Small Farm Holder Cropping Systems Influence Microbial Profiles in an Equatorial Rainforest Agroecosystem
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Soil Properties
3.2. Microbial Diversity
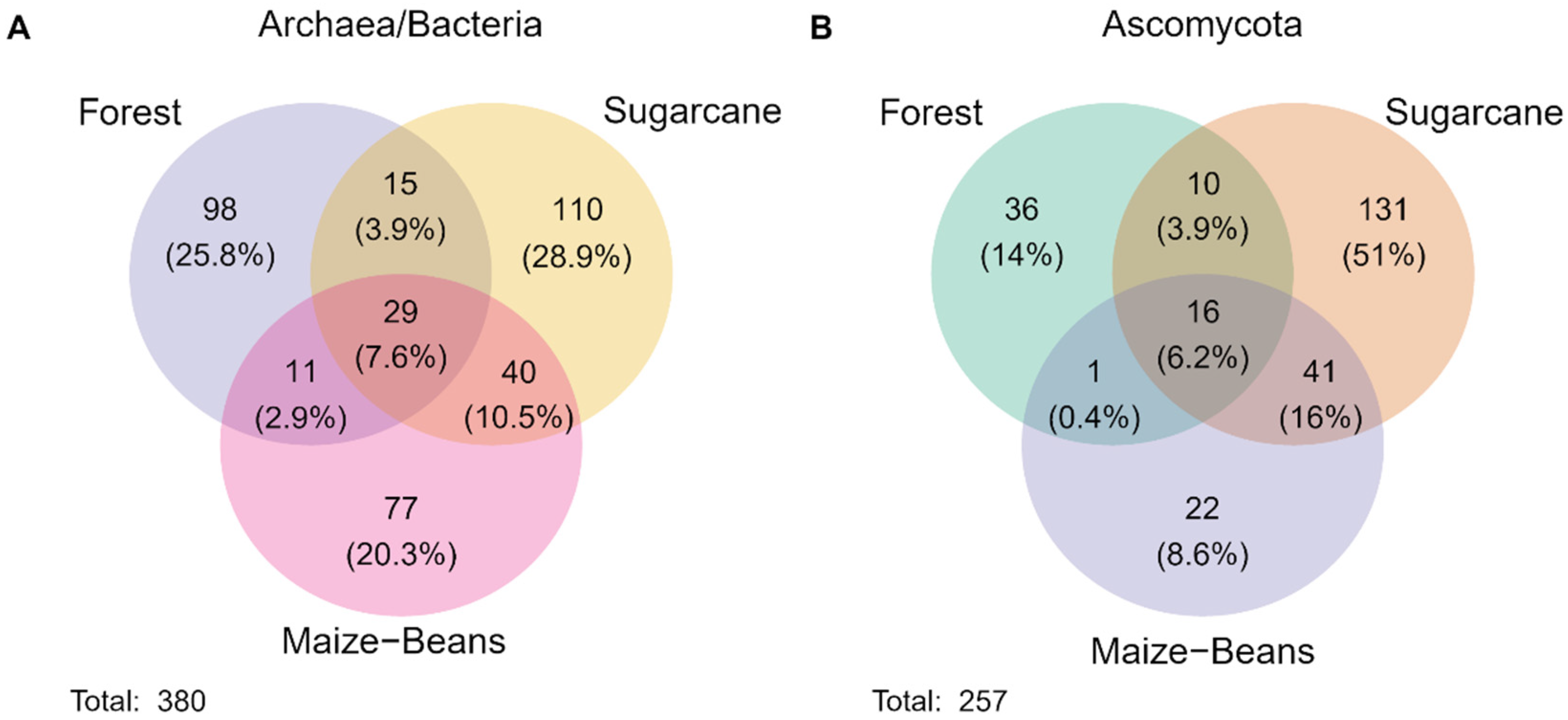
3.3. Microbial Functional Traits
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatoon, H.; Solanki, P.; Narayan, M.; Tewari, L.; Rai, J.; Hina Khatoon, C. Role of microbes in organic carbon decomposition and maintenance of soil ecosystem. Int. J. Chem. Stud. 2017, 5, 1648–1656. [Google Scholar]
- Amolo, R.A.; Sigunga, D.O.; Owuor, P.O. Evaluation of soil properties of sugarcane zones and cropping systems for improved productivity in Western Kenya. Int. J. Agron. Agric. Res. 2017, 11, 1–16. [Google Scholar]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land. Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Tian, G.; Kang, B.T.; Kolawole, G.O.; Idinoba, P.; Salako, F.K. Long-term effects of fallow systems and lengths on crop production and soil fertility maintenance in West Africa. Nutr. Cycl. Agroecosyst. 2005, 71, 139–150. [Google Scholar] [CrossRef]
- Nassary, E.K.; Baijukya, F.; Ndakidemi, P.A. Intensification of common bean and maize production through rotations to improve food security for smallholder farmers. J. Agric. Food Res. 2020, 2, 100040. [Google Scholar] [CrossRef] [PubMed]
- Krause, H.; Fasse, A.; Grote, U. Nutrient-dense crops for rural and peri-urban smallholders in Kenya—A regional social accounting approach. Sustainability 2019, 11, 3017. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Sombroek, W.G.; Braun, H.M.H.; van der Pouw, B.J.A. Exploratory Soil Map and Agroclimatic Zone Map of Kenya, 1980. Scale 1:1,000,000; Kenya Soil Survey: Nairobi, Kenya, 1982; pp. 1–56. ISBN 9032701622. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Available online: https://obrl-soil.github.io/wrbsoil2022/index.html (accessed on 9 March 2024).
- Kenya Meteorological Department. KMD Map Room. Climate. Available online: http://kmddl.meteo.go.ke:8081/maproom/Climatology/index.html (accessed on 8 March 2024).
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soil. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods, 2nd ed.; Cambridge University Press: Wallingford, UK, 1993; pp. 70–74. ISBN 0-85198-821-0. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 192–224. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Omoro, L.; Starr, M.; Pellikka, P.K.E. Tree biomass and soil carbon stocks in indigenous forests in comparison to plantations of exotic species in the Taita Hills of Kenya. Silva Fenn. 2013, 47, 935. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Asemaninejad, A.; Weerasuriya, N.; Gloor, G.B.; Lindo, Z.; Thorn, G. New primers for discovering fungal diversity using nuclear large ribosomal DNA. PLoS ONE 2016, 11, e0159043. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C. Silva Taxonomic Training Data Formatted for DADA2 (Silva Version 132). Available online: https://zenodo.org/records/1172783 (accessed on 8 November 2021).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://cran.r-project.org/package=rstatix (accessed on 8 November 2021).
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]

| Soil Chemistry | pH | % N | TN | % OC | SOC | BD | % Sand | % Silt | % Clay | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cropping System | ||||||||||
| Kruskal–Wallis p-value | 0.016 | 0.293 | 0.324 | 0.034 | 0.033 | 0.282 | 0.03 | 0.088 | 0.081 | |
| Dunn’s Pairwise Comparisons | F–MB | * | ns | ns | ns | ns | ns | * | ns | ns |
| F–Sc | * | ns | ns | ns | ns | ns | * | ns | ns | |
| MB–Sc | ns | ns | ns | * | ns | ns | ns | ns | ns | |
| Location | ||||||||||
| Kruskal–Wallis p-value | 0.002 | 0.125 | 0.233 | 0.024 | 0.038 | 0.059 | <0.001 | <0.001 | <0.001 | |
| Dunn’s Pairwise Comparisons | Ma–Bu | ** | ns | ns | ns | ns | ns | ns | ns | ns |
| Ma–Ik | ns | ns | ns | ns | ns | ns | *** | ** | ** | |
| Ma–Tw | * | ns | ns | ns | ns | ns | ns | ns | ns | |
| Bu–Ik | ns | ns | ns | * | ns | ns | **** | **** | *** | |
| Bu–Tw | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Ik–Tw | ns | ns | ns | ns | ns | ns | ** | * | * | |
| Richness (S) | Fisher’s α (S) | Shannon (H′) | InvSimpson (λ−1) | ||
|---|---|---|---|---|---|
| Archaea/ Bacteria | F | 153 | 17.1 | 4.7 | 73.0 |
| MB | 157 | 17.7 | 4.7 | 64.7 | |
| Sc | 194 | 33.0 | 4.8 | 75.3 | |
| Fungi (Ascomycota) | F | 63 | 6.9 | 2.9 | 8.1 |
| MB | 80 | 9.8 | 3.3 | 9.5 | |
| Sc | 132 | 15.5 | 4.1 | 28.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matindu, C.; Weerasuriya, N.M.; Muyekho, F.N.; Creed, I.F.; Thorn, R.G.; Sifuna, A.W. Small Farm Holder Cropping Systems Influence Microbial Profiles in an Equatorial Rainforest Agroecosystem. Agronomy 2024, 14, 646. https://doi.org/10.3390/agronomy14040646
Matindu C, Weerasuriya NM, Muyekho FN, Creed IF, Thorn RG, Sifuna AW. Small Farm Holder Cropping Systems Influence Microbial Profiles in an Equatorial Rainforest Agroecosystem. Agronomy. 2024; 14(4):646. https://doi.org/10.3390/agronomy14040646
Chicago/Turabian StyleMatindu, Christine, Nimalka M. Weerasuriya, Francis N. Muyekho, Irena F. Creed, R. Greg Thorn, and Anthony W. Sifuna. 2024. "Small Farm Holder Cropping Systems Influence Microbial Profiles in an Equatorial Rainforest Agroecosystem" Agronomy 14, no. 4: 646. https://doi.org/10.3390/agronomy14040646






