Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Field Experiments and Vegetal Material
2.2. Water and Soil Physicochemical Properties
2.3. Inoculation of Tomato and Jalapeño Pepper Seedlings with Rhizobacteria
2.4. Plant Growth Conditions
2.5. Disease Incidence and Insect Pest Monitoring in Tomato and Jalapeño Pepper Plants
2.6. Growth Promotion and Yields in Tomato and Jalapeño Pepper Plants
2.7. Data Analysis
3. Results
3.1. Potential Phytopathogen Detection in the Experimental Soil
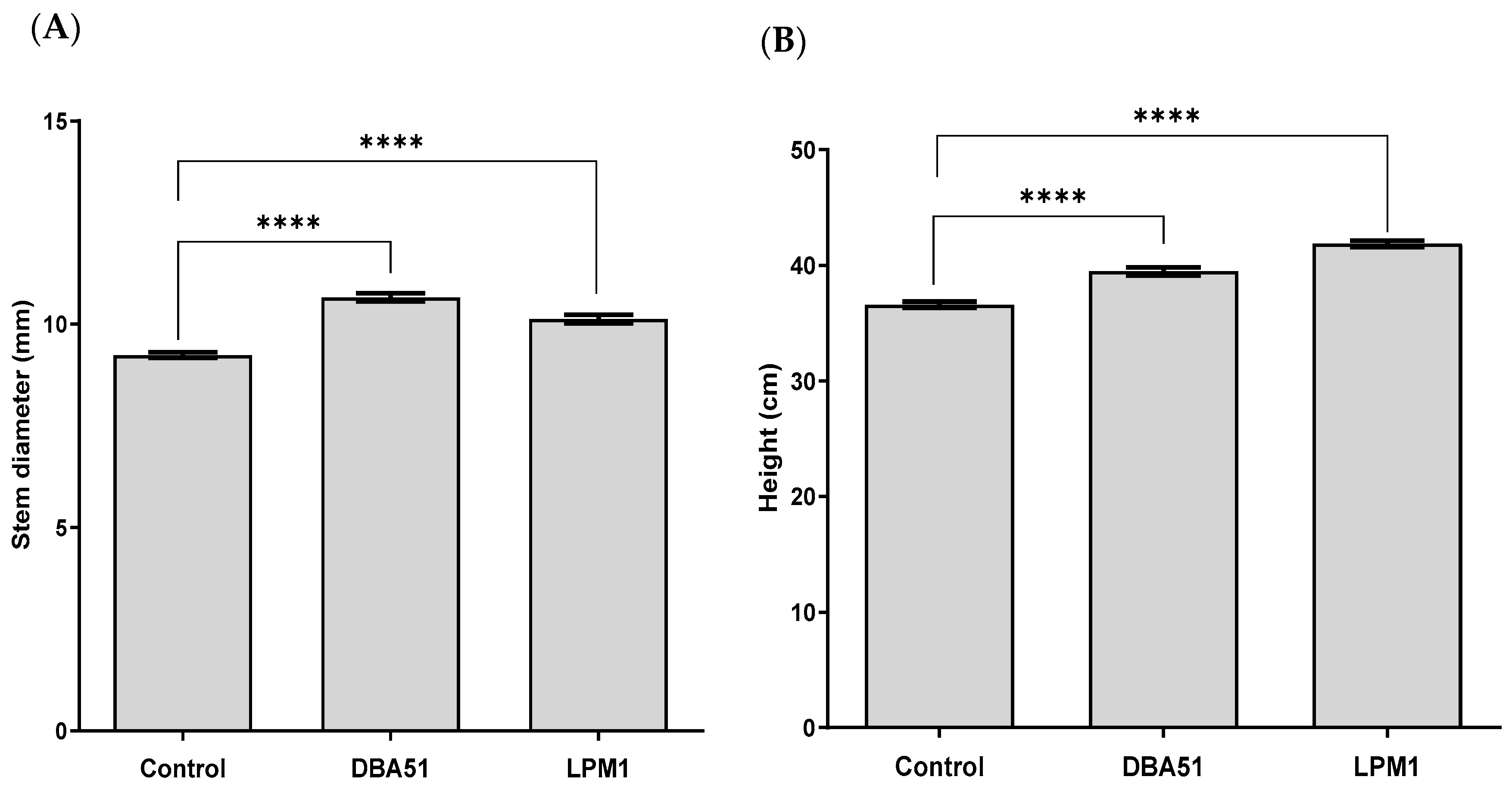
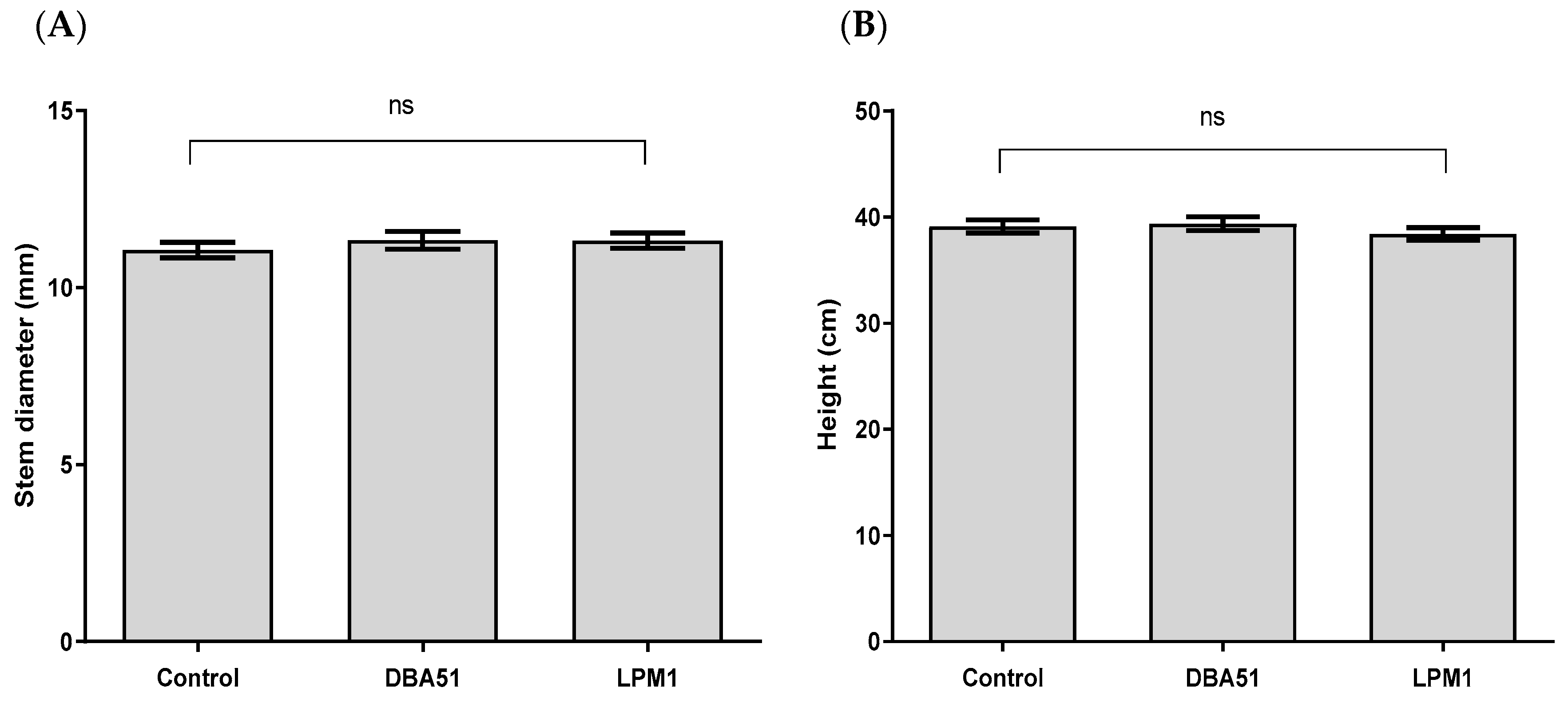
3.2. Vegetative Growth Promotion by PGPR Treatments in Tomato and Jalapeño Pepper Plants
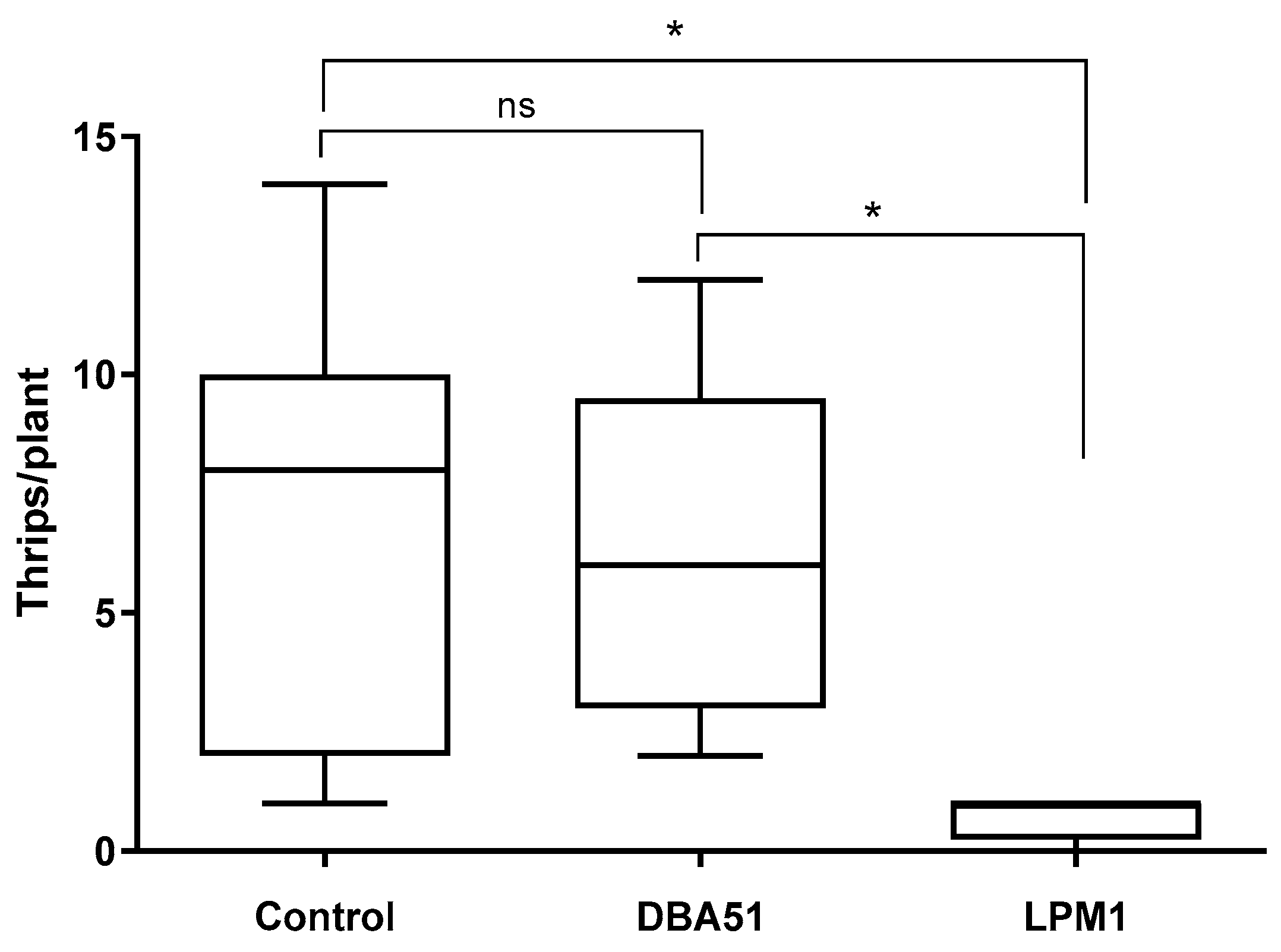
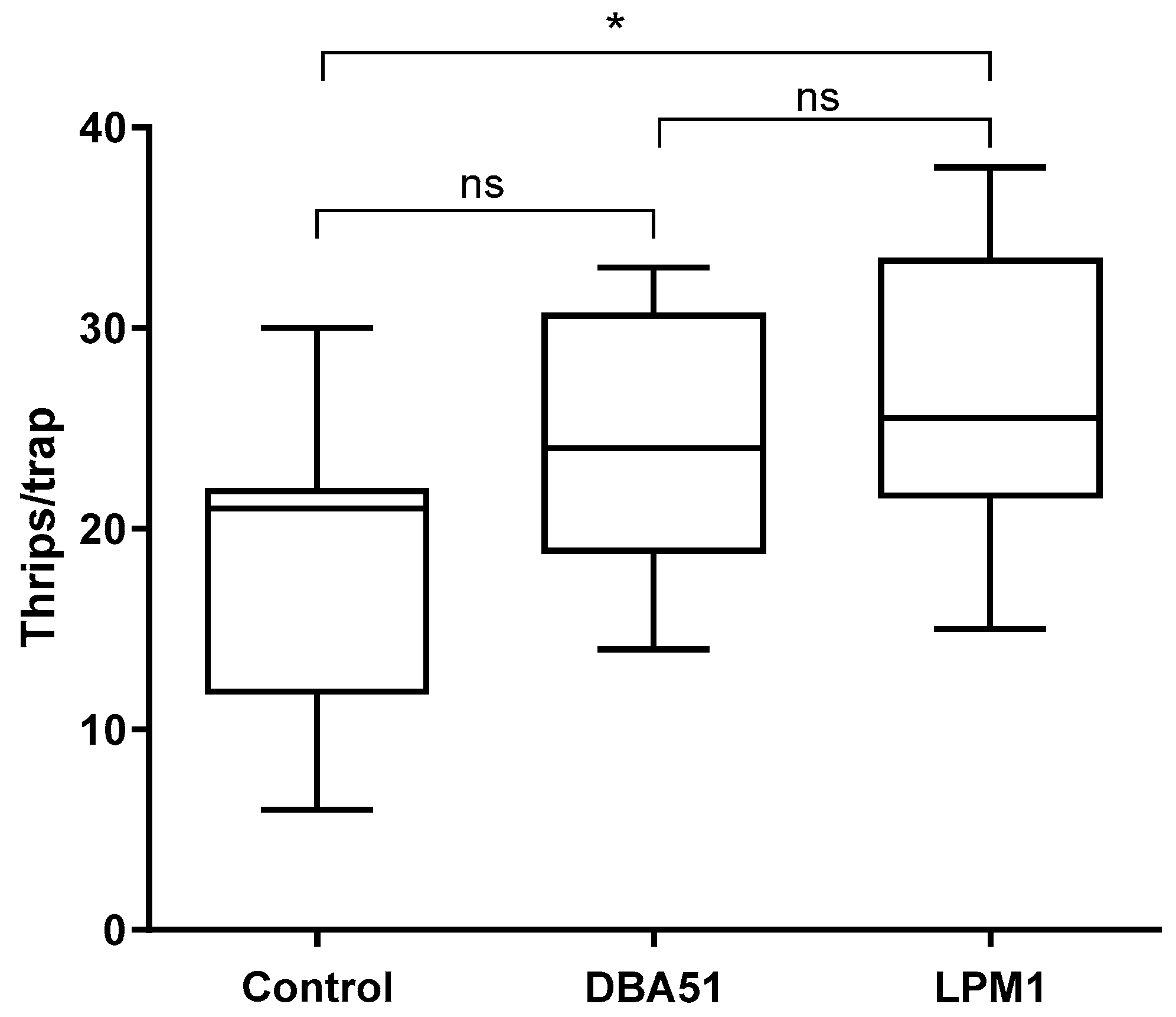
3.3. Disease Incidence in Tomato Plants and Pest Monitoring in Jalapeño Pepper Plants
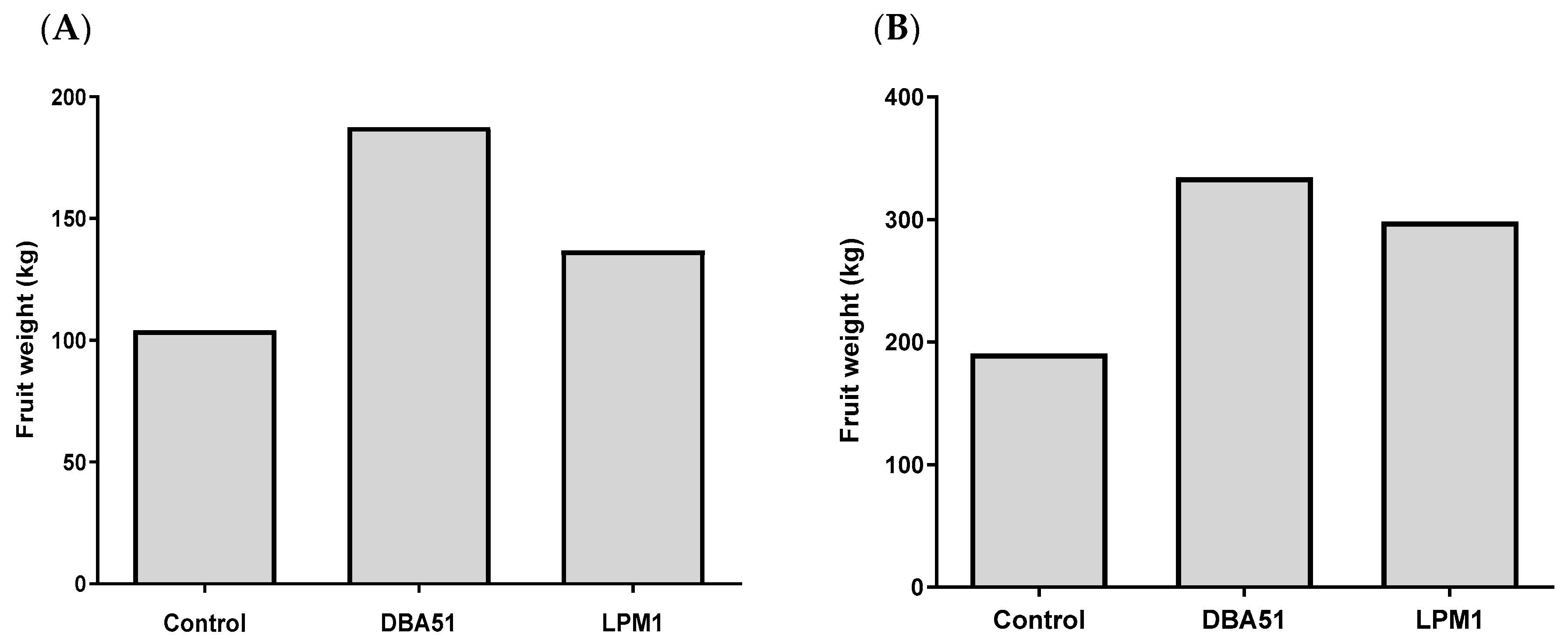
3.4. Increased Crop Yield in Tomato and Jalapeño Pepper by PGPR Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere bacterial signalling: A love parade beneath our feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.; Davidson, G.; Grant, W.P.; Greaves, J.; Tatchell, G.M. Microbial biopesticides for integrated crop management: An assessment of environmental and regulatory sustainability. Trends Food Sci. Technol. 2008, 19, 275–283. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Chin-A-Woeng, T.F.; Bloemberg, G.V. Microbe–plant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Kalsoom, M.; Adnan, M.; Toor, M.; Zulfiqar, A. Plant growth promoting rhizobacteria and their mechanisms involved in agricultural crop production: A review. SunText Rev. Biotechnol. 2020, 1, 1–6. [Google Scholar]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Feng, H.; Fu, R.; Hou, X.; Lv, Y.; Zhang, N.; Liu, Y.; Xu, Z.; Miao, Y.; Krell, T.; Shen, Q. Chemotaxis of beneficial rhizobacteria to root exudates: The first step towards root–microbe rhizosphere interactions. Int. J. Mol. Sci. 2021, 22, 6655. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Ortiz-Castro, R.; Flores-Olivas, A.; Moggio, I.; Arias, E.; Valenzuela-Soto, J.H. Fluorescent detection of pyrene-stained Bacillus subtilis LPM1 rhizobacteria from colonized patterns of tomato roots. Photochem. Photobiol. Sci. 2020, 19, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Schoebitz, M.; López, M.D.; Roldán, A. Bioencapsulation of microbial inoculants for better soil-plant fertilization. A review. Agron. Sust. Dev. 2013, 33, 751–765. [Google Scholar] [CrossRef]
- Ortega-Ortega, Y.; Sarmiento-López, L.G.; Baylón-Palomino, A.; Vázquez-Lee, J.; Maldonado-Bonilla, L.D.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Enterobacter sp. DBA51 produces ACC deaminase and promotes the growth of tomato (Solanum lycopersicum L.) and tobacco (Nicotiana tabacum L.) plants under greenhouse condition. Curr. Res. Microb. Sci. 2023, 6, 100207. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Flores, A.; Ruíz-Salas, C.E.; Vázquez-Lee, J.; Baylón-Palomino, A.; Mounzer, O.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bacillus subtilis LPM1 differentially promotes the growth of bell pepper (Capsicum annuum L.) varieties under shade house. Cogent Food Agric. 2023, 9, 2232165. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 2014, 3, 53–66. [Google Scholar]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Cisternas, J.; Rodriguez, A.; Vega-Gálvez, A.; Bravo, J. Importance of crop phenological stages for the efficient use of PGPR inoculants. Sci. Rep. 2021, 11, 19548. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Castro, A.; Ferrera-Cerrato, R.; Alarcón, A.; Almaraz-Suárez, J.J.; Delgadillo-Martínez, J.; Jiménez-Fernández, M.; García-Barradas, O. Crecimiento y eficiencia fotoquímica del fotosistema II en plántulas de 2 variedades de Capsicum annuum L. inoculadas con rizobacterias u hongos micorrícicos arbusculares. Rev. Argent. Microbiol. 2018, 50, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Mechanisms used by plant growth-promoting bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Růzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; He, L.; Huang, R. The coordination of ethylene and other hormones in primary root development. Front. Plant Sci. 2019, 10, 459007. [Google Scholar] [CrossRef] [PubMed]
- Artyszak, A.; Gozdowski, D. Application of growth activators and Plant Growth-Promoting Rhizobacteria as a method of introducing a “farm to fork” strategy in crop management of winter oilseed. Sustainability 2021, 13, 3562. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, V.; Kitto, S.L.; Caplan, J.L.; Hsueh, Y.-H.; Kearns, D.B.; Wu, Y.S.; Bais, H.P. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 2012, 160, 1642–1661. [Google Scholar] [CrossRef] [PubMed]
- Dolkar, D.; Dolkar, P.; Angmo, S.; Chaurasia, O.P.; Stobdan, T. Stress tolerance and plant growth promotion potential of Enterobacter ludwigii PS1 isolated from Seabuckthorn rhizosphere. Biocatal. Agric. Biotechnol. 2018, 14, 438–443. [Google Scholar] [CrossRef]
- Anand, G.; Bhattacharjee, A.; Shrivas, V.L.; Dubey, S.; Sharma, S. ACC deaminase positive Enterobacter mediated mitigation of salinity stress, and plant growth promotion of Cajanus cajan: A lab to field study. Physiol. Mol. Biol. Plants 2021, 27, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Senapati, A.; Sharma, L.; Nayak, A.K.; Kumar, A.; Kumar, U.; Prabhukarthikeyan, S.R.; Mitra, D.; Sagarika, M.S. Understanding rice growth-promoting potential of Enterobacter spp. isolated from long-term organic farming soil in India through a supervised learning approach. Curr. Res. Microb. Sci. 2021, 2, 100035. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, B.; Bachani, P.; Singh, A.; Mishra, S. Enterobacter hormaechei as plant growth-promoting bacteria for improvement in Lycopersicum esculentum. Curr. Microbiol. 2021, 78, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, B.; Mishra, S.; Singh, A. Enterobacter hormaechei (MF957335) enhanced yield, disease and salinity tolerance in tomato. Arch. Microbiol. 2021, 203, 2659–2667. [Google Scholar] [CrossRef]

| Parameters | Results | mEq/L | ppm |
|---|---|---|---|
| EC (dS/m) | 1.28 | - | - |
| pH | 7.68 | - | - |
| Hardness | 33.91 | - | - |
| Ca | - | 3.70 | 74.1 |
| Mg | - | 3.12 | 37.9 |
| Na | - | 5.01 | 115 |
| K | - | 0.81 | 31.7 |
| S-SO4 | - | 1.89 | 60.5 |
| HCO3 | - | 8.21 | 501 |
| Cl | - | 2.47 | 86.5 |
| CO3 | - | 0.00 | 0.00 |
| N-NO3 | - | 0.17 | 2.38 |
| B | - | - | 0.10 |
| Fe | - | - | 0.0020 |
| Mn | - | - | 1.7630 |
| Cu | - | - | 0.0010 |
| Zn | - | - | 0.0010 |
| As | - | - | 0.0005 |
| Parameters | Results |
|---|---|
| Soil texture | Sandy, clay, loam |
| EC (dS/m) | 1.15 |
| pH | 7.38 |
| Bulk density (g/cm3) | 1.13 |
| Organic matter (%) | 2.06 |
| Carbonates (%) | 1.78 |
| Saturation point (%) | 55.0 |
| Field capacity (%) | 29.4 |
| Permanent wilting point (%) | 17.5 |
| Hydraulic conductivity (cm/h) | 2.00 |
| P-Bray (ppm) | 48.3 |
| K (ppm) | 403 |
| Ca (ppm) | 5101 |
| Mg (ppm) | 1015 |
| Na (ppm) | 378 |
| Fe (ppm) | 18.6 |
| Zn (ppm) | 6.55 |
| Mn (ppm) | 19.8 |
| Cu (ppm) | 3.59 |
| B (ppm) | 2.12 |
| S (ppm) | 17.5 |
| N-NO3 (ppm) | 39.2 |
| Health of soil (mg CO2/kg) | 309 |
| Type of Pathogen | Identity | (CFU/g Soil) |
|---|---|---|
| Bacterial cell density | - | - |
| Fungal cell density | ||
| Colletotrichum sp. | 4.1 × 103 | |
| Fusarium sp. | 1.3 × 103 | |
| Pythium sp. | 3 × 102 | |
| Nematodes density | (individuals/100 g soil) | |
| Pratylenchus sp. | 11 | |
| Tylenchulus sp. | 33 | |
| Tylenchus sp. | 99 | |
| Nacobbus sp. | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Délano-Frier, J.P.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions. Agronomy 2024, 14, 702. https://doi.org/10.3390/agronomy14040702
Délano-Frier JP, Flores-Olivas A, Valenzuela-Soto JH. Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions. Agronomy. 2024; 14(4):702. https://doi.org/10.3390/agronomy14040702
Chicago/Turabian StyleDélano-Frier, John Paul, Alberto Flores-Olivas, and José Humberto Valenzuela-Soto. 2024. "Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions" Agronomy 14, no. 4: 702. https://doi.org/10.3390/agronomy14040702
APA StyleDélano-Frier, J. P., Flores-Olivas, A., & Valenzuela-Soto, J. H. (2024). Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions. Agronomy, 14(4), 702. https://doi.org/10.3390/agronomy14040702






