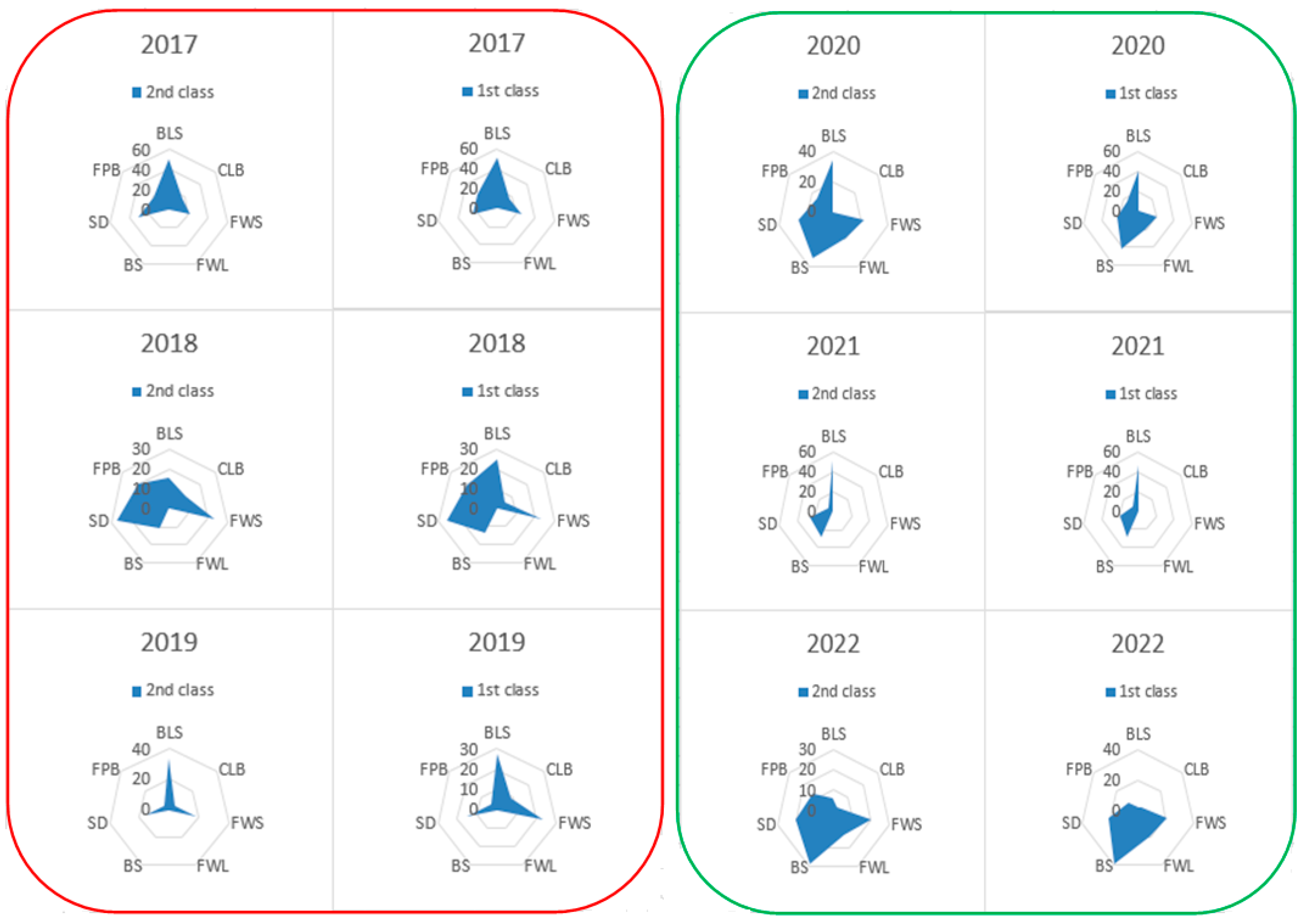
3.2. The Occurrence of Fungal Diseases in the Years of the Study
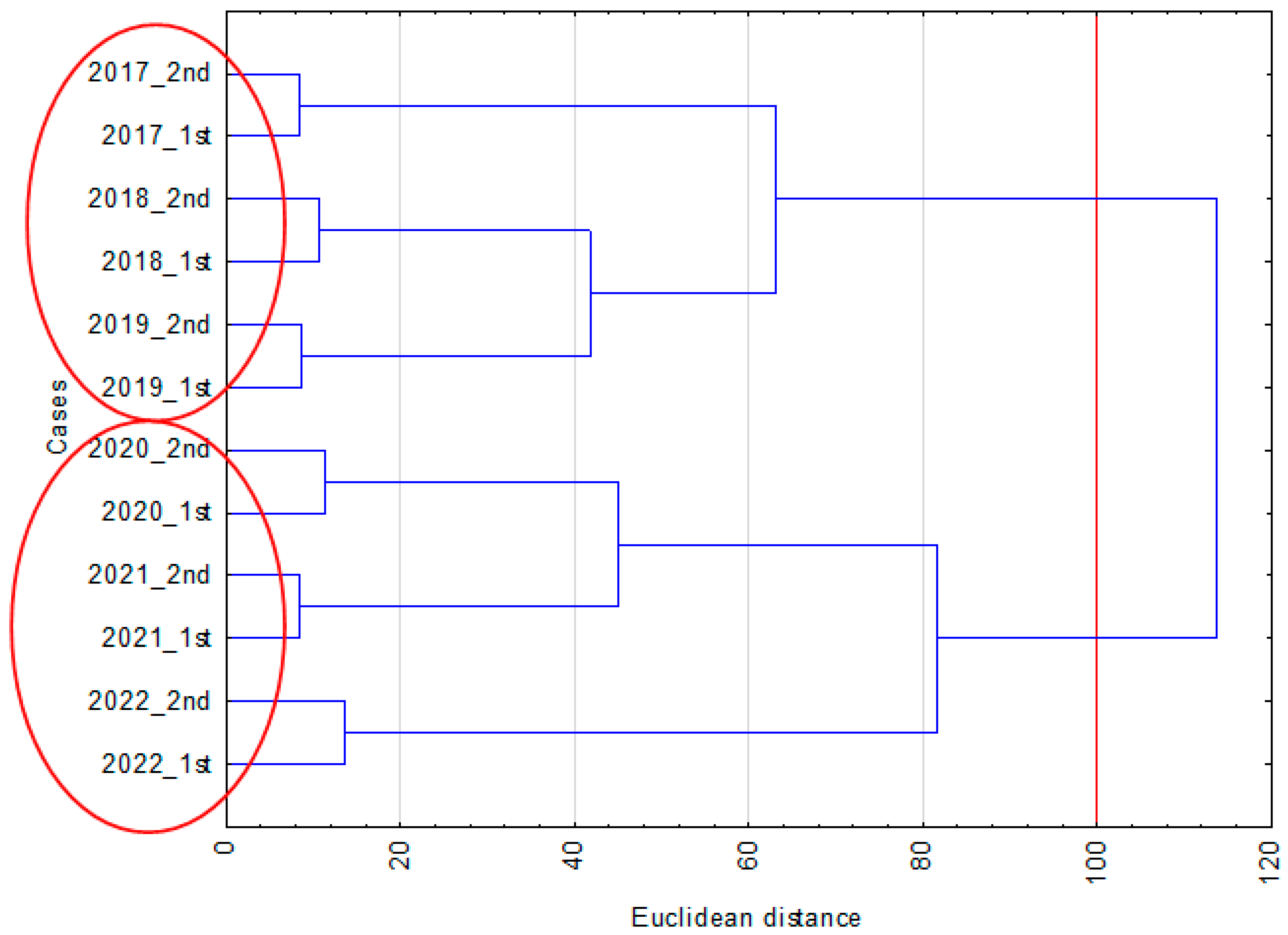
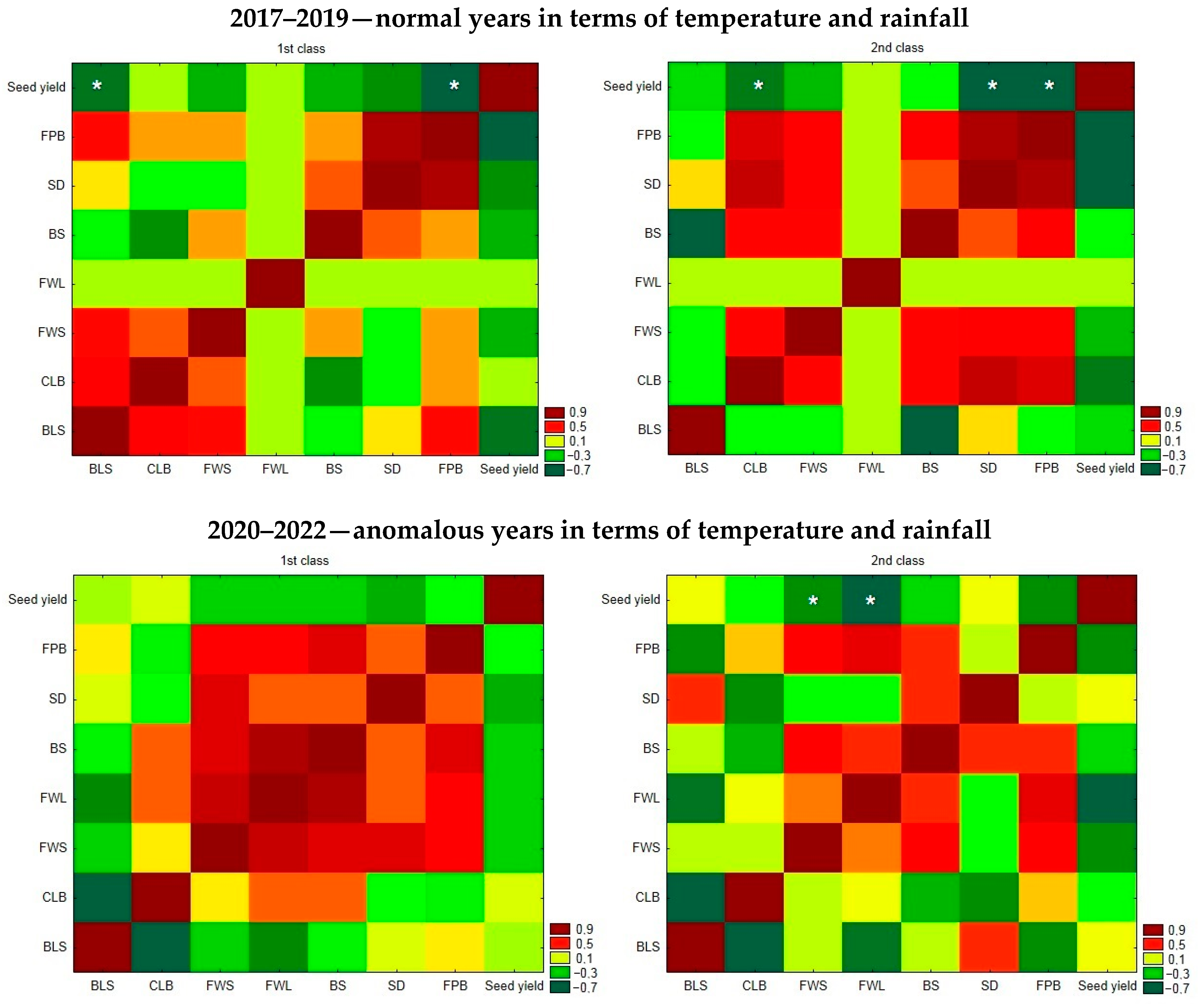
3.3. Relationship between Yield and the Occurrence of Diseases in Each Year
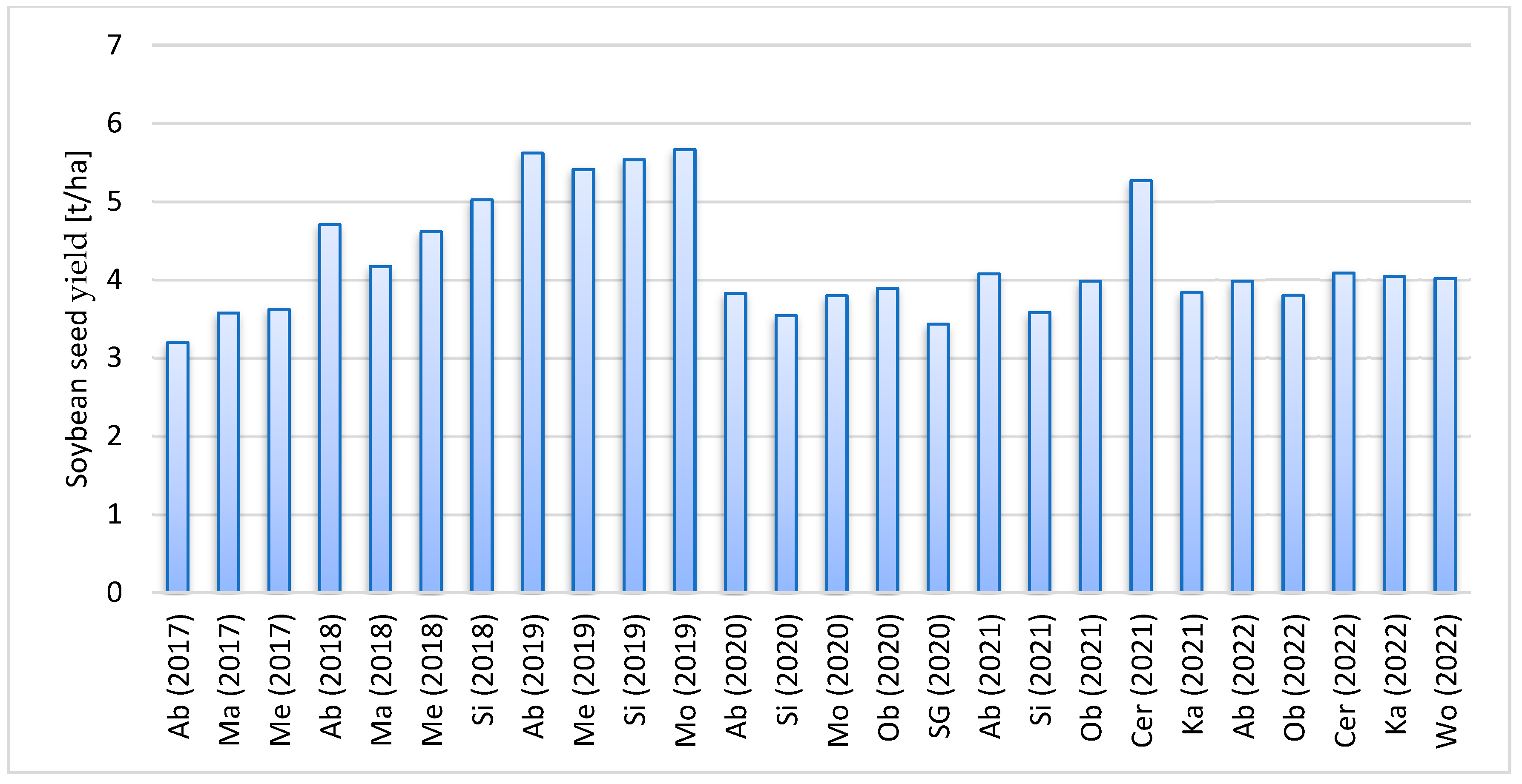
3.4. Yield and Coefficients of Variation of Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Nendel, C.; Reckling, M.; Debaeke, P.; Schulz, S.; Berg-Mohnicke, M.; Constantin, J.; Fronzek, S.; Hoffmann, M.; Jakšić, S.; Kersebaum, K.; et al. Future area expansion outweighs increasing drought risk for soybean in Europe. Glob. Chang. Biol. 2023, 29, 1340–1358. [Google Scholar] [CrossRef]
- Short-Term Outlook for EU Agricultural Markets in 2022. Available online: https://agriculture.ec.europa.eu/system/files/2022-10/short-term-outlook-autumn-2022_en_1.pdf (accessed on 1 February 2024).
- Coleman, K.; Whitmore, A.P.; Hassall, K.L.; Shield, I.; Semenov, M.A.; Dobermann, A.; Bourhis, Y.; Eskandary, A.; Milne, A.E. The potential for soybean to diversify the production of plant-based protein in the UK. Sci. Total Environ. 2021, 767, 144903. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat (accessed on 31 December 2023).
- FAOSTAT 2022. Available online: http://www.fao.org/faostat (accessed on 1 February 2024).
- Hufnagel, J.; Reckling, M.; Ewert, F. Diverse approaches to crop diversification in agricultural research. A review. Agron. Sustain. Dev. 2020, 40, 14. [Google Scholar] [CrossRef]
- Kothari, K.; Battisti, R.; Boote, K.J.; Archontoulis, S.V.; Confalone, A.; Constantin, J.; Cuadra, S.V.; Debaeke, P.; Faye, B.; Grant, B.; et al. Are soybean models ready for climate change food impact assessments? Eur. J. Agron. 2022, 135, 126482. [Google Scholar] [CrossRef]
- Lin, F.; Chhapekar, S.S.; Vieira, C.C.; Da Silva, M.P.; Rojas, A.; Lee, D.; Liu, N.; Pardo, E.M.; Lee, Y.-C.; Dong, Z.; et al. Breeding for disease resistance in soybean: A global perspective. Theor. Appl. Genet. 2022, 135, 3773–3872. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Thakur, S.; Nagre, S.P.; Anand, K.; Saraswat, S.; Mohare, S. Review of soybean breeding for disease and insect resistance. Front. Crop Improv. 2022, 10, 541–548. [Google Scholar]
- Andrijanić, Z.; Nazzicari, N.; Šarčević, H.; Sudarić, A.; Annicchiarico, P.; Pejić, I. Genetic Diversity and Population Structure of European Soybean Germplasm Revealed by Single Nucleotide Polymorphism. Plants 2023, 12, 1837. [Google Scholar] [CrossRef] [PubMed]
- Fagodiya, R.K.; Trivedi, A.; Fagodia, B.L. Impact of weather parameters on Alternaria leaf spot of soybean incited by Alternaria alternata. Sci. Rep. 2022, 12, 6131. [Google Scholar] [CrossRef] [PubMed]
- Kleczewski, N.; Plewa, D.; Kangas, C.; Phillippi, E.; Kleczewski, V. First Report of Red Crown Rot of Soybeans Caused by Calonectria ilicicola (Anamorph: Cylindrocladium parasiticum) in Illinois. Plant Dis. 2019, 103, 1777. [Google Scholar] [CrossRef]
- Heeb, L.; Jenner, E.; Cock, M.J.W. Climate-smart pest management: Building resilience of farms and landscapes to changing pest threats. J. Pset Sci. 2019, 92, 951–969. [Google Scholar] [CrossRef]
- Roth, M.G.; Webster, R.W.; Mueller, D.S.; I Chilvers, M.; Faske, T.R.; Mathew, F.M.; A Bradley, C.; Damicone, J.P.; Kabbage, M.; Smith, D.L. Integrated Management of Important Soybean Pathogens of the United States in Changing Climate. J. Integr. Pest Manag. 2020, 11, 17. [Google Scholar] [CrossRef]
- Walsh, M.K.; Backlund, P.; Buja, L.; DeGaetano, A.; Melnick, R.; Prokopy, L.; Takle, E.; Todey, D.; Ziska, L. Climate Indicators for Agriculture; Agriculture Technical Bulletin: Washington, DC, USA, 2020. [Google Scholar]
- Davis, E.L.; Tylka, G.L. Soybean cyst nematode disease. Plant Heal. Instr. 2021. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Smith, D.L.; Sikora, E.J.; Robertson, A.E. (Eds.) A Farmer’s Guide to Soybean Diseases; American Phytopathological Society: St. Paul, MN, USA, 2016. [Google Scholar]
- Hartman, G.L.; Hill, C.B. Diseases of soybean and their management. In The Soybean; Singh, G., Ed.; CABI: Wallingford, UK, 2010; pp. 276–299. [Google Scholar]
- Research Centre for Cultivar Testing Methodology. Available online: https://coboru.gov.pl/index_en (accessed on 1 February 2024).
- Dłużniewska, J.; Klimek-Kopyra, A.; Czech, T.; Dobrowolski, J.W.; Dacewicz, E. The Use of Coherent Laser Stimulation of Seeds and a Fungal Inoculum to Increase the Productivity and Health of Soybean Plants. Agronomy 2021, 11, 1923. [Google Scholar] [CrossRef]
- Węgorek, P.; Korbas, M.; Zamojska, J.; Kierzek, R.; Piszczek, J.; Pieczul, K. Odporność agrofagów na środki ochrony roślin. s. 87–127. [W]: “Integrowana ochrona upraw rolniczych. Podstawy integrowanej ochrony”, (M. Mrówczyński, red.); Powszechne Wydawnictwo Rolnicze i Leśne: Poznań, Poland, 2013; p. 219. [Google Scholar]
- Miętus, M.; Owczarek, M.; Filipiak, J. Warunki termiczne na obszarze Wybrzeża i Pomorza w świetle wybranych klasyfikacji, Materiały Badawcze IMGW. S. Meteorologia 2002, 36, 1–56. [Google Scholar]
- Kaczorowska, Z. Opady w Polsce w przekroju wieloletnim. Przegląd Geogr. IG PAN 1962, 33, 1–112. [Google Scholar]
- Stanisz, A. Przystępny Kurs Statystyki: Z Zastosowaniem STATISTICA PL na Przykładach z Medycyny. T. 3. Analizy Wie-Lowymiarowe; StatSoft: Tulsa, OK, USA, 2007. [Google Scholar]
- Gass, T.; Schori, A.; Fossati, A.; Soldati, A.; Stamp, P. Cold tolerance of soybean (Glycine max (L.) Merr.) during the reproductive phase. Eur. J. Agron. 1996, 5, 71–88. [Google Scholar] [CrossRef]
- Setiyono, T.; Weiss, A.; Specht, J.; Bastidas, A.; Cassman, K.; Dobermann, A. Understanding and modeling the effect of temperature and daylength on soybean phenology under high-yield conditions. Field Crop. Res. 2007, 100, 257–271. [Google Scholar] [CrossRef]
- Parent, B.; Tardieu, F. Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytol. 2012, 194, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Boote, K.J. Improving soybean cultivars for adaptation to climate change and climate variability. In Crop Adaptation to Climate Change; Wiley-Blackwell: Oxford, UK, 2011; pp. 370–395. [Google Scholar] [CrossRef]
- Câmara, G.M.S.; Sediyama, T.; Dourado-Neto, D.; Bernardes, M.S. Influence of fotoperiod and air temperature on the growth, flowering and maturation of the soybean (Glycine max. L. Merrill). Sci. Agric. 1997, 54, 149–154. Available online: https://www.scienceopen.com/document?vid=f604f4c0-fa07-4018-afaa-ff18d7c9b5aa (accessed on 2 August 2022). [CrossRef]
- Kumar, A.; Pandey, V.; Shekh, A.; Kumar, M. Growth and yield response of soybean (Glycine max L.) in relation to temperature, photoperiod and sunshine duration at Anand, Gujarat, India. Am. Eur. J. Agron. 2008, 1, 45–50. Available online: http://www.idosi.org/aeja/1(2)08/6.pdf?q=birla-institute-of-technology-mesra-ranchi-835215-india (accessed on 2 August 2022).
- Staniak, M.; Stępień-Warda, A.; Czopek, K.; Kocira, A.; Baca, E. Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress. Agronomy 2021, 11, 520. [Google Scholar] [CrossRef]
- Markowski, A. Influence of initial seed moisture and temperature conditions during germination and emergence on seedling survival and yields of soybean (Glycine max L. Merrill). Acta Agrobot. 1982, 35, 43–59. [Google Scholar] [CrossRef][Green Version]

| % of Normal Rainfall | <50 | 50–74 | 75–89 | 90–110 | 111–125 | 126–150 | >150 |
|---|---|---|---|---|---|---|---|
| Rainfall characterization of month | Extremely dry | Very dry | Dry | Average | Rainy | Very rainy | Extremely rainy |
| Month | 10-Day Period | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) * | P (mm) ** | Ndp (−) | T (°C) * | P (mm) ** | Ndp (−) | T (°C) * | P (mm) ** | Ndp (−) | T (°C) * | P (mm) ** | Ndp (−) | T (°C) * | P (mm) ** | Ndp (−) | T (°C) * | P (mm) ** | Ndp (−) | ||
| April | 1 | 11.0 | 37.8 | 3 | 10.5 | 3.4 | 2 | 9.1 | 14.4 | 2 | 8.7 | 0 | 0 | 5.1 | 6.4 | 6 | 4.7 | 24.6 | 9 |
| 2 | 5.5 | 42.6 | 7 | 15.1 | 5.6 | 3 | 7.9 | 5.4 | 3 | 10.1 | 0.2 | 1 | 4.9 | 34.1 | 9 | 7.0 | 6.2 | 5 | |
| 3 | 6.3 | 60.0 | 8 | 15.4 | 2.6 | 1 | 11.9 | 69.6 | 6 | 10.3 | 7 | 2 | 7.3 | 9.6 | 5 | 9.1 | 14.8 | 5 | |
| May | 1 | 9.4 | 45.8 | 5 | 17.4 | 20.2 | 2 | 9.7 | 14.2 | 3 | 10.5 | 19.8 | 6 | 10.4 | 14.2 | 5 | 13.8 | 11.0 | 3 |
| 2 | 14.6 | 24.6 | 4 | 14.8 | 32.8 | 5 | 11.9 | 69.6 | 6 | 11.2 | 24.6 | 6 | 14.4 | 54.6 | 6 | 16.2 | 0 | 0 | |
| 3 | 17.1 | 58.2 | 7 | 19.3 | 0.0 | 0 | 14.4 | 167.4 | 9 | 11.8 | 90.6 | 9 | 13.3 | 28.8 | 7 | 14.7 | 9.4 | 3 | |
| June | 1 | 17.8 | 33.0 | 2 | 20.2 | 72.6 | 6 | 20.1 | 1.4 | 1 | 15.6 | 29.6 | 6 | 16.5 | 4.8 | 3 | 18.0 | 43.6 | 7 |
| 2 | 18.4 | 28.2 | 3 | 19.9 | 15.1 | 3 | 23.3 | 21.2 | 4 | 19.4 | 35.4 | 8 | 19.5 | 7.8 | 1 | 19.1 | 29.6 | 4 | |
| 3 | 22.0 | 19.0 | 2 | 16.1 | 73.9 | 7 | 23.3 | 72.0 | 1 | 19.9 | 44.2 | 5 | 22.4 | 78.8 | 5 | 22.8 | 0 | 0 | |
| July | 1 | 18.8 | 45.2 | 5 | 19.4 | 9.2 | 3 | 18.9 | 3.0 | 1 | 20.2 | 19.6 | 4 | 22.5 | 27.2 | 2 | 20.6 | 57.2 | 6 |
| 2 | 19.9 | 29.6 | 5 | 20.1 | 124.2 | 9 | 17.8 | 31.6 | 4 | 17.4 | 52.2 | 6 | 21.9 | 102.8 | 9 | 19.9 | 10.6 | 4 | |
| 3 | 21.4 | 18.4 | 5 | 22.9 | 29.2 | 3 | 21.5 | 21.2 | 2 | 20.5 | 5.8 | 3 | 21.8 | 29.9 | 5 | 22.3 | 44.2 | 5 | |
| August | 1 | 24.2 | 2.0 | 1 | 23.8 | 32.6 | 3 | 20.4 | 60.0 | 5 | 22.0 | 8.4 | 2 | 19.8 | 116 | 7 | 21.2 | 0.4 | 1 |
| 2 | 21.2 | 90.2 | 4 | 22.0 | 10.8 | 2 | 19.7 | 49.4 | 5 | 21.6 | 58 | 3 | 20.2 | 24.4 | 3 | 22.7 | 8.6 | 3 | |
| 3 | 18.5 | 16.2 | 4 | 19.2 | 35.8 | 3 | 21.6 | 12.0 | 1 | 20.1 | 12.6 | 3 | 16.0 | 85.4 | 9 | 21.7 | 75.2 | 4 | |
| September | 1 | 16.1 | 47.8 | 6 | 18.9 | 34.2 | 3 | 17.6 | 70.0 | 5 | 16.2 | 28.6 | 6 | 15.2 | 1.2 | 1 | 17.1 | 24.4 | 3 |
| 2 | 14.9 | 113.6 | 8 | 17.9 | 20.8 | 2 | 14.0 | 6.0 | 2 | 17.7 | 0 | 0 | 15.9 | 31.2 | 4 | 13.4 | 23 | 6 | |
| 3 | 11.0 | 75.8 | 4 | 11.3 | 41.6 | 7 | 13.5 | 5.4 | 3 | 14.9 | 48 | 6 | 13.2 | 14.4 | 3 | 11.6 | 20.9 | 7 | |
| Avg T/ total P | 16.0 ↑ | 788 ↑ | 13.8 ↑ | 18.0 ↑ | 565 ↑ | 10.7 ↓ | 16.5 ↑ | 694 ↑ | 10.5 ↓ | 16.0 ↑ | 485 ↑ | 12.7 ↓ | 15.6 ↑ | 67 | 15.0 ↑ | 16.4 ↑ | 404 ↓ | 12.5 ↓ | |
| Apr–Sep: long-term average temperature from 1991–2020 (T): 15.5 °C, precipitation total (P): 449 mm; number of days with precipitation ≥0.1 mm: 13.3 | |||||||||||||||||||
| Year | Disease | Growing Period | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | |||||||||||
| 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | ||
| BBCH | BBCH 1–15 | BBCH 16–51 | BBCH 51–69 | BBCH 70–89 | ||||||||||||
| 2017 | SD * | |||||||||||||||
| CLB | ||||||||||||||||
| FWS | ||||||||||||||||
| FPB | ||||||||||||||||
| 2018 | SD | |||||||||||||||
| BLS | ||||||||||||||||
| CLB | ||||||||||||||||
| FWS | ||||||||||||||||
| BS | ||||||||||||||||
| FPB | ||||||||||||||||
| 2019 | SD | |||||||||||||||
| BLS | ||||||||||||||||
| CLB | ||||||||||||||||
| FWS | ||||||||||||||||
| FPB | ||||||||||||||||
| 2020 | SD | |||||||||||||||
| BLS | ||||||||||||||||
| FWS | ||||||||||||||||
| FWL | ||||||||||||||||
| BS | ||||||||||||||||
| FPB | ||||||||||||||||
| 2021 | SD | |||||||||||||||
| BLS | ||||||||||||||||
| FWS | ||||||||||||||||
| BS | ||||||||||||||||
| FPB | ||||||||||||||||
| 2022 | SD | |||||||||||||||
| BLS | ||||||||||||||||
| CLB | ||||||||||||||||
| FWS | ||||||||||||||||
| FWL | ||||||||||||||||
| BS | ||||||||||||||||
| FPB | ||||||||||||||||
| Cultivar | Class | Average Yield ± Standard Deviation | Cv(%) |
|---|---|---|---|
| 2017–2019 | |||
| Aligator | 2 | 4.68 ± 0.28 | 6 |
| Merlin | 1 | 4.45 ± 0.33 | 8 |
| Abelina | 1 | 4.51 ± 0.43 | 10 |
| 2020–2022 | |||
| Acardia | 2 | 4.36 ± 0.67 | 16 |
| Viola | 2 | 3.80 ± 0.37 | 10 |
| Abelina | 1 | 3.96 ± 0.41 | 10 |
| Obelix | 1 | 3.89 ± 0.46 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Kopyra, A.; Skowera, B.; Dacewicz, E.; Boligłowa, E.; Kulig, B.; Znój, K. Occurrence of Diseases and Seed Yield of Early Maturing Soybean Cultivars Grown under the Conditions of Central Europe. Agronomy 2024, 14, 534. https://doi.org/10.3390/agronomy14030534
Klimek-Kopyra A, Skowera B, Dacewicz E, Boligłowa E, Kulig B, Znój K. Occurrence of Diseases and Seed Yield of Early Maturing Soybean Cultivars Grown under the Conditions of Central Europe. Agronomy. 2024; 14(3):534. https://doi.org/10.3390/agronomy14030534
Chicago/Turabian StyleKlimek-Kopyra, Agnieszka, Barbara Skowera, Ewa Dacewicz, Elżbieta Boligłowa, Bogdan Kulig, and Katarzyna Znój. 2024. "Occurrence of Diseases and Seed Yield of Early Maturing Soybean Cultivars Grown under the Conditions of Central Europe" Agronomy 14, no. 3: 534. https://doi.org/10.3390/agronomy14030534
APA StyleKlimek-Kopyra, A., Skowera, B., Dacewicz, E., Boligłowa, E., Kulig, B., & Znój, K. (2024). Occurrence of Diseases and Seed Yield of Early Maturing Soybean Cultivars Grown under the Conditions of Central Europe. Agronomy, 14(3), 534. https://doi.org/10.3390/agronomy14030534








