Abstract
Perennial grains have been proposed as a soil-healthy alternative to annual grains. Intermediate wheatgrass (Thinopyrum intermedium), whose seed is currently sold under the trade name Kernza®, and silflower (Silphium integrifolium), which is in the early stages of domestication at The Land Institute in Central Kansas, lack characterization for their deficiency symptoms. This has complicated attempts to assess the causes of visible stress on plants in the field and the greenhouse. By growing Th. intermedium and S. integrifolium in a set of hydroponic solutions, each containing all but one selected nutrient—including nitrogen, phosphorous, potassium, calcium, magnesium, sulfur, iron, boron, zinc, copper, molybdenum, and manganese—we were able to assess the effects of twelve different nutrient deficiencies across the two species. Visible symptoms were described and documented via photographs. The effects of the deficiencies on height, leaf biomass, root biomass, gas exchange and photosynthesis (silflower), and resin production (silflower) were measured. Calcium, nitrogen, and potassium were found to alter growth responses in intermediate wheatgrass; in silflower, growth, resin production, and photosynthetic traits were affected by many nutrient deficient treatments. Our results suggest that further work addressing how symptoms might look at the time of flowering, seed production, and in the field at different concentrations of key nutrients would help ongoing plant-breeding efforts.
1. Introduction
Perennial grain crops have been shown to reduce soil erosion, limit nutrient runoff, and sequester carbon in soils when planted as an alternative to annual grain crops [1,2,3]. Two novel perennial grain crops are under domestication at The Land Institute—intermediate wheatgrass (Thinopyrum intermedium), the plant that produces Kernza® grain, and silflower (Silphium integrifolium), a drought-tolerant native oilseed. Both lack characterization of their symptoms for nutrient deficiencies. This presents a challenge when assessing the causes of visible abiotic or biotic stress on plants in farmer and research fields and the greenhouse. Furthermore, little is known about how specific nutrient deficiencies may affect the physiological traits of the plants, thereby impacting their potential yield in suboptimal soil conditions.
Most widely grown annual crops have rigorous documentation of their specific nutrient deficiencies’ symptoms [4,5]. Namely, in annual crops, nutrient stress has been implicated in reductions in seed yield [6,7], biomass [8], and, in some cases, pest and pathogen defense [9,10]. Zinc (Zn) deficiency was found to reduce grain yield in wheat, independent of drought conditions [6], and nitrogen (N) deficiency has been tied to grain yield reduction in maize [7], wheat [11], and many other crops [12]. In wheat, the timing of N deficiency in the growth cycle influences its effects on grain yield; low nitrogen levels early on were found to be non-limiting, but closer to seed production, plants needed higher N to maintain yields [11]. A review by Hermans et al. details the role that N, phosphorus (P), potassium (K), and magnesium (Mg) deficiencies play in reducing biomass [8].
Nutrients have also been shown to modulate disease and pest resistance in plants. Huber et al. suggest that most nutrients, when increased to baseline, will have a pest- and pathogen-inhibiting effect due to improved plant nutrition; however, some appear to have greater effects than others on specific pathogens [13]. For example, calcium (Ca) and boron (B) can decrease susceptibility to disease by strengthening cell walls and membranes, respectively, and Ca applications can be an important tool in controlling Fusarium wilts [13]. Oil palm, which produces secondary metabolites toxic to spider mites, can better defend against pests with B, the nutritional prerequisite for producing the defense compound, cyanidin [13]. N (depending on form), P, K, and Ca applications have been shown to improve resistance to pathogens [9], although Dordas et al. tell a complex story where baseline nutrition improves disease resistance, but higher fertilization may increase disease severity. This is the case for P, which may worsen the severity of several diseases, including Sclerotinia in multiple vegetables, Bremia in lettuce, and flag smut in wheat [10]. It is important to note that the relationship between a nutrient and host resistance can be complicated by the levels of other nutrients (as in the case of K deficiency, which can be induced by high Ca or Mg concentrations) and by pathogens themselves, which may restrict nutrient uptake [14].
For silflower, symptoms of biotic stresses (rust and other diseases) result in a reduction of net CO2 uptake, changes in leaf defenses (resin), and, ultimately, yield reduction [15]. The resin (terpenes) present in silflower has been hypothesized to be related to pest defense, and resin production seems to be induced by rust infection [16]. In general, the synthesis of terpenes is one of the plant’s responses to attack in numerous plant–pathogen interactions. Terpenes act as specialized or generalized pathogen inhibitors [17]; however, these responses can be modulated by the C:N (carbon/nutrient) balance of the plant [18] and their synthesis and accumulation; thus, terpenes are ultimately affected by nutrient availability. Within this context, the effect of micronutrients on carbon-based secondary metabolites (CBSMs) is not fully understood and may depend on specific nutrients and their concentration.
The early external symptoms of some silflower diseases are not always clear and can be confused with responses to abiotic stress. Silflower plants with Silphium clear vein (SCV) disease [19] were initially believed to be suffering from a B deficiency due to the similarity in symptoms. B-deficient plants can experience leaf-width reduction and constriction, yellowing near the leaf tip, and dying of leaves near the edges. These symptoms approximate symptoms described as SCV: leaf-width reduction, leaf-tip constriction, toothiness of leaf margins, mosaic pattern, and twisting of stalks and leaves. After completing this study, we were able to identify clear differences between the observed B and SCV symptoms, including a greater yellowing in B-deficient plants, and a production of clear and large veins in SCV-afflicted plants. However, this initial misdiagnosis drove the need for better descriptions of the physical symptoms of nutrient deficiencies in silflower.
Our objectives were to (1) characterize the effects of macro- and micronutrient stresses on the visible morphology, plant height, and biomass for Th. intermedium and S. integrifolium; (2) investigate gas exchange parameters and resin production in S. integrifolium due to their relationship with pest defense and plant yield [15].
2. Materials and Methods
2.1. Seed Starting, Nutrient Solution Development, and Treatments
Intermediate wheatgrass half-sibling seeds used in this study were provided by Lee Dehaan from the Kernza breeding program at The Land Institute. Silflower seeds were half-siblings originating from David Van Tassel’s oilseeds breeding program at The Land Institute in Salina, KS, USA.
Th. intermedium and S. integrifolium seeds were started in a 2:1 perlite/turface mixture in cell trays (28 × 54.3 cm tray, containing 72 cells 3.8 cm square and 5.7 cm deep) with double-distilled water added at the bottom of each tray, as needed, to keep roots hydrated. Once the two-true leaf stage was reached, plants were given a water-soluble solution containing all nutrients, according to Kauffman et al. [20], with the exception of the replacement of manganese chloride with manganese (II) sulfate to supply the same concentration of manganese (Mn) ions, which was substituted for human safety. More nutrient solution was added as needed to the bottom of each tray to keep roots hydrated.
At 5 weeks post-planting, individual plants were removed from the perlite/turface mixture, and each was transferred to a 3″ plastic net pot (Growneer, Shanghai, China) suspended in a 32 oz. clear plastic cup for a total of 5 plants per treatment. Cups were filled with full nutrient solution deficient in one nutrient (pH: 7.4) up to the bottoms of the net pots to create conditions for nutrient deficiency. A minor adjustment to the sulfur-deficient solution was made which retained an ion concentration of 0.2% relative to control (instead of 0% like all other deficient solutions) due to lack of availability of formulations that supplied manganese ions without sulfate (Table 1). Aluminum foil was wrapped around the sides and top of each cup to prevent algal growth. Control plants received the same treatment, except they were kept in full nutrient solution for the duration of the experiment. There was no added root aeration; preliminary experiments suggested that adding aeration was not necessary.The control solution contained all nutrients in deionized, reverse-osmosis water; the 12 treatment solutions contained all nutrients except one specific nutrient each: N, P, K, Ca, Mg, Zn, B, Mn, sulfur (S), iron (Fe), copper (Cu), and molybdenum (Mo). Solution was added as needed throughout the growth process to keep roots hydrated (2–3 times per week), and cups were emptied and cleaned at any sign of potential microbial growth. This was not a major concern: only a few cups showed signs and were cleaned promptly. The greenhouse was set to heat to 20.0 °C during the day and 15.5 °C at night, and cool at 25.6 °C during the day and 24.4 °C at night. The mean temperature was 25.1 °C; however, the minimum temperature of 14.4 °C and maximum temperature of 40.6 °C exceeded the set limits. The greenhouse was set to dehumidify at 70% relative humidity. Overhead lights were set to be on a 12 h cycle, from 6:00 to 18:00.

Table 1.
Nutrient concentrations in full solution. Modified nutrient deficient solutions had the concentration of each nutrient reduced to 0 (M).
Throughout the growth process, visible symptoms were documented via written descriptions. Brief notes were taken at least once per week, and overhead photographs were taken at transplanting and two weeks post-transplanting.
2.2. Gas Exchange
Four weeks post-transplanting, leaf CO2 gas-exchange was measured on silflower plants by sampling one attached and fully expanded leaf per plant. Gas exchange was measured with an LI-6800 gas exchange system (LICOR, Lincoln, NE, USA), following Ravetta et al. [21]. Measurements were taken midday between 10 a.m. and 1 p.m. outside the greenhouse, with settings at 50% air relative humidity, 400 ppm CO2 concentration, and 2000 mol m−2 s−1 PPFD.
2.3. Growth Responses and Resin Production
After 5 weeks of growing in treatment solution, height was measured, final photographs were taken (using a Canon EOS Rebel T6 Digital SLR), and descriptions of visual symptoms were written. Biomass was divided into roots and leaves and then dried (in a drying room set at 15 C with 40% humidity) and weighed. To assess resin content in silflower, aboveground biomass was dried at 40 C until constant weight and then ground and weighed; 4 g per sample was placed in a Soxhlet apparatus with 250 mL of dichloromethane for 4 h [22]. Resin content was calculated as percent resin of dry leaves based on weight.
2.4. Statistical Analysis
Differences in gas exchange variables, growth responses, and resin production between nutrient deficient treatments and the control were analyzed in R, using Kruskal–Wallis one-way ANOVA and post hoc Dunnett’s tests for multiple comparisons at a significance of α = 0.05 [23,24].
3. Results
3.1. Intermediate Wheatgrass
3.1.1. Aboveground Intermediate Wheatgrass Nutrient Deficiency Descriptions
N-deficient leaves turned yellow evenly and died off, starting at the leaf tip (Figure 1B). N-deficient plants continued to produce new green leaves, but they were stunted and quickly yellowed and then died back. K-deficient leaves emerged mildly yellowed (Figure 1C). Older K-deficient leaves yellowed at the edges and sometimes between veins; some leaves died back from the tips or developed brown dead spots. P-deficient leaves emerged green but eventually turned red-purple and died, remaining red-tinted compared to the dead leaves of control plants (Figure 1D). Some P-deficient plants had leaves that yellowed in large splotches before dying instead of turning red-purple, but dead leaves were still slightly red-tinted compared to control plants (Figure 1A). Ca-deficient plants produced no new growth after being transplanted into Ca-deficient solution (Figure 1E). Ca-deficient leaves yellowed or browned, curled over, and died at roughly three weeks post-transplanting. S-deficient plants were slightly and evenly yellow across the plant and across each leaf (Figure 1F). Mg-deficient leaves had yellow, tan, and reddish-brown splotches and streaks across their surfaces (Figure 1G). New Mg-deficient leaves emerged somewhat splotched. Most Mg-deficient leaves partially died back from the leaf tip. Fe-deficient leaves yellowed between the veins (Figure 1H). Older leaves remained green, but new leaves emerged progressively more yellow. B-deficient plants were slightly yellowed, primarily between veins, and had leaf die-back that began at the base of the leaf (Figure 1I). Zn-deficient plants had yellow-white streaking between veins and/or small white necrotic spots (Figure 1J). There were no visual differences in aboveground biomass between Cu-, Mn-, and Mo-deficient intermediate wheatgrass plants and the control plants.
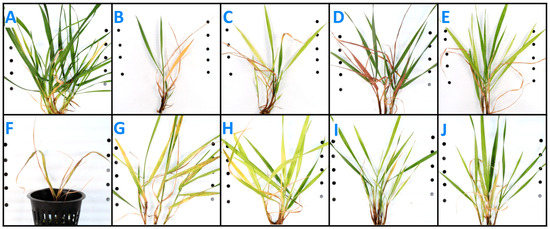
Figure 1.
Intermediate wheatgrass aboveground symptoms at 5 weeks post-transplanting into nutrient deficient solution (except calcium—photo taken at 3.5 weeks post-transplanting): (A) control, (B) nitrogen, (C) potassium, (D) phosphorus, (E) sulfur, (F) calcium, (G) magnesium, (H) iron, (I) boron, and (J) zinc.
3.1.2. Belowground Intermediate Wheatgrass Nutrient Deficiency Descriptions
N-deficient plants had visibly fewer thick roots (Figure 2B) than control plants (Figure 2A). K-deficient roots appeared shorter and darker than control roots (Figure 2C). Plants grown in P-deficient solution had longer and finer roots with reduced branching compared to controls (Figure 2D). Once plants were moved to a Ca-deficient solution, they put on no new root biomass. Existing roots became brown and mushy once calcium was lacking (Figure 2E). S-deficient roots were visibly similar to control roots (Figure 2F). Mg- and B-deficient roots appeared to have reduced branching compared to the controls (Figure 2G and Figure 2I, respectively). Fe-deficient plants appeared to put on fewer roots than the controls (Figure 2H). The roots of S-, Zn-, Cu-, Mn-, and Mo-deficient plants were visibly similar to those of control plants (S-deficient, Figure 2F; Zn-deficient, Figure 2J).

Figure 2.
Intermediate wheatgrass belowground symptoms at 5 weeks post-transplanting into nutrient deficient solution (except calcium—photo taken at 3.5 weeks post-transplanting): (A) control, (B) nitrogen, (C) potassium, (D) phosphorus, (E) sulfur, (F) calcium, (G) magnesium, (H) iron, (I) boron, and (J) zinc.
3.1.3. Intermediate Wheatgrass Growth Responses
The growth of Th. intermedium was greatly affected by the deficiency of multiple key nutrients. The height of Th. intermedium was significantly reduced when potassium and nitrogen were missing from the nutrient solution, with the loss of nitrogen reducing plant height by 40% (Figure 3). At harvest time, Ca-deficient leaves were too shriveled and dry to obtain accurate height measurements. Calcium deficiency produced a 5-fold reduction in aboveground biomass. Belowground biomass was significantly reduced when plants were grown without calcium, producing an 11-fold reduction in biomass. Root-to-shoot ratio significantly increased by 80% in the absence of nitrogen.
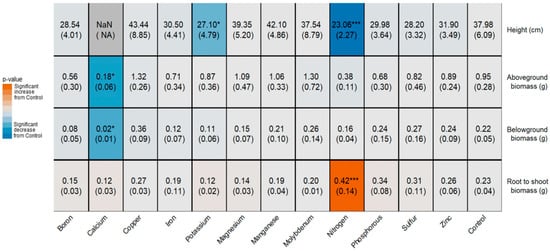
Figure 3.
Nutrient deficiency effects on Th. intermedium height, biomass, and root to shoot ratio; significant increase (red) or decrease (blue) compared to control. Mean and asterisk (denoting p-value significance: * p < 0.05 and *** p < 0.001) are on the first line and (standard deviation) is on the second line of the block.
3.2. Silflower
3.2.1. Aboveground Silflower Nutrient Deficiency Descriptions
N-deficient leaves (Figure 4B) were visibly smaller and evenly yellow-green compared to the control leaves (Figure 4A). New leaves on N-deficient plants emerged relatively lighter green, and some older leaves turned brown and died off. K-deficient leaves were ruffled and had multi-shade brown dead spots creeping in from the leaf tips and edges (Figure 4C). Some leaves had large yellow patches between veins. New leaves emerged visibly smaller but otherwise appeared normal. Older leaves were often symptomatic. P-deficient leaves were smaller than control leaves, but they emerged a similar shade of dark green (Figure 4D). Primarily older leaves died back from the edges or in brown patches. S-deficient leaves were smaller than the controls and slightly yellowed across the leaf surface (Figure 4E); older leaves did not die off like N-deficient leaves. Ca-deficient plants produced no new growth after transplanting into Ca-deficient solution, and plants died shortly after, appearing dried out aboveground (Figure 4F). Mg-deficient leaves were ruffled and necrotic at the edges (Figure 4G). Areas around the central vein and immediate offshoot veins were dark green. On many leaves, the areas in between these veins were lighter green, yellow, or even a burnt ochre. The undersides of leaf veins had brown patches, but the veins appeared normal from the top. In Fe-deficient plants, areas between the leaf veins turned yellow; this was most severe in younger leaves which emerged light yellow to white near the end of treatment (Figure 4H). In B-deficient plants, yellowing occurred mostly between the main leaf veins and was most severe near the leaf tip (Figure 4I). Leaves occasionally formed dead patches, ranging in color from brown to white, which most often formed at the leaf tips or edges. New leaves emerged with restricted width, and the edges sometimes looked constricted, like a rubber band was stretched across their width. Not all symptoms were present on every leaf or plant. Zn-deficient leaves had light green marbling, especially between the veins (Figure 4J). Zn-deficient leaves sometimes had a dark green mid-vein and immediate branching veins surrounded by slightly lighter green leaf tissue. There were no observed visual differences in aboveground biomass between Cu-, Mn-, and Mo-deficient silflower plants and the control plants.
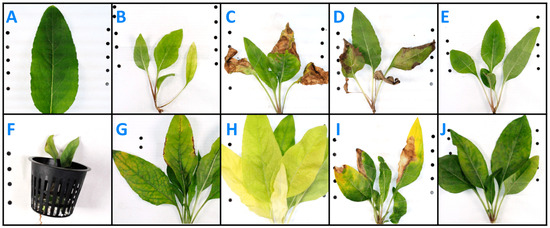
Figure 4.
Silflower aboveground symptoms at 5 weeks post-transplanting into nutrient deficient solution (except calcium—photo taken at 2 weeks post-transplanting): (A) control, (B) nitrogen, (C) potassium, (D) phosphorus, (E) sulfur, (F) calcium, (G) magnesium, (H) iron, (I) boron, and (J) zinc.
3.2.2. Belowground Silflower Nutrient Deficiency Descriptions
Some K-deficient plants had brown and slightly mushy roots compared to the controls (Figure 5C). P-deficient roots appeared to branch less than the controls (Figure 5D). As in wheatgrass, Ca-deficient plants put on no new root biomass after transplanting, and existing roots became brown and mushy (Figure 5F). Mg-deficient roots appeared to branch less than the controls (Figure 5G). B-deficient plants had small brown bumps along their roots (Figure 5I). Visually, they had fewer roots overall. The roots of N-, S-, Fe-, Zn-, Cu-, Mn-, and Mo-deficient plants were visually similar to those of the control roots (Figure 5B,E,H,J; and not pictured, respectively).
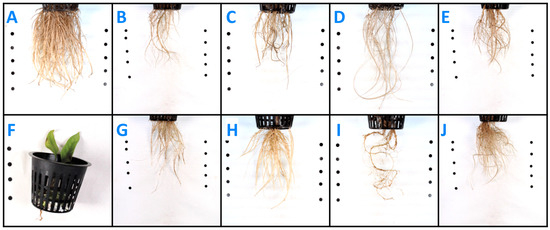
Figure 5.
Silflower belowground symptoms at 5 weeks post-transplanting into nutrient deficient solution (except calcium—photo taken at 2 weeks post-transplanting): (A) control, (B) nitrogen, (C) potassium, (D) phosphorus, (E) sulfur, (F) calcium, (G) magnesium, (H) iron, (I) boron, and (J) zinc.
3.2.3. Silflower Growth Responses, Physiology, and Resin Content
S. integrifolium responded to several nutrient deficiencies with significant reductions in growth responses. Plants that received B-, Fe-, K-, Mg-, N-, P-, S-, and Zn-deficient solutions all had significant reductions in height, belowground biomass, and aboveground biomass (Figure 6). Compared to controls, B-deficient plants had reduced above and belowground biomass by 4- and 5-fold, respectively. Fe- and S-deficient plants had half the belowground biomass of control plants at five weeks, while K-deficient plants had approximately 10% of the belowground biomass. The most extreme reduction in aboveground biomass was seen in P-deficient plants (87% decrease), followed by N-deficient plants (79% decrease). These treatments also produced the biggest reductions in height—each roughly halving the average height of the controls. K- and Mg-deficient plants each also had significantly reduced root-to-shoot ratios compared to control plants. For the same amount of aboveground biomass, the K- and Mg-deficient plants decreased their belowground biomass by ~65% and 73%, respectively, compared to the controls. All S. integrifolium plants lacking calcium died 1.5 weeks into treatment, so the numerical data for these plants are missing.
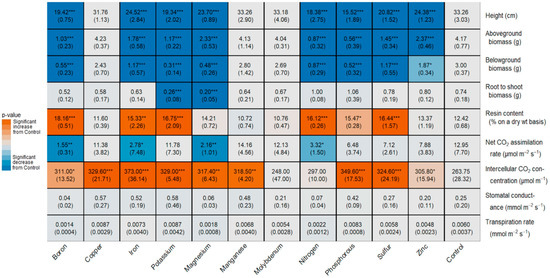
Figure 6.
Nutrient deficiency effects on S. integrifolium height, biomass, root-to-shoot ratio, resin, and photosynthetic traits; significant increase (red) or decrease (blue) compared to control. Mean and asterisk (denoting p-value significance: * p < 0.05; ** p < 0.01; and *** p < 0.001) are on the first line, and the standard deviation is in parentheses on the second line of the block.
N-, P-, S-, K-, B-, and Fe-deficient plants accumulated more resin than the controls (Figure 6). This was most pronounced in B-deficient plants, which had increased resin production, approximately 146% that of control plants.
Nutrient treatments did not significantly affect the transpiration rate (E) or stomatal conductance (gs), but they did affect the net CO2 uptake (A) and intercellular CO2 concentration (Ci) (Figure 6). The net CO2 uptake was significantly lower than that of the controls in N-, Fe-, Mg-, and Bo-deficient plants (B- and Mg-deficient plants showed the greatest reductions, 88% and 83%, respectively).
4. Discussion
Ca deficiency had a large and fatal effect on both silflower and intermediate wheatgrass plants, likely due to its role in cell wall development and immobility within the plant [25]. While visible Ca deficiency can be rare, studies in wheat and sunflower suggest that Ca can be a limiting nutrient in grain development and that Ca deficiency can increase disease susceptibility, respectively [26,27]. This points to a potential benefit from ensuring that the Ca needs of intermediate wheatgrass and silflower are met where they are grown.
Growers and researchers have already recognized the need for the N fertilization of intermediate wheatgrass stands, applying N at a rate of 61.0 to 96.4 kg/ha when grown for grain [28]. The root-to-shoot ratio increased dramatically for Th. intermedium plants grown without N in this study; however, field studies in Th. intermedium and other perennial grasses have not shown a significant response to N fertilization [29] or shown a significant decrease in the root-to-shoot ratio with N fertilization [30]. This suggests that soil dynamics, water availability, and plant age may have a greater effect on the root-to-shoot ratio than N availability on its own, at least at the N concentrations used in these studies.
S. integrifolium had large and significant reductions in height and biomass for most nutrient deficiencies investigated in this study, some of which are regularly applied as fertilizer or have been investigated for their effects at the field level. Silphium perfoliatum, a close relative of S. integrifolium that is crossed with silflower in The Land Institute’s perennial oilseeds breeding program, is currently researched and grown in Poland and other sites in Europe as a forage and biogas crop. S. perfoliatum produces more oilseed when fertilized with N and more dry matter when fertilized with CaCO3 in acidic soils or with sewage sludge [31]. N fertilization rates have been investigated in Minnesota- and Kansas-grown silflower, with positive effects on seed yield and negative effects on harvest index [16,32]. Nutrients in this study beyond N and Ca could be the foci of future studies to address their contributions to oilseed yield and harvest index.
As expected, significant effects of nutrient deficiencies were found on silflower’s gas exchange parameters. N is a major component of the photosynthetic apparatus, and its deficiency can reduce the content of light-harvesting proteins and reduce Rubisco and other photosynthetic enzymes directly involved in carboxylation. Similarly, Fe has a very high demand as a cofactor in photosynthetic electron transport [33]. Mg is the central atom of the chlorophyll molecule, and fluctuations in its levels in the chloroplast regulate the activity of key photosynthetic enzymes. Mg is also involved in carbohydrate transport from the leaves, and sugar accumulation in leaves that results from the reduction in transport is considered an early response to Mg deficiency. Mg deficiency is visible in root growth, resulting in a significant reduction in the root/shoot ratio [8]. These changes have also been described for K deficiency. P is necessary for the metabolism of adenosine triphosphate (ATP), nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), nucleic acids, and phospholipids, and it plays a major role in plant growth, signal transduction, and photosynthesis [34].
For N, Mg, and B, the reduction in net CO2 uptake (assimilation) was accompanied by a concomitant reduction in gs (Figure 7), showing coordination between the biochemical and gas exchange phases of photosynthesis. The limitation of the Fe supply did not produce stomatal closure, and photosynthesis was most likely limited by carboxylation and not by CO2 intake. Furthermore, the intercellular CO2 concentration was highest in Fe limited plants (141% that of control plants, Figure 6), also pointing to the net CO2 uptake being limited by carboxylation.
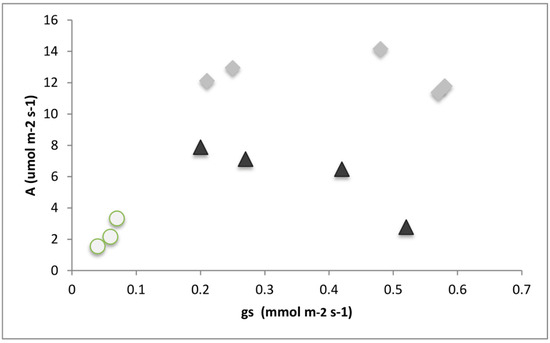
Figure 7.
Net CO2 uptake (A) and stomatal conductance (gs) measured on silflower plants under different nutrient deficiency treatments. White circles have both low gs and low A (B, Mg, and N); black triangles have high gs and low A (Fe, P, S, and Zn); gray diamonds have high gs and high A (control, Cu, K, Mg, and Mo). n = 5 plants in each treatment.
While there was no statistical reduction in the net CO2 uptake for P-, S-, and Zn-deficient plants, the CO2 uptake tended to be lower than expected by the leaves’ stomatal conductance (Figure 7). For all of these elements, the internal CO2 concentration was higher than that of the control plants, also pointing to reductions in the carboxylation processes. Deficiencies in other nutrients (B, Cu, Fe, K, Mg, Mn, P, and S) also resulted in increased intercellular CO2 concentrations (Figure 6).
As with other carbon-based secondary metabolites (CBSMs), resin production and accumulation tends to conform with the tenets of the C/Nutrient Balance Hypothesis [18]. This and the growth-differentiation hypotheses [35,36] state that moderate environmental restrictions that limit plant growth more than photosynthesis should result in a larger pool of carbohydrates available for the synthesis of carbon-based secondary metabolites. While the theory predicts changes in resin production with nitrogen availability, there is contradictory evidence concerning nitrogen effects on the production of resins. Most studies have been performed for conifers, and, in general, they report no effects [37] or a positive relationship [38,39] between nitrogen fertilization and oleoresin production. Still, in the Asteraceae family, resin-content increases with N deficiencies have been found in Grindelia [40].
Within this framework, nutrient availability could affect resin production if growth were more responsive to nitrogen restrictions than assimilation, resulting in an increase in the availability of carbohydrates for resin biosynthesis under some deficient conditions. Our results are in accordance with the physiological mechanisms proposed in these theories. Deficiencies of those elements that resulted in reductions in biomass were found to increase percent resin, with the exception of Zn and Mg, both of which had significantly reduced biomass yet no significant change in percent resin compared to control plants. We do not have an explanation for Zn and Mg, but our hypothesis is that both of these elements may be important in one of the steps of the biosynthesis of terpenes. In particular, for Zn, a significant increase in the total phenols and flavonoids content (other CBSM) has been found in Nigella sativa with enhanced Zn availability [41]. Culturing geranium at Zn 0.250 g/m3 was the critical concentration for maximum content of total essential monoterpene oil(s). At Zn 0.005–0.250 g/m3, the net photosynthetic rate, and the contents of chlorophyll and essential monoterpene oil(s) were affected [42]. These findings suggest that Zn may be involved in resin production and suggest methods to find critical thresholds for Zn and Mg availability for silflower terpene production.
The enhanced uptake of some micronutrients by AMF has been found to increase terpene production [40]. Although this experiment cannot corroborate those findings for silflower, the hypothesis that AMF can improve terpene defenses in silflower should be tested in future experiments.
Visual symptoms of nutrient deficiency in intermediate wheatgrass and silflower are very similar to documented symptoms in related grain crops, such as wheat [5] and sunflower [43]. Treatments in our study that did not produce visual symptoms in this experiment were those whose nutrients were at relatively low concentrations in the control solution (Cu, Mn, and Mo); it is expected that if plants had spent a longer duration in a nutrient deficient treatment, they could eventually show signs of deficiency. For treatments that produced visual symptoms, these were easily identified by week four of treatment. Future research could address this gap by keeping plants in nutrient deficient solutions for longer, and/or starting the plants directly on a deficient solution instead of a solution with full nutrients. In the case of nutrients whose deficiencies are observed in growers’ fields or for those that are known or suspected to be limiting, symptoms could be confirmed at different concentrations relevant to production soils. This work is underway for nitrogen fertilization in intermediate wheatgrass [28] and silflower [44]. Plants in this study were terminated prior to flowering, when symptoms could be more pronounced, and seed yield, one of the most relevant measurements for a grain crop. Both of these present opportunities for future research that investigates nutrient deficiency later in the plants’ development and as the stand ages in a perennial planting.
This study was designed to build tools to identify common nutrient stresses and differentiate them from diseases. This is especially relevant in silflower, where recently identified viruses (Dahlia mosaic virus; Dahlia common mosaic virus; and an endogenous plant pararetroviral sequence—DvEPRS) and herbicide (2,4-D and Dicamba) sensitivity (Figure 8) described as Silphium clear vein (SCV) were initially confounded with potentially similar symptoms of B deficiency [19]. SCV is characterized by wide, clear veins; twisting of leaves; yellowing at the leaf tips; and mottling. A section of a stem may show symptoms and then demonstrate recovery in newer growth. SCV will also reduce flowering when twisting symptoms are severe. B deficiency overlaps with SCV’s symptoms of yellowing and mottling, but the leaves do not twist like SCV leaves do—they constrict and pinch in. B deficiency also induces necrotic patches on leaves, a phenomenon that is not seen in SCV. Before completing this study and complementary work in SCV, we were unsure how these symptoms overlapped and differed; characterizing B deficiency via images and written descriptions has helped to differentiate these stressors from one another.

Figure 8.
Silflower with (A) boron deficiency and with (B) Silphium clear vein symptoms in field plants treated with 2,4-D. These were initially confounded due to overlapping symptoms, including constriction of leaf width and yellowing at the leaf tip.
The images and written descriptions of all nutrient deficiency symptoms were collected to help growers and researchers identify stresses in their fields. These results were formatted into open-access guides written for the general public [45,46]. These guides are accessible as additions to collections of extension materials for growers at Kernza.org and through the Land Institute’s Silphium Civic Science community through CitSci.org. The results of this paper, along with responses from growers and researchers to the open-access guides, encourage further research to investigate the responses of Th. intermedium and S. integrifolium to abiotic stressors under different growing conditions, different plant ages, and different levels of nutrient deficiency.
Aside from needing to start from scratch relatively, investigating nutrient deficiencies in novel perennial grain crops poses particular challenges. These new crops have vast genetic diversity within each species and within the breeding germplasm at this stage of domestication, necessitating more replicates and larger experimental designs to quantify more exact nutrient needs. Further, perenniality produces difficulties in assessing nutrient needs due to the longer and variable plant life cycles and vernalization requirements for seed set and maturity. Nutrient storage re-mobilization between growth cycles adds a layer of complexity not seen in annual crops. This was recently demonstrated in silflower, where the selection for yield has altered the patterns of storage and remobilization of N [47]; similar changes in storage throughout lifespans and between accessions may be occurring for other nutrients. Seed yield, while an important measure of a plant’s response to nutrient deficiency, could not be assessed with the design of this hydroponic study due to time frame and vernalization requirements of both species. We hope future studies will assess this response in field conditions.
5. Conclusions
Many treatments in silflower produced reductions in key growth responses, increased resin production, and altered photosynthetic traits. Visual symptoms overlapped with a number of our greenhouse- and field-grown plants, suggesting that further research on identifying optimal nutrient conditions could benefit ongoing domestication efforts.
This paper represents the first major effort to document the symptoms of multiple nutrient stressors in perennial grain crops and lays the foundation for future work targeting key limiting nutrients, including N, Ca, and K, for both crops; and, additionally, B, Fe, Mg, P, S, and Zn for silflower. Since these crops are novel, there has been very little work performed in many areas required to support healthy and productive growth. Outside N [16,28,29,30,32] (and Ca in silflower’s close relative [31]), there were no previously published nutrient recommendations for these crops. Because they are still early in the research phase, they lack trial by farmers in optimal management; most research focuses primarily on improving the seed source over the agronomy and management.
Responses from growers and researchers to the open-access guides [45,46] have emphasized a demand for research in the area of abiotic stressors in perennial grain crops. Future studies will need to address seed yield responses to nutrient deficiencies, investigate optimal conditions for key nutrients beyond N and Ca, and make use of both greenhouse and field settings. As there are many possible first targets, priority should be given to nutrients that growers and researchers identify as issues in their plants; thus, research in this area will be strongest if participatory. Research into the nutrient needs and abiotic stressors of perennial grain crops is a necessary step in advancing plant breeding, pathology, and physiology efforts and in building support resources for the people who grow intermediate wheatgrass and silflower.
Author Contributions
Conceptualization, A.B. and M.K.T.; methodology, A.B., D.R. and M.K.T.; software, A.B. and Y.T.; formal analysis, A.B.; investigation, A.B. and D.R.; data curation, A.B. and Y.T.; writing—original draft preparation, A.B., D.R. and M.K.T.; writing—review and editing, A.B., Y.T. and M.K.T.; visualization, A.B., Y.T. and D.R.; supervision, M.K.T.; project administration, A.B.; funding acquisition, M.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank The Perennial Agriculture Project in conjunction with the Malone Family Land Preservation Foundation and the Land Institute for providing funding and resources.
Data Availability Statement
The raw data and R code supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
Thank you to the Land Preservation Foundation and the Land Institute for providing funding and resources. Thank you to Lee DeHaan and David Van Tassel for providing seeds and advising on experimental design; Brandon Schlautman, Spencer Barriball, and Ebony Murrell for advising on experimental design; Edy Cheremond for guidance on data analysis; and Stacy Holt Jr. for assisting with photography. The Land Institute operates on land taken by treaty from the Kaw Nation in 1825, which is also the ancestral homeland of the Comanche, Osage, and other presently living peoples. The Meskwaki Nation has a longstanding relationship with S. integrifolium as a medicinal plant.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Crews, T.E.; Carton, W.; Olsson, L. Is the Future of Agriculture Perennial? Imperatives and Opportunities to Reinvent Agriculture by Shifting from Annual Monocultures to Perennial Polycultures. Glob. Sustain. 2018, 1, e11. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased Food and Ecosystem Security via Perennial Grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef]
- Soto-Gómez, D.; Pérez-Rodríguez, P. Sustainable Agriculture through Perennial Grains: Wheat, Rice, Maize, and Other Species. A Review. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- Adotey, N.; McClure, A.; Raper, T.; Florence, R. Visual Symptoms: A Handy Tool in Identifying Nutrient Deficiency in Corn, Cotton and Soybean. 2021. Available online: https://utia.tennessee.edu/publications/wp-content/uploads/sites/269/2023/10/W976.pdf (accessed on 20 March 2024).
- Snowball, K.; Robson, A.D. Nutrient Deficiencies and Toxicities in Wheat: A Guide for Field Identification; International Maize and Wheat Improvement Center: México, México, 1991; ISBN 978-968-6127-48-5. [Google Scholar]
- Bagci, S.A.; Ekiz, H.; Yilmaz, A.; Cakmak, I. Effects of Zinc Deficiency and Drought on Grain Yield of Field-Grown Wheat Cultivars in Central Anatolia. J. Agron. Crop Sci. 2007, 193, 198–206. [Google Scholar] [CrossRef]
- Binder, D.L.; Sander, D.H.; Walters, D.T. Maize Response to Time of Nitrogen Application as Affected by Level of Nitrogen Deficiency. Agron. J. 2000, 92, 1228–1236. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How Do Plants Respond to Nutrient Shortage by Biomass Allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Bhaduri, D.; Rakshit, R.; Chakraborty, K. Primary and Secondary Nutrients-a Boon to Defense System against Plant Diseases. Int. J. Bio-Resour. Stress Manag. 2014, 5, 461. [Google Scholar] [CrossRef]
- Dordas, C. Role of Nutrients in Controlling Plant Diseases in Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Ravier, C.; Meynard, J.-M.; Cohan, J.-P.; Gate, P.; Jeuffroy, M.-H. Early Nitrogen Deficiencies Favor High Yield, Grain Protein Content and N Use Efficiency in Wheat. Eur. J. Agron. 2017, 89, 16–24. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G.; Durand, J.-L.; Louarn, G. Quantifying Crop Responses to Nitrogen and Avenues to Improve Nitrogen-Use Efficiency. In Crop Physiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 161–206. ISBN 978-0-12-417104-6. [Google Scholar]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between Nutrition, Plant Diseases and Pests. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 283–298. ISBN 978-0-12-384905-2. [Google Scholar]
- Park, J.W.; Melgar, J.C.; Kunta, M. Plant Nutritional Deficiency and Its Impact on Crop Production. In Bioactive Molecules in Plant Defense: Signaling in Growth and Stress; Jogaiah, S., Abdelrahman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 231–258. ISBN 978-3-030-27165-7. [Google Scholar]
- Turner, M.; Ravetta, D.; Van Tassel, D. Effect of Puccinia Silphii on Yield Components and Leaf Physiology in Silphium Integrifolium: Lessons for the Domestication of a Perennial Oilseed Crop. Sustainability 2018, 10, 696. [Google Scholar] [CrossRef]
- Vilela, A.; González-Paleo, L.; Turner, K.; Peterson, K.; Ravetta, D.; Crews, T.; Van Tassel, D. Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium Integrifolium, a Sunflower Substitute. Sustainability 2018, 10, 638. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. 16-Role of Terpenes in Plant Defense to Biotic Stress. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Delhi, India, 2021; pp. 401–417. ISBN 978-0-12-822919-4. [Google Scholar]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon/Nutrient Balance of Boreal Plants in Relation to Vertebrate Herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Cassetta, E.; Peterson, K.; Bever, J.D.; Brandvain, Y.; VanTassel, D.; Lubin, T.K.; Alexander, H.M.; Byers, D.L.; Schiffner, S.; Turner, K. Adaptation of Pathogens to Their Local Plant Host, Silphium Integrifolium, along a Precipitation Gradient. Ecosphere 2023, 14, e4565. [Google Scholar] [CrossRef]
- Kauffman, P.B.; Labavitch, J.; Anderson-Prouty, A.; Ghosheh, N.S. Laboratory Experiments in Plant Physiology; Macmillan Publishers: New York, NY, USA, 1975. [Google Scholar]
- Ravetta, D.A.; Vilela, A.E.; Gonzalez-Paleo, L.; Van Tassel, D.L. Unpredicted, Rapid and Unintended Structural and Functional Changes Occurred during Early Domestication of Silphium Integrifolium, a Perennial Oilseed. Planta 2023, 258, 18. [Google Scholar] [CrossRef]
- Ravetta, D.A.; Anouti, A.; McLaughlin, S.P. Resin Production of Grindelia Accessions under Cultivation. Ind. Crops Prod. 1996, 5, 197–201. [Google Scholar] [CrossRef]
- Quacchia, A.; Ferracini, C.; Bonelli, S.; Balletto, E.; Alma, A. Can the Geranium Bronze, Cacyreus marshalli, become a threat for European biodiversity? Biodivers. Conserv. 2008, 17, 1429–1437. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Branco, C.A.; Soares, P.V.; Correr-Sobrinho, L.; Haiter-Neto, F.; Fernandes-Neto, A.J.; Soares, C.J. Radiodensity of base, liner and luting dental materials. Clin. Oral Investig. 2006, 10, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.D. The Calcium Conundrum. Both Versatile Nutrient and Specific Signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef]
- Adcock, K.G.; Gartrell, J.W.; Brennan, R.F. CALCIUM DEFICIENCY OF WHEAT GROWN IN ACIDIC SANDY SOIL FROM SOUTHWESTERN AUSTRALIA. J. Plant Nutr. 2001, 24, 1217–1227. [Google Scholar] [CrossRef]
- Chrominski, A.; Abia, J.A.; Smith, B.N. Calcium Deficiency and Gibberellic Acid Enhance Susceptibility of Pumpkin and Sunflower Seedlings to Sclerotinia Sclerotiorum Infection. J. Plant Nutr. 1987, 10, 2181–2193. [Google Scholar] [CrossRef]
- Jungers, J.M.; DeHaan, L.R.; Betts, K.J.; Sheaffer, C.C.; Wyse, D.L. Intermediate Wheatgrass Grain and Forage Yield Responses to Nitrogen Fertilization. Agron. J. 2017, 109, 462–472. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W.; Ghimire, R.P. Root Biomass, Root/Shoot Ratio, and Soil Water Content under Perennial Grasses with Different Nitrogen Rates. Field Crops Res. 2017, 210, 183–191. [Google Scholar] [CrossRef]
- Li, S.; Jensen, E.S.; Liu, N.; Zhang, Y.; Dimitrova Mårtensson, L.-M. Species Interactions and Nitrogen Use during Early Intercropping of Intermediate Wheatgrass with a White Clover Service Crop. Agronomy 2021, 11, 388. [Google Scholar] [CrossRef]
- Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyżaniak, M.; Dębowski, M. Silphium Perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. [Google Scholar] [CrossRef]
- Schiffner, S.; Jungers, J.M.; Hulke, B.S.; Van Tassel, D.L.; Smith, K.P.; Sheaffer, C.C. Silflower Seed and Biomass Responses to Plant Density and Nitrogen Fertilization. Agrosyst. Geosci. Environ. 2020, 3, e20118. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Carswell, M.C. Metabolic Aspects of the Phosphate Starvation Response in Plants. In Plant Responses to Environmental Stresses: From Phytohormones to Genome Reorganization: From Phytohormones to Genome Reorganization; Lerner, H.R., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 1999; pp. 349–372. ISBN 978-1-351-42410-3. [Google Scholar]
- Loomis, W. Growth-Differentiation Balance vs. Carbohydrate-Nitrogen Ratio. Proc. Am. Soc. Hortic. Sci. 1932, 29, 240–245. [Google Scholar]
- Lorio, P.L. Growth-Differentiation Balance: A Basis for Understanding Southern Pine Beetle-Tree Interactions. For. Ecol. Manag. 1986, 14, 259–273. [Google Scholar] [CrossRef]
- Muzika, R.M.; Pregitzer, K.S.; Hanover, J.W. Changes in Terpene Production Following Nitrogen Fertilization of Grand Fir (Abies Grandis (Dougl.) Lindl.) Seedlings. Oecologia 1989, 80, 485–489. [Google Scholar] [CrossRef]
- Björkman, C.; Larsson, S.; Gref, R. Effects of Nitrogen Fertilization on Pine Needle Chemistry and Sawfly Performance. Oecologia 1991, 86, 202–209. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.; Palomäki, V.; Holopainen, T. Effects of Nitrogen Fertilization on Secondary Chemistry and Ectomycorrhizal State of Scots Pine Seedlings and on Growth of Grey Pine Aphid. J. Chem. Ecol. 1996, 22, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Wassner, D.; Ravetta, D. Nitrogen Availability, Growth, Carbon Partition and Resin Content in Grindelia Chiloensis. Ind. Crops Prod. 2007, 25, 218–230. [Google Scholar] [CrossRef]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Casabianca, H.; Hosni, K. Responses of Nigella Sativa L. to Zinc Excess: Focus on Germination, Growth, Yield and Yield Components, Lipid and Terpene Metabolism, and Total Phenolics and Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 1664–1675. [Google Scholar] [CrossRef]
- Misra, A.; Srivastava, A.; Srivastava, N.; Khan, A. Zn-Acquisition and Its Role in Growth, Photosynthesis, Photosynthetic Pigments, and Biochemical Changes in Essential Monoterpene Oil (s) of Pelargonium Graveolens. Photosynthetica 2005, 43, 153–155. [Google Scholar] [CrossRef]
- Christin, H.; Petty, P.; Ouertani, K.; Burgado, S.; Lawrence, C.; Kassem, M.A. Influence of Iron, Potassium, Magnesium, and Nitrogen Deficiencies on the Growth and Development of Sorghum (Sorghum Bicolor L.) and Sunflower (Helianthus Annuus L.) Seedlings. J. Biotech. Res 2009, 1, 64–71. [Google Scholar]
- Vilela, A. (Museo Egidio Feruglio, Consejo Nacional de Investigaciones Científicas y Técnicas, Fontana 140, Trelew 9100, Chubut, Argentina). Personal Communication, 2023.
- Brekalo, A. Nutrient Deficiency in Intermediate Wheatgrass; Zenodo: 2022. Available online: https://zenodo.org/records/5914997 (accessed on 22 March 2024).
- Brekalo, A. Nutrient Deficiency in Silphium Integrifolium; Zenodo: 2022. Available online: https://zenodo.org/records/5915000 (accessed on 22 March 2024).
- Gonzalez-Paleo, L.; Ravetta, D.A.; Vilela, A.E.; Van Tassel, D. Domestication effects on nitrogen allocation, internal recycling and nitrogen use efficiency in the perennial new crop Silphium integrifolium (Asteraceae). Ann. Appl. Biol. 2023, 182, 397–411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).