Yellow Nutsedge (Cyperus esculentus L.) as an Agricultural Crop in Brazil: Tuber Dormancy Breaking
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location
2.2. Cyperus Esculentus Tubers
2.3. Experimental Design and Dormancy-Breaking Treatments
2.4. Seedling Evaluations
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Treatments for Breaking Dormancy in Cyperus esculentus Tubers
3.2. Effect of Dormancy-Breaking Treatments on the Initial Development of Cyperus esculentus Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais no Brasil: Nativas E Exóticas, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2002; 512p. [Google Scholar]
- Spósito, R.C.A. Cyperus esculentus L. Para Obtenção de Bioprodutos com Aplicação Biotecnológica. Ph.D. Thesis, Universidade Federal da Bahia, Salvador, Brazil, 2018. [Google Scholar]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology. Pedobiologia 1978, 18, 296–297. [Google Scholar] [CrossRef]
- Arafat, S.A.; Gaafar, A.; Basuny, A.M.; Nassef, S. Chufa Tubers (Cyperus esculentus L.): As a New Source of Food. World Appl. Sci. J. 2009, 7, 151–156. [Google Scholar]
- CRDO—Consejo Regulador de la Denominación de Origen Chufa de Valencia. Chufa de Valencia. Available online: http://www.chufadevalencia.org (accessed on 1 March 2024).
- Zhang, S.; Li, P.; Wei, Z.; Cheng, Y.; Liu, J.; Yang, Y.; Wang, Y.; Mu, Z. Cyperus (Cyperus esculentus L.): A Review of Its Compositions, Medical Efficacy, Antibacterial Activity and Allelopathic Potentials. Plants 2022, 11, 1127. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger nut (Cyperus esculentus L.): Nutrition, processing, function and applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, E.R.; Obioma, N.; Christophe, O.I. The Phytochemical Composition and Some Biochemical Effects of Nigerian Tigernut (Cyperus esculentus L.) Tuber. Pak. J. Nutr. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Adejuyitan, J.A.; Otunola, E.T.; Akande, E.A.; Bolarinwa, I.F.; Oladokun, F.M. Some physicochemical properties of flour obtained from fermentation oftigernut (Cyperus esculentus) sourced from a market in Ogbomoso, Nigeria. Afr. J. Food Sci. 2009, 3, 51–55. [Google Scholar]
- Pereira, W. Prevenção e Controle da Tiririca em Áreas Cultivadas com Hortaliças. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/107342/1/cnph-documentos-15-prevencao-e-controle-da-tiririca-em-areas-cultivadas-com-hortalicas-fl-07825.pdf> (accessed on 17 January 2024).
- Azania, C.A.M.; Azania, A.A.P.M.; Pavani, M.C.M.D.; Alves, P.L.C.A. Desenvolvimento da tiririca (Cyperus rotundus) influenciado pela presença e ausência de palha de cana-de-açúcar e herbicida. Planta Daninha 2006, 24, 29–35. [Google Scholar] [CrossRef][Green Version]
- Fontes, P.C.R.; Finger, F.L. Dormência dos tubérculos, crescimento da parte aérea e tuberização da batateira. Inf. Agropecuário 1999, 20, 24–29. [Google Scholar]
- Ferreira, G.A.P.; Mendes, K.F. Mecanismo de dormência e germinação escalonada das sementes de plantas daninhas. In Mecanismos de Sobrevivência das Plantas Daninhas: Reprodução, Dispersão e Dormência, 1st ed.; Mendes, K.F., Ed.; Brazil Publishing: Curitiba, Brazil, 2022; pp. 177–199. [Google Scholar]
- Finger, F.L.; Fontes, P.C.R.; Puiatti, M. Dormência e tuberização. In Olericultura: Teoria e Prática, 2nd ed.; Fontes, P.C.R., Nick, C., Eds.; DAA/UFV: Viçosa, Brazil, 2021; pp. 51–56. [Google Scholar]
- Thomas, P.E.L.; Henson, I.E. Influence of Climate and Soil Moisture on Tuber Dormancy of Cyperus esculentus. Int. J. Pest Manag. Part C 1968, 14, 271–276. [Google Scholar] [CrossRef]
- Huang, J.; Gómez-Dans, J.L.; Huang, H.; Ma, H.; Wu, Q.; Lewis, P.E.; Xie, X. Assimilation of remote sensing into crop growth models: Current status and perspectives. Agric. For. Meteorol. 2019, 276–277, 107609. [Google Scholar]
- Du, K.; Huang, J.; Wang, W.; Zeng, Y.; Li, X.; Zhao, F. Monitoring Low-Temperature Stress in Winter Wheat Using TROPOMI Solar-Induced Chlorophyll Fluorescence. IEEE Trans. Geosci. Remote Sens. 2024, 62, 4402111. [Google Scholar] [CrossRef]
- He, M.; Ren, T.; Jin, Z.D.; Deng, L.; Liu, H.; Cheng, Y.Y.; Chang, H. Precise analysis of potassium isotopic composition in plant materials by multi-collector inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2023, 209, 106781. [Google Scholar] [CrossRef]
- Qiu, S.; Yang, H.; Zhang, S.; Huang, S.; Zhao, S.; Xu, X.; Banwart, S.A. Carbon storage in an arable soil combining field measurements, aggregate turnover modeling and climate scenarios. Catena 2023, 220, 106708. [Google Scholar] [CrossRef]
- BRAZIL; Ministério da Agricultura, Pecuária e Abastecimento—MAPA. Regras Para Análise de Sementes. Secretaria de Defesa Agropecuária; MAPA/ACS: Brasília, Brazil, 2009; 395p.
- Maguire, J.D. Speed of germination aid in selection and evaluation for seeding emergence and vigor. Crop Sci 1962, 2, 76–177. [Google Scholar] [CrossRef]
- Labouriau, L.G. Seeds Germination; OEA: Washington, DC, USA, 1983; p. 173. [Google Scholar]
- Bisognin, D.A.; Centenaro, R.; Missio, E.L. Quebra de dormência e de dominância apical em batata. Hortic. Bras. 1996, 14, 23–26. [Google Scholar]
- Ravisankar, D.; Chinnamuthu, C.R.; Srimathi, P. Influence of growth promoting substances on dormancy breaking and sprouting of purple nutsedge (Cyperus rotundus L.) tuber. Res. J. Agric. Sci. 2012, 3, 1213–1216. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 858p. [Google Scholar]
- Liu, B.; Li, H.; Chen, W.; Wang, Y.; Chen, Y.; Wei, X. Dormancy break, sprouting and later tuber reproduction in response to different tuber sizes of tiger nut (Cyperus esculentus L.). R. Soc. Open Sci. 2024, 11, 231616. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Bajwa, R.; Rabbani, N.; Anjum, T. Comparative tolerance of rice (Oryza sativa L.) genotypes to purple nutsedge (Cyperus rotundus L.) allelopathy. Allelopath. J. 2007, 20, 157–166. [Google Scholar]
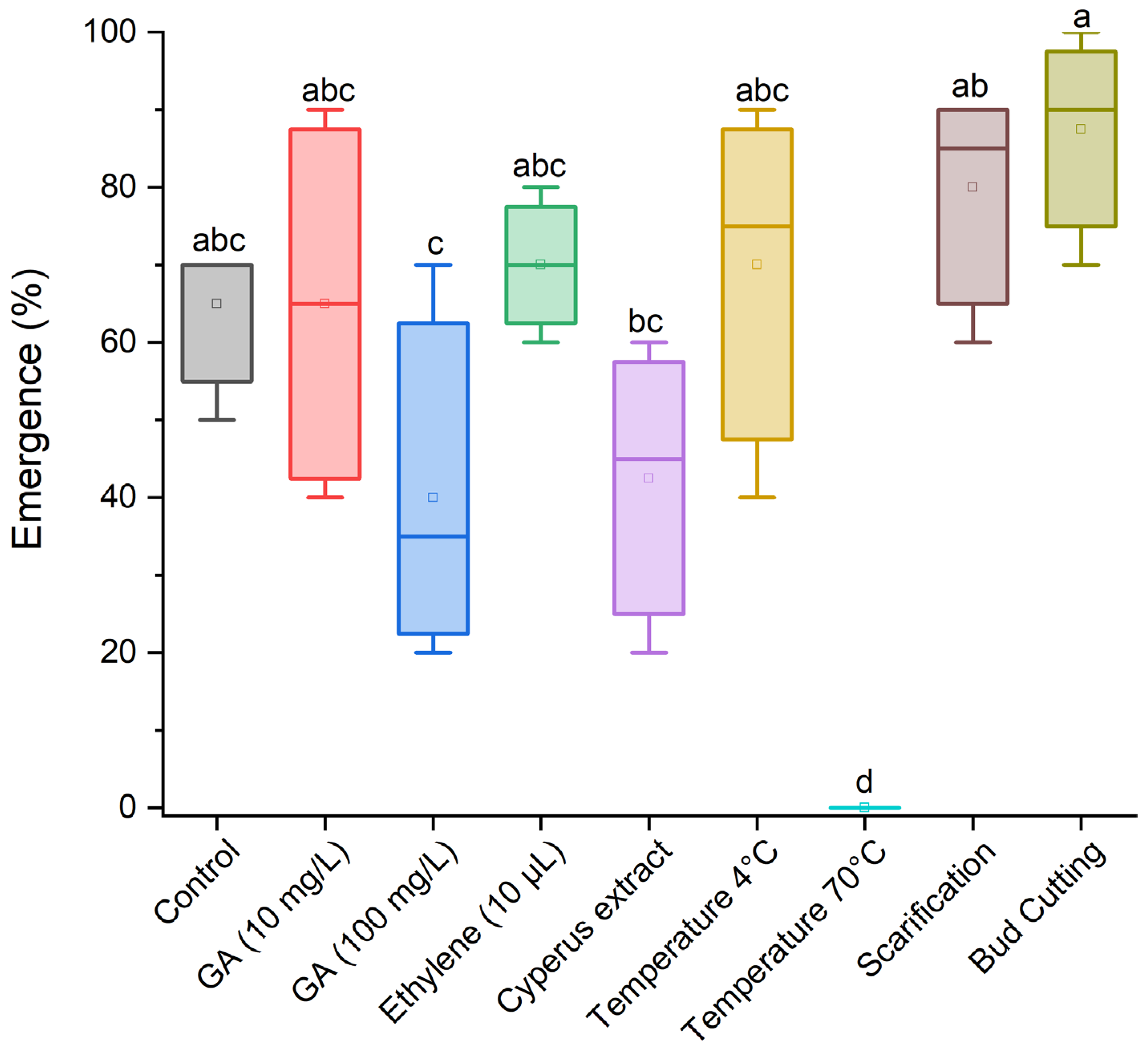
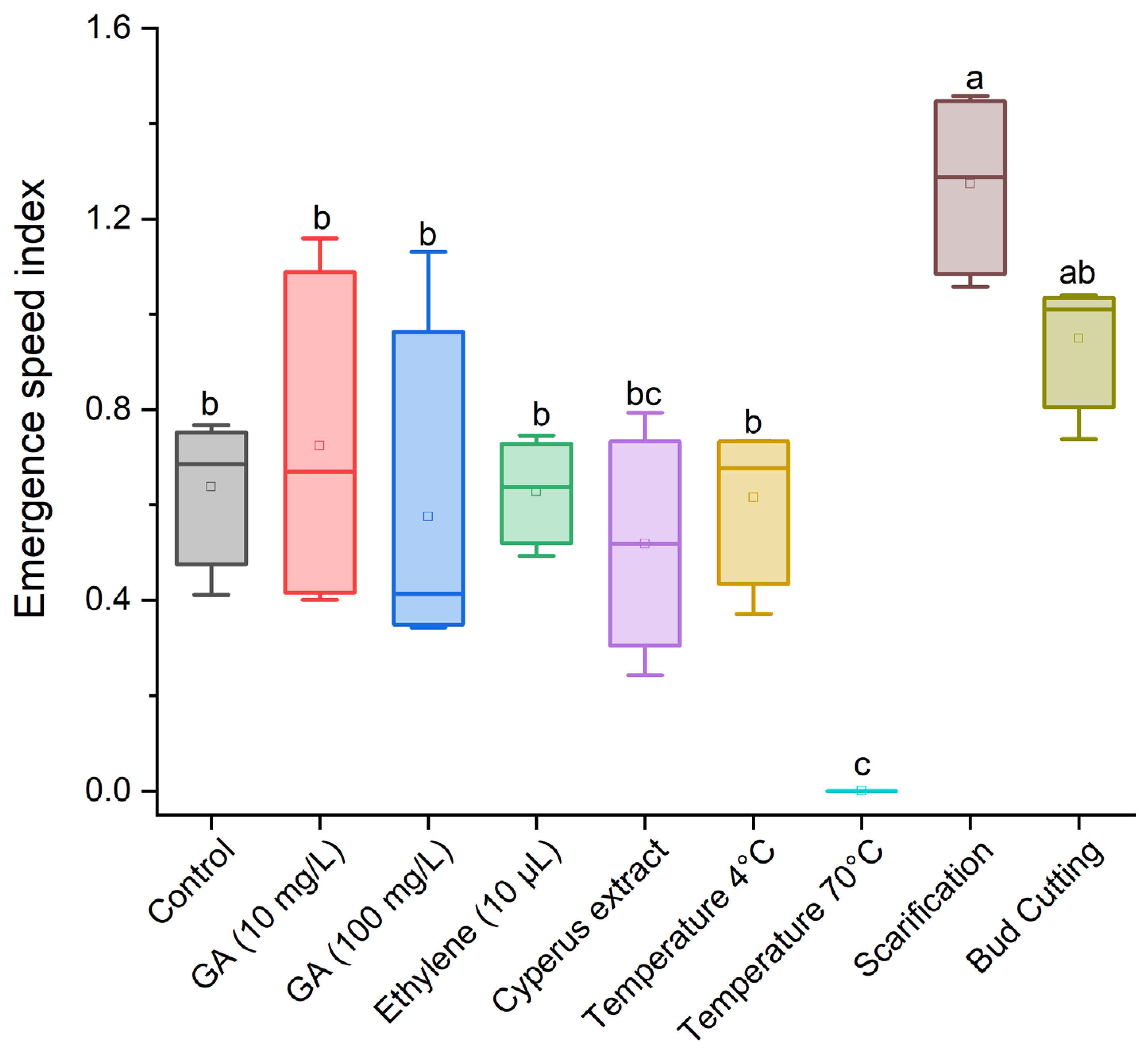
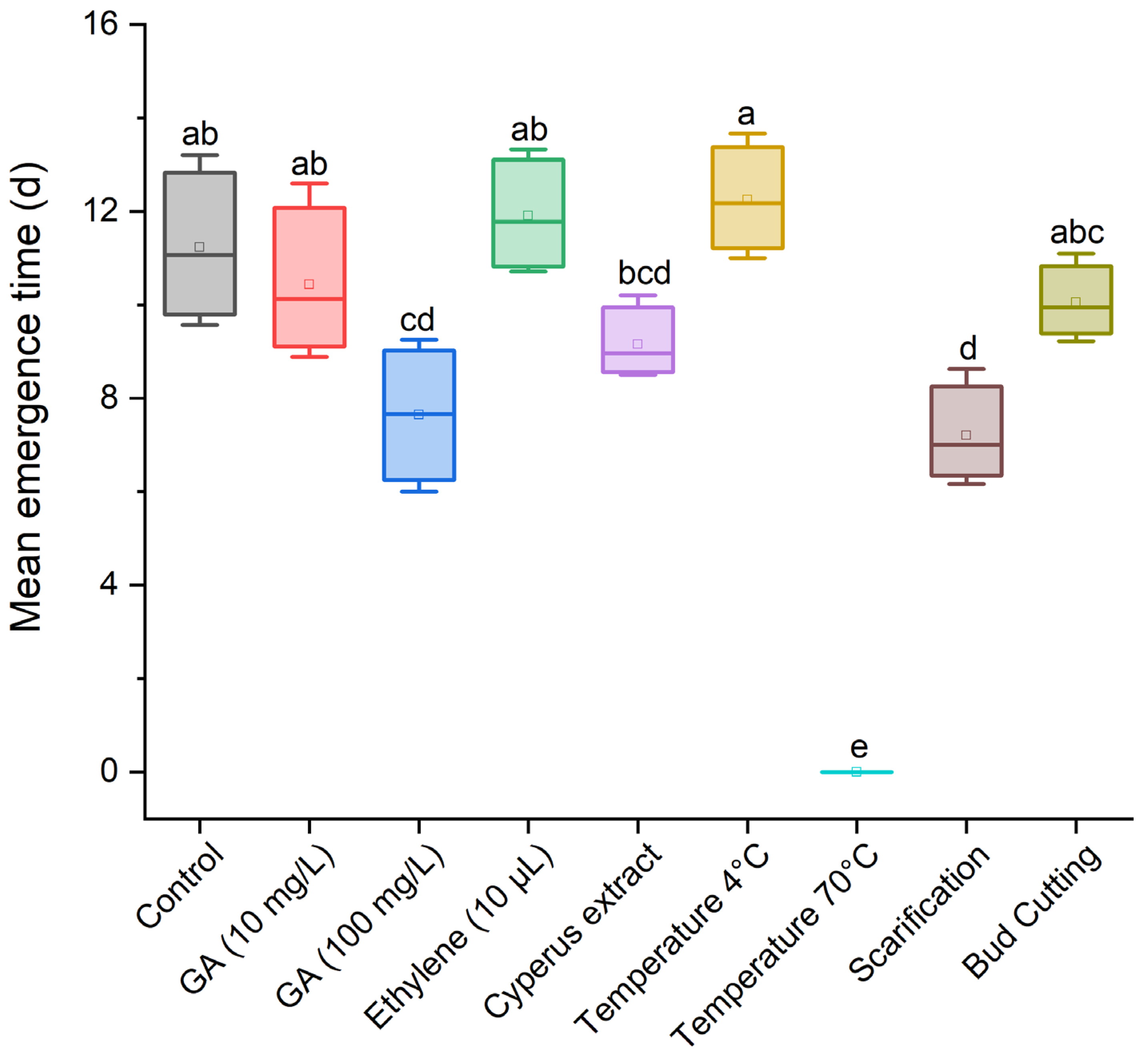
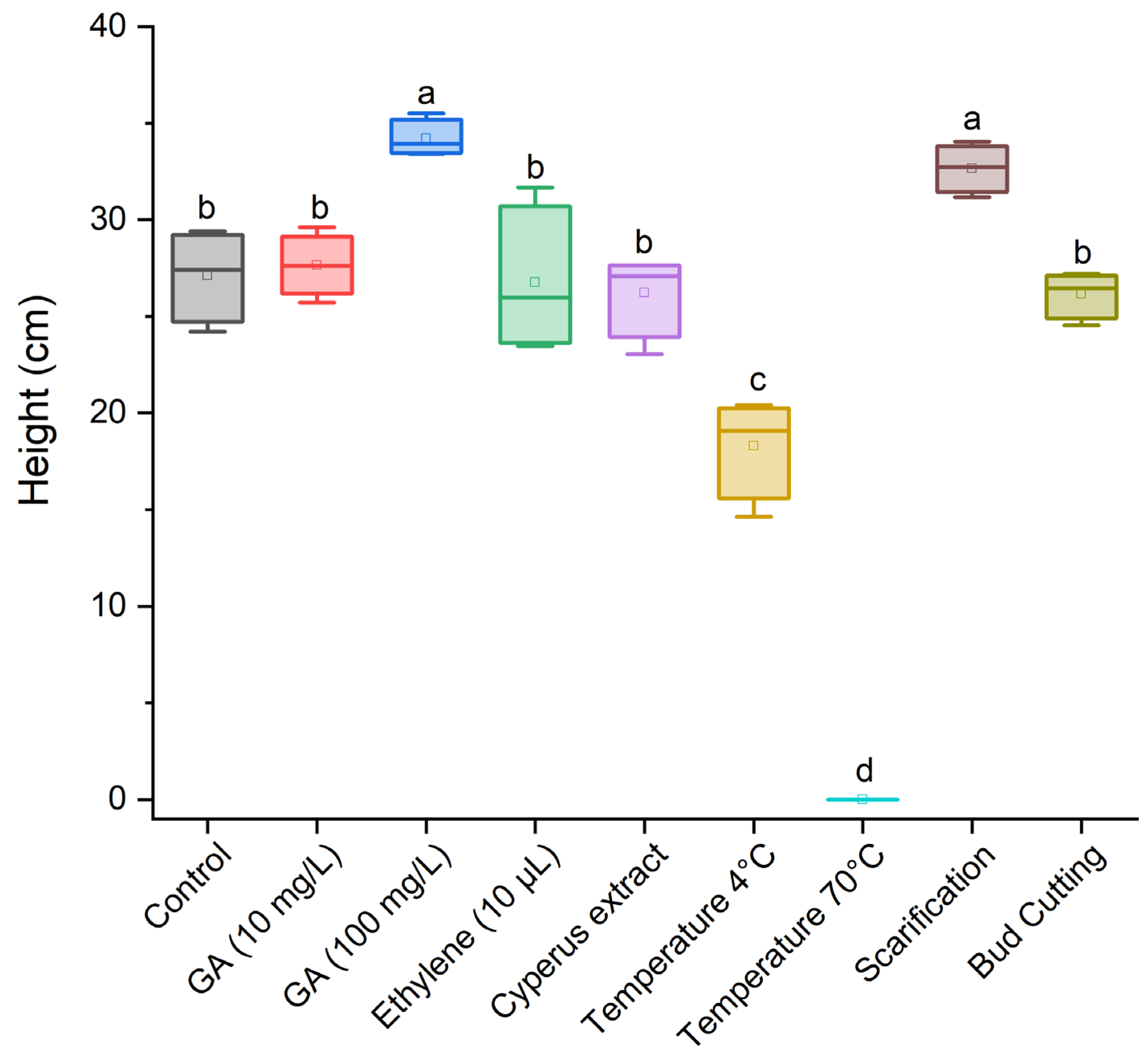
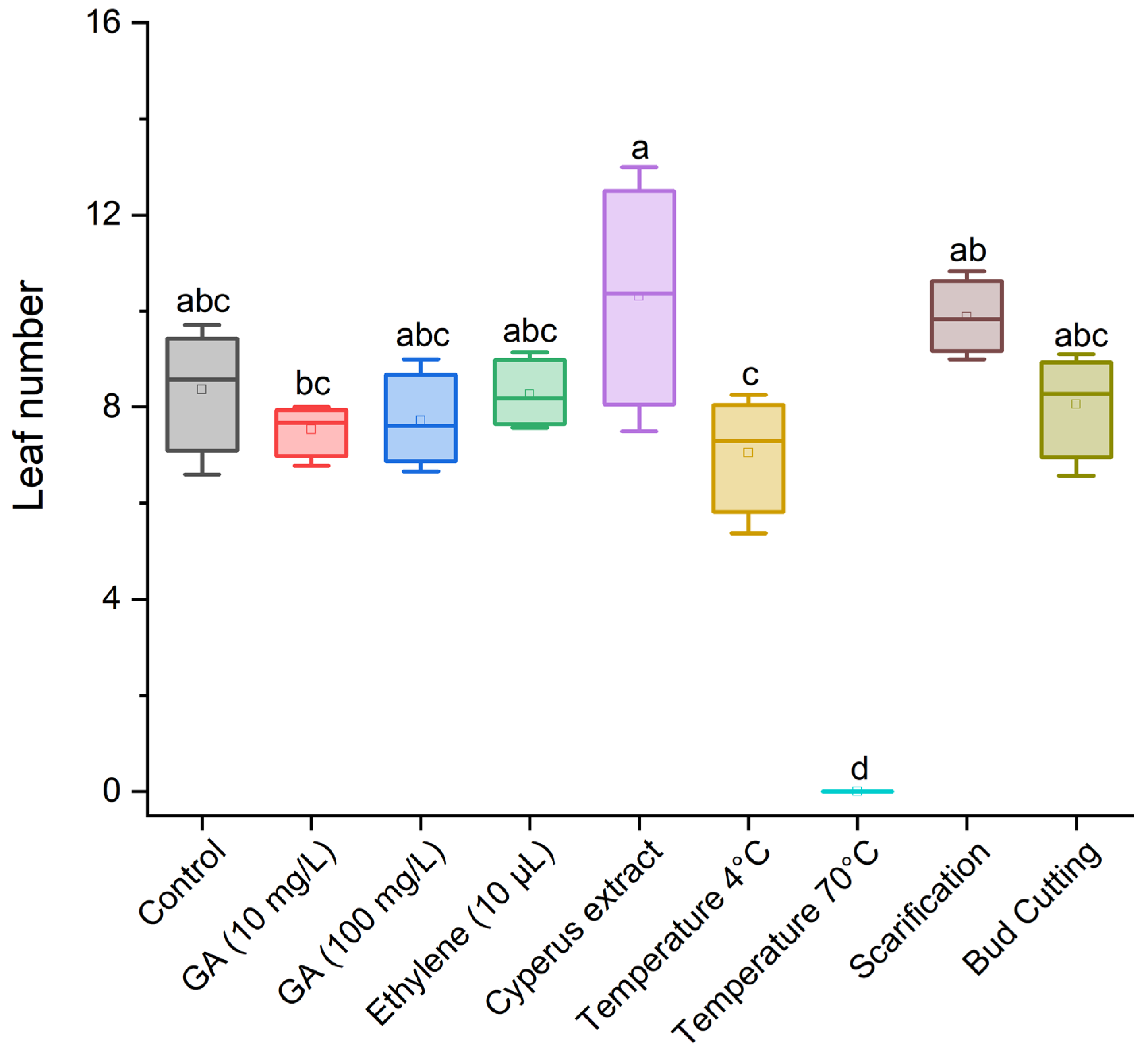
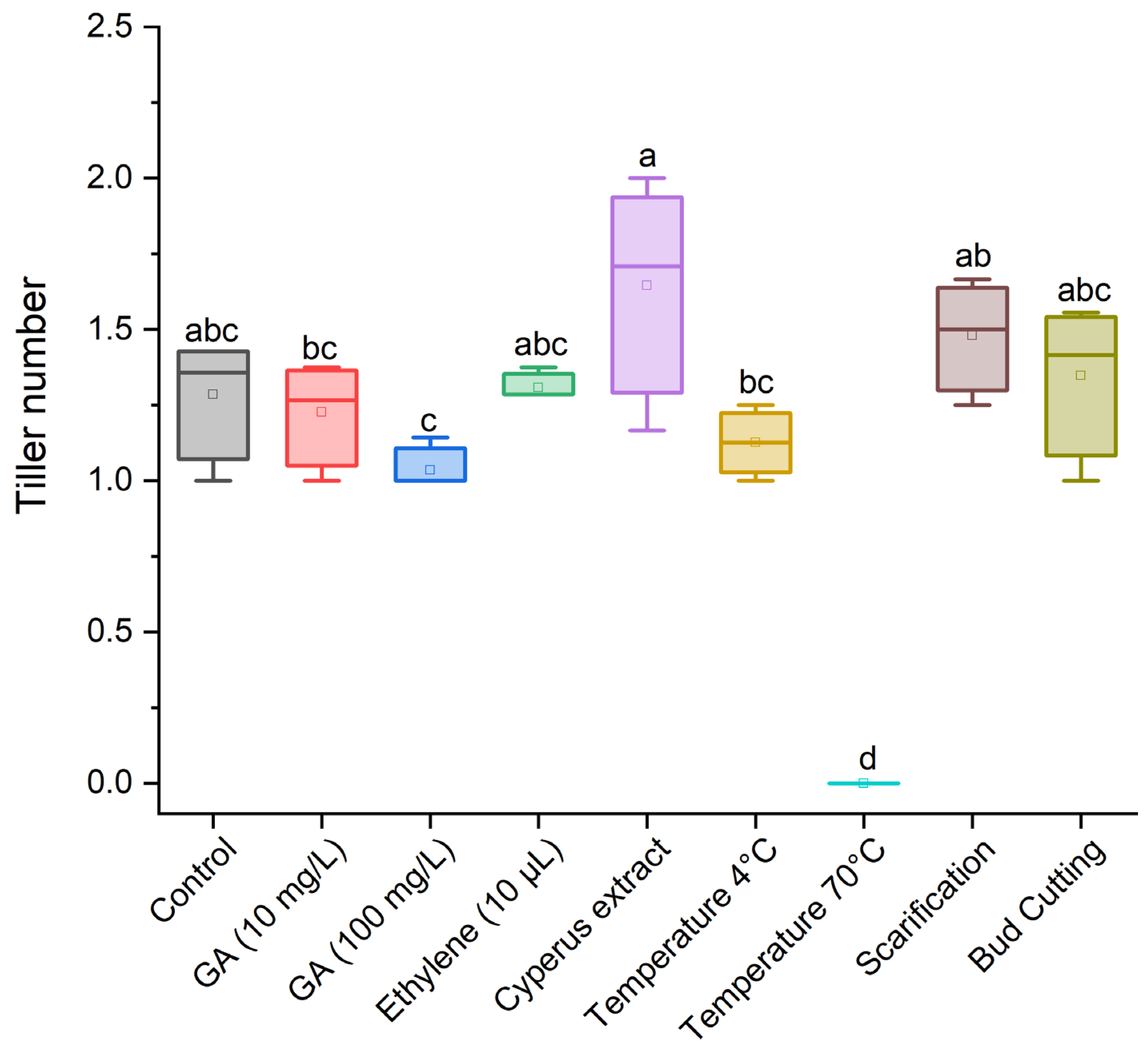

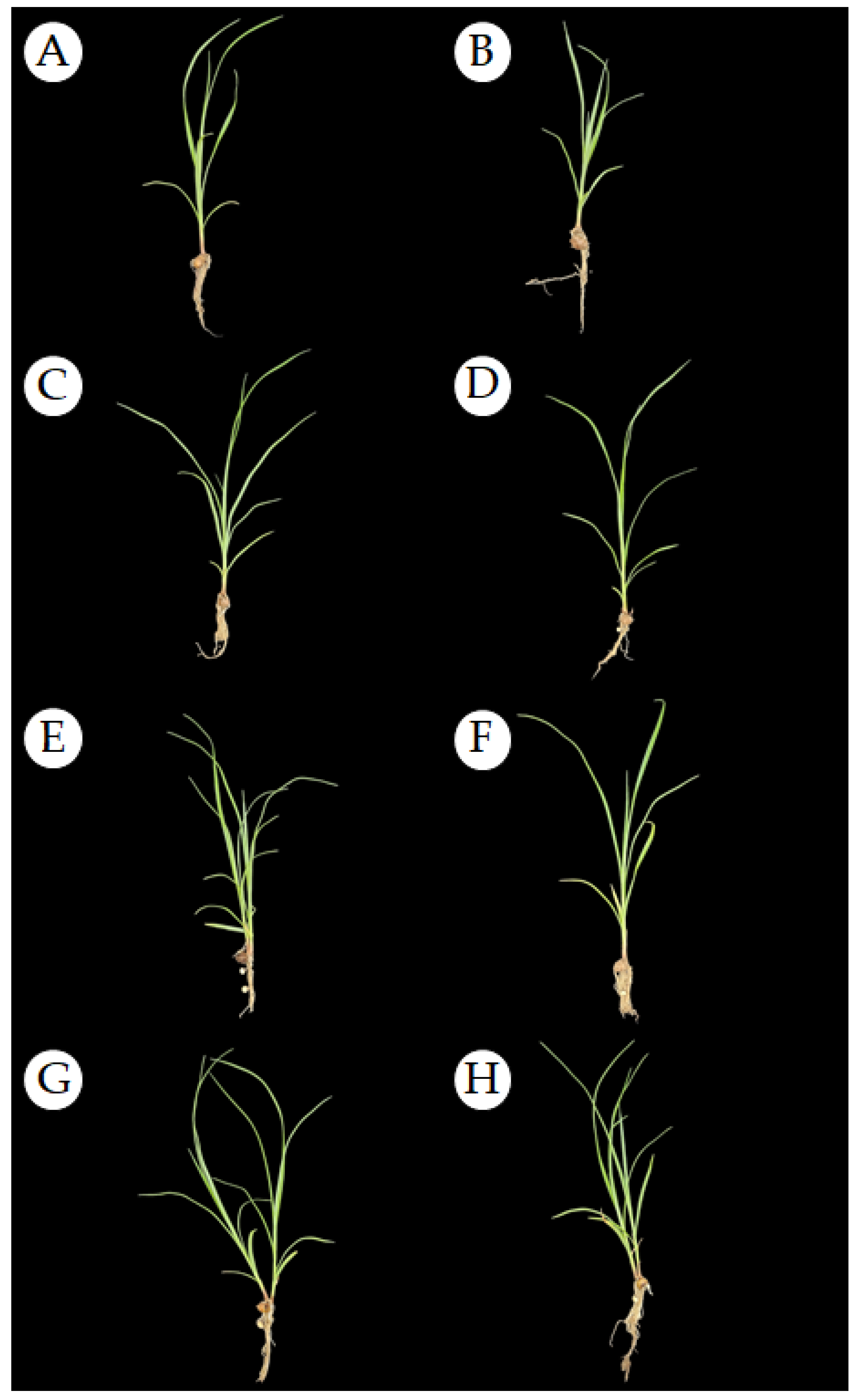
| Control (no dormancy-breaking treatment) |
| Immersion in gibberellic acid (GA) at 10 mg L−1 |
| Immersion in GA at 100 mg L−1 |
| 100 μL Ethylene |
| Immersion in purple nutsedge extract (Cyperus rotundus) |
| Conditioning at 4 °C |
| Conditioning at 70 °C |
| Scarification |
| Bud cutting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoi Junior, M.A.; da Silva, R.S.; de Sousa, R.N.; Pinto, C.M.F.; Ribeiro, W.S.; Mendes, K.F. Yellow Nutsedge (Cyperus esculentus L.) as an Agricultural Crop in Brazil: Tuber Dormancy Breaking. Agronomy 2024, 14, 688. https://doi.org/10.3390/agronomy14040688
Godoi Junior MA, da Silva RS, de Sousa RN, Pinto CMF, Ribeiro WS, Mendes KF. Yellow Nutsedge (Cyperus esculentus L.) as an Agricultural Crop in Brazil: Tuber Dormancy Breaking. Agronomy. 2024; 14(4):688. https://doi.org/10.3390/agronomy14040688
Chicago/Turabian StyleGodoi Junior, Márcio Antônio, Rebeca Soares da Silva, Rodrigo Nogueira de Sousa, Cleide Maria Ferreira Pinto, Wellington Souto Ribeiro, and Kassio Ferreira Mendes. 2024. "Yellow Nutsedge (Cyperus esculentus L.) as an Agricultural Crop in Brazil: Tuber Dormancy Breaking" Agronomy 14, no. 4: 688. https://doi.org/10.3390/agronomy14040688
APA StyleGodoi Junior, M. A., da Silva, R. S., de Sousa, R. N., Pinto, C. M. F., Ribeiro, W. S., & Mendes, K. F. (2024). Yellow Nutsedge (Cyperus esculentus L.) as an Agricultural Crop in Brazil: Tuber Dormancy Breaking. Agronomy, 14(4), 688. https://doi.org/10.3390/agronomy14040688








