Physiological and Antioxidative Effects of Strontium Oxide Nanoparticles on Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Plant Extract
2.3. Green Synthesis and Characterization of SrO-NPs
2.4. Application of SrO-NPs on Wheat and Their Growing Condition
2.5. Measuring the Chlorophyll Value
2.6. Morphological Observations
2.7. Cell Membrane Damage
2.8. H2O2, MDA, and Stress Enzyme Analysis
2.9. Data Analysis
3. Results and Discussion
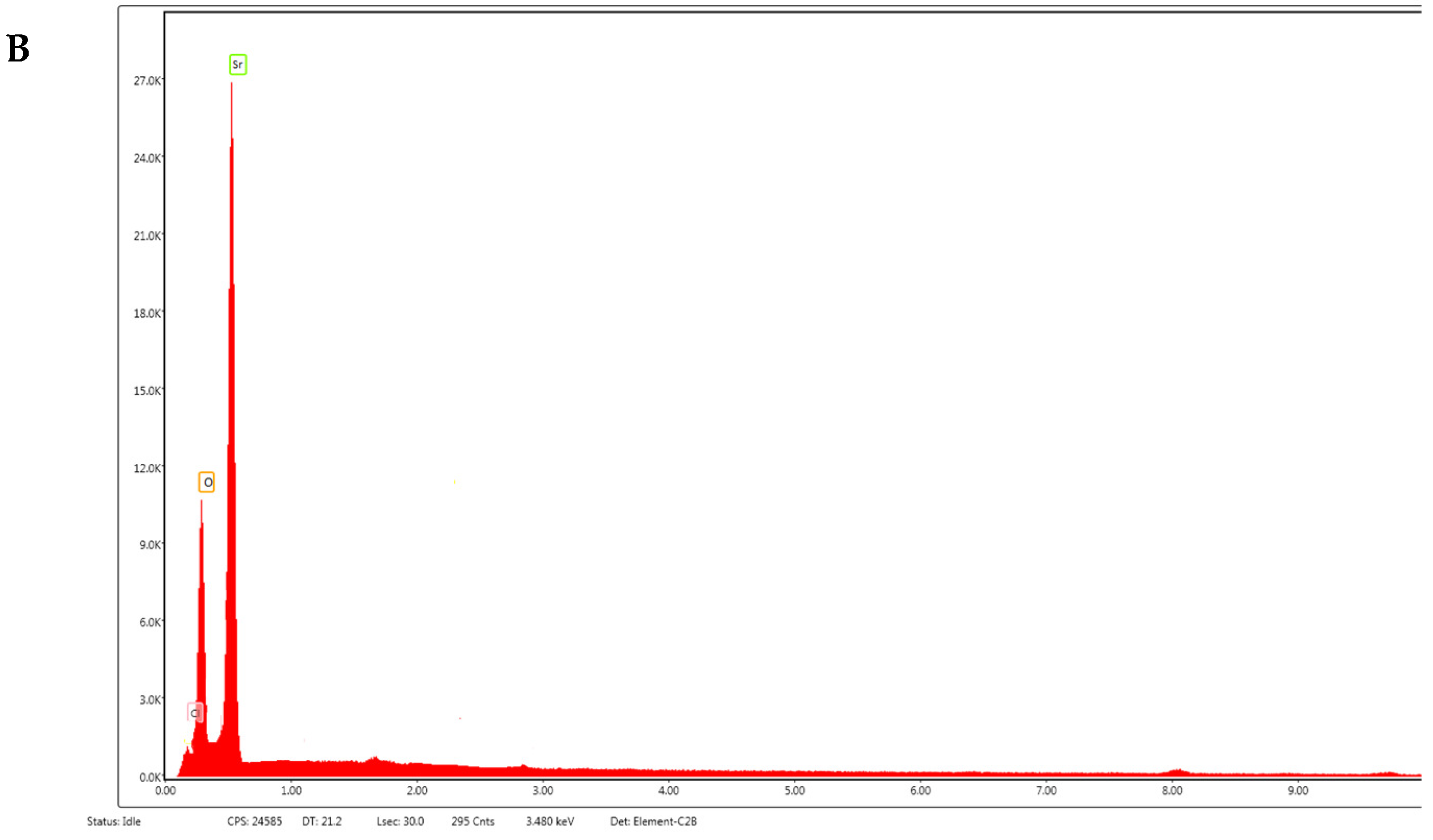
3.1. Synthesis and Characterization of SrO-NPs
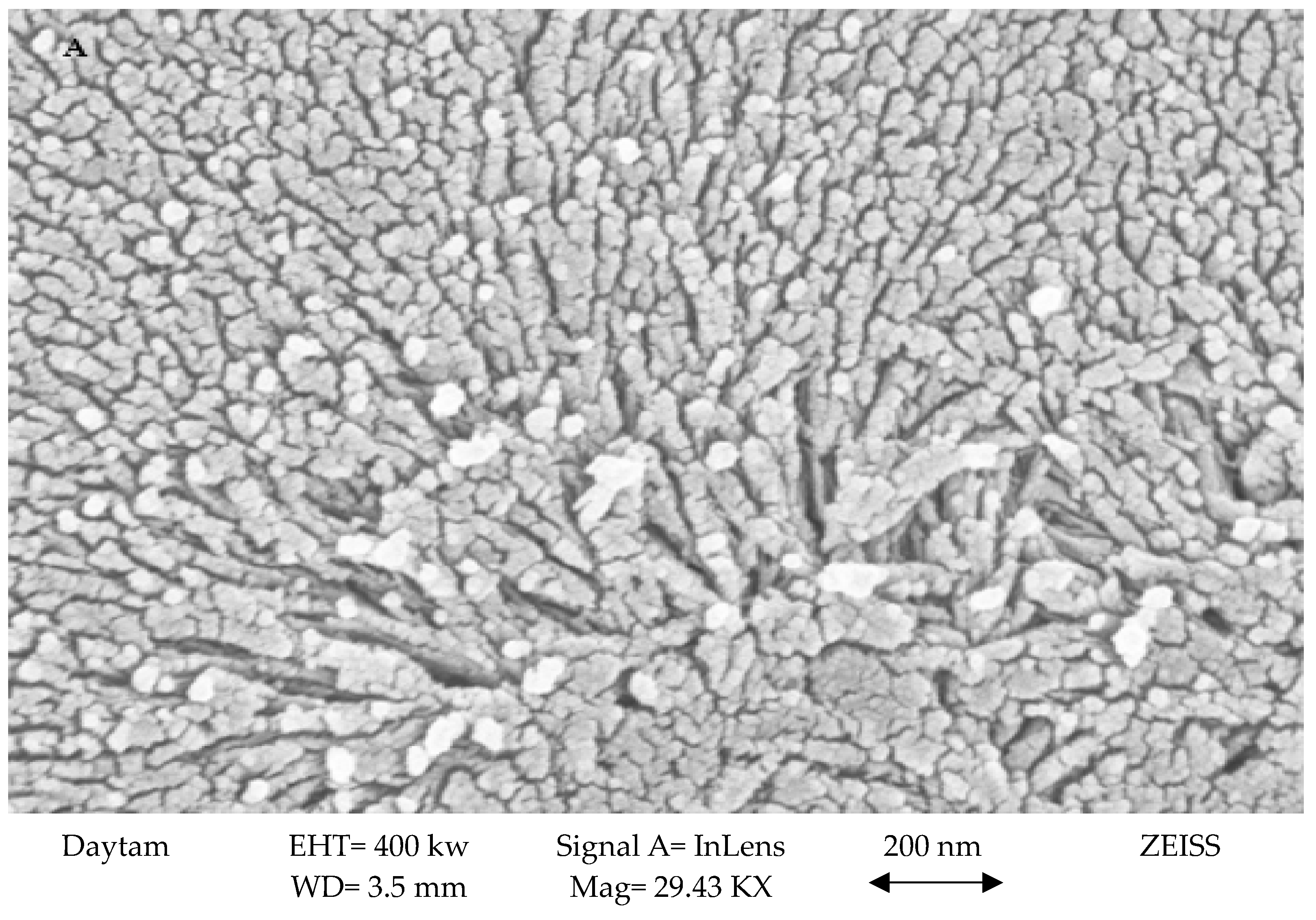
3.1.1. SEM Analysis
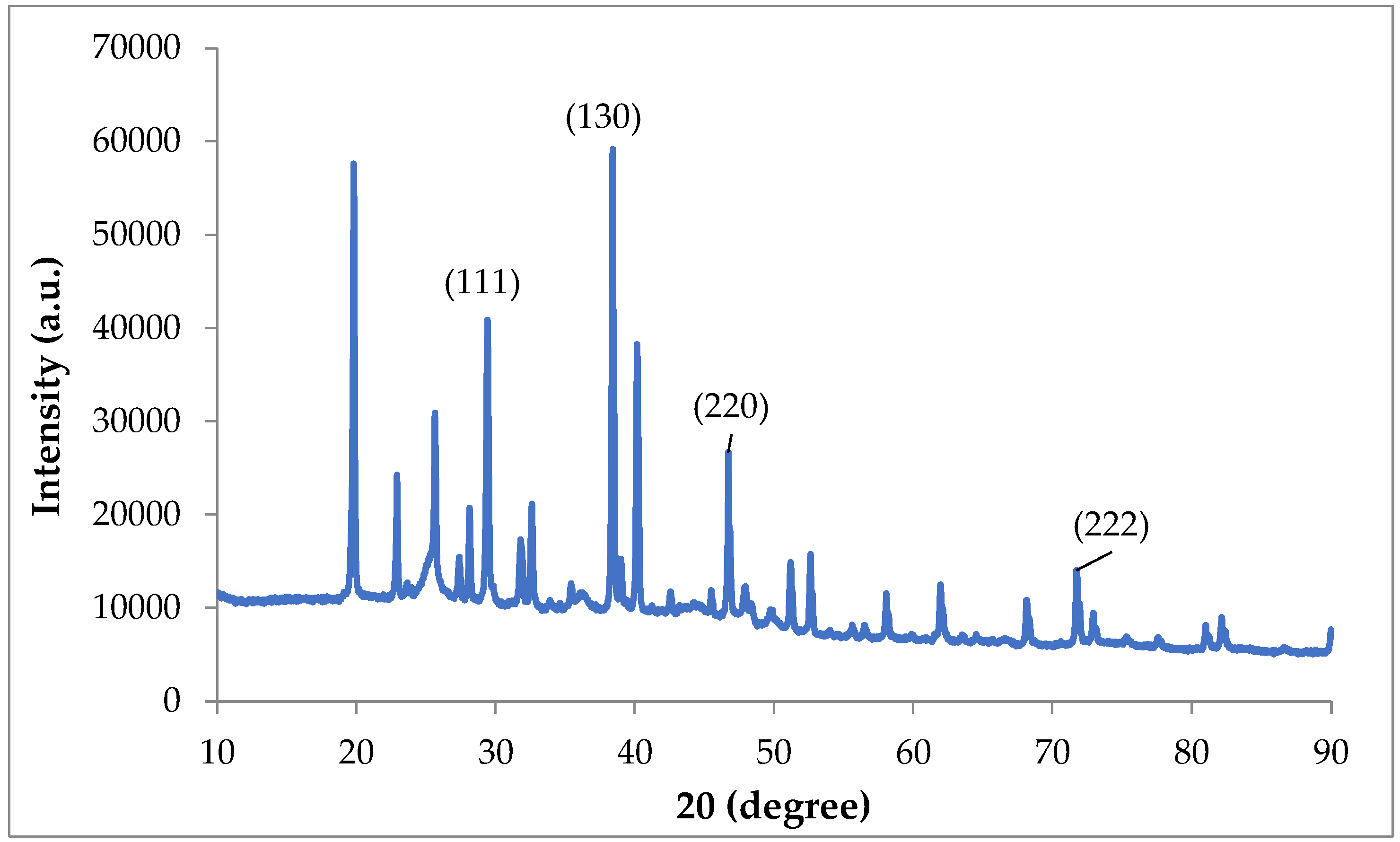
3.1.2. XRD of ZnO-NPs
3.1.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.2. Physiological Effect on Wheat of SrO-NPs
3.2.1. Shoot Length
3.2.2. Shoot Fresh Weight
3.2.3. Number of Roots
3.2.4. Root Length
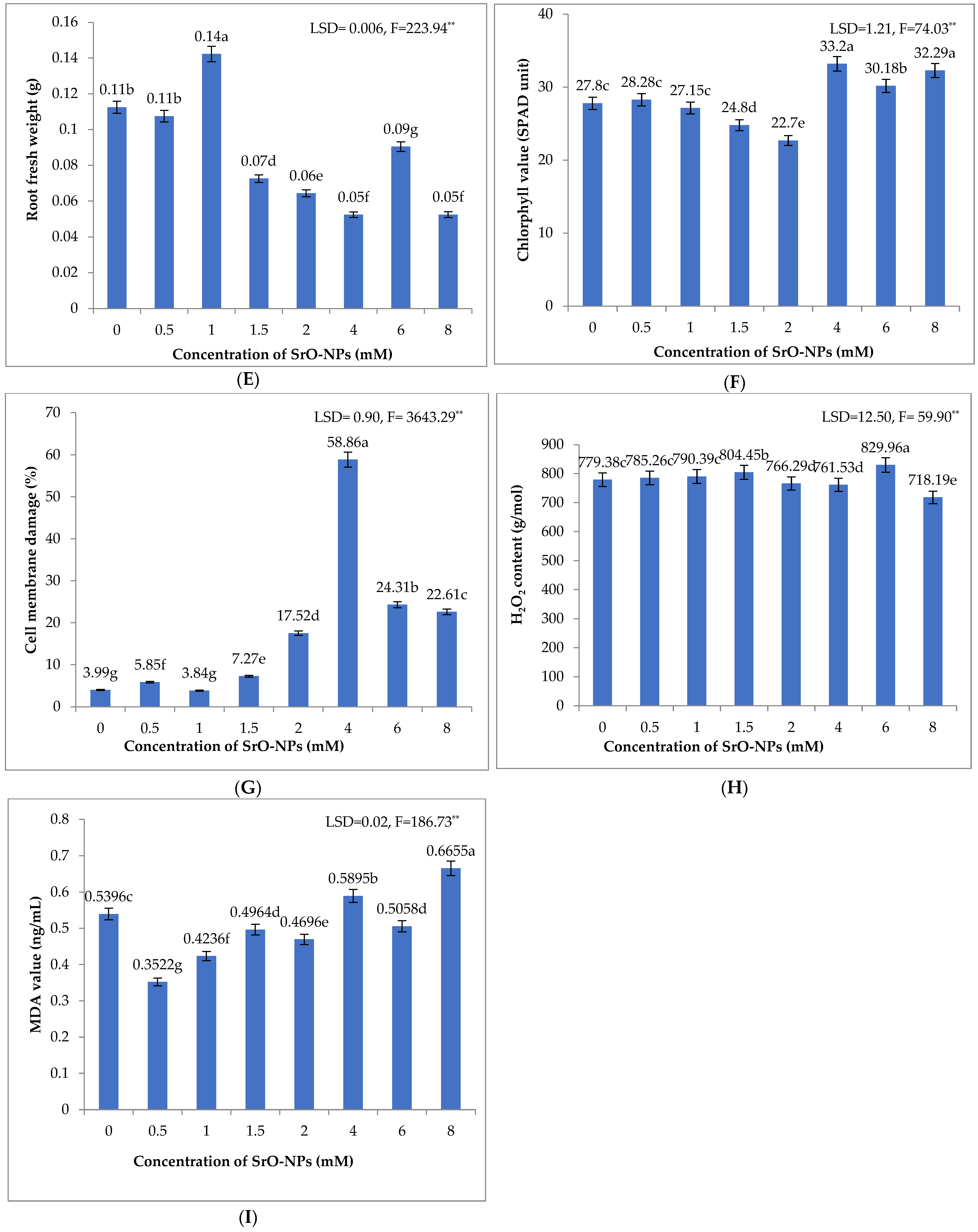
3.2.5. Fresh Root Weight
3.2.6. Chlorophyll Value
3.2.7. Cell Membrane Damage
3.2.8. H2O2 Value
3.2.9. MDA Value
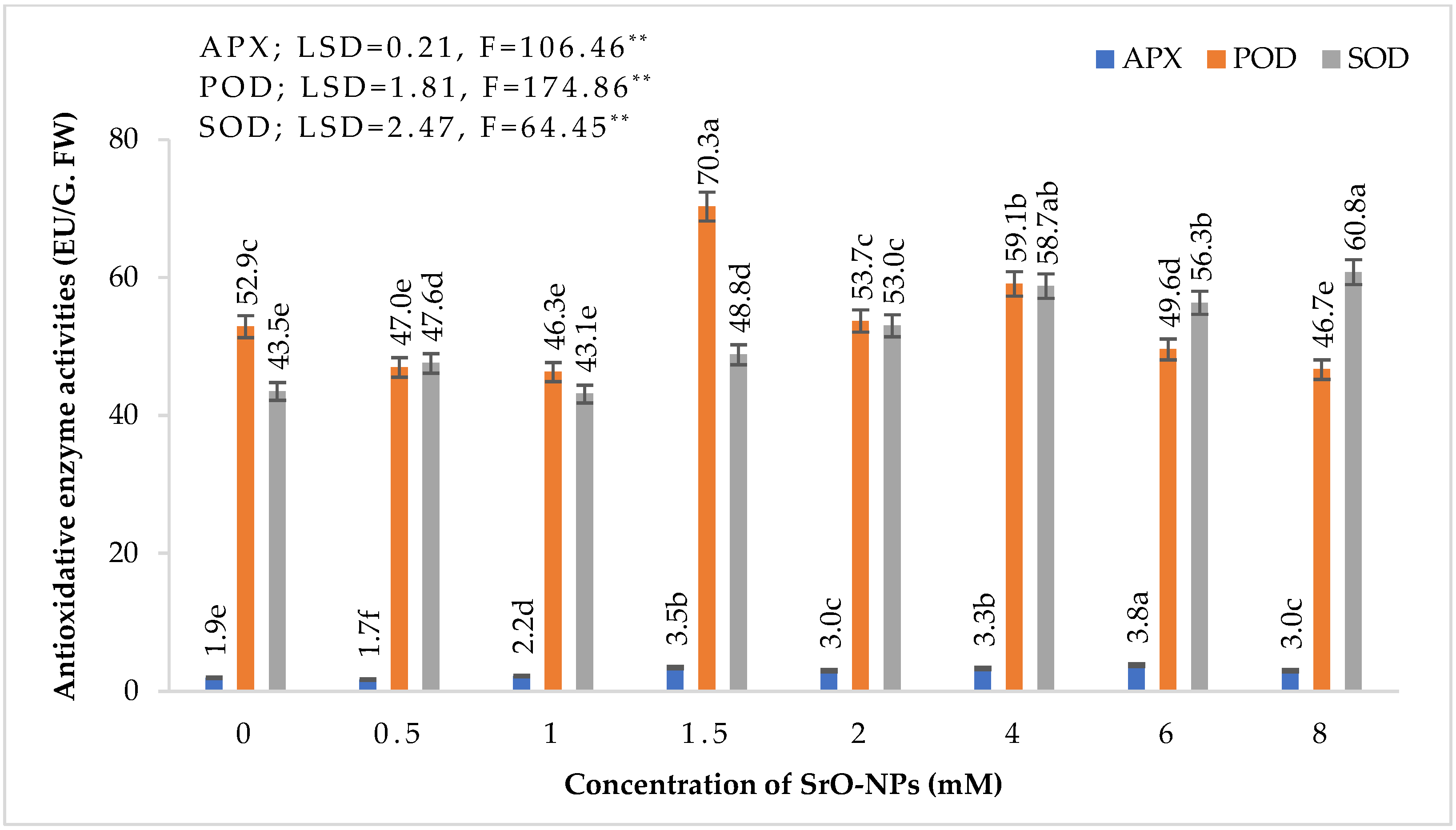
3.3. Antioxidant Effect on Wheat of SrO-NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moshawih, S.; Abdullah Juperi, R.a.N.A.; Paneerselvam, G.S.; Ming, L.C.; Liew, K.B.; Goh, B.H.; Al-Worafi, Y.M.; Choo, C.-Y.; Thuraisingam, S.; Goh, H.P. General health benefits and pharmacological activities of Triticum aestivum L. Molecules 2022, 27, 1948. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Iqbal, M.; Asif, M.; Hirani, A.H.; Goyal, A. Growing wheat on saline lands: Can a dream come true? Aust. J. Crop Sci. 2013, 7, 515–524. [Google Scholar]
- Uthayakumaran, S.; Wrigley, C. Wheat: Grain-quality characteristics and management of quality requirements. In Cereal Grains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–134. [Google Scholar]
- Delporte, F.; Mostade, O.; Jacquemin, J. Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell Tissue Organ Cult. 2001, 67, 73–80. [Google Scholar] [CrossRef]
- Jadia, C.D.; Fulekar, M. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters, and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Van Hoeck, A.; Horemans, N.; Van Hees, M.; Nauts, R.; Knapen, D.; Vandenhove, H.; Blust, R. β-Radiation stress responses on growth and antioxidative defense system in plants: A study with strontium-90 in Lemna minor. Int. J. Mol. Sci. 2015, 16, 15309–15327. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.; Ma, H.; Chaudhary, S.; Nuruzaman, M.; Azeem, I.; Mehmood, F.; Rahman, S.U.; Aiwang, D.; Sun, C. Nanotechnology in precision agriculture: Advancing towards sustainable crop production. Plant Physiol. Biochem. 2023, 206, 108244. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef]
- Haliloğlu, K.; Türkoğlu, A.; Balpınar, Ö.; Nadaroğlu, H.; Alaylı, A.; Poczai, P. Effects of Zinc, Copper and Iron Oxide Nanoparticles on Induced DNA Methylation, Genomic Instability and LTR Retrotransposon Polymorphism in Wheat (Triticum aestivum L.). Plants 2022, 11, 2193. [Google Scholar] [CrossRef]
- Koçak, R.; Okcu, M.; Haliloğlu, K.; Türkoğlu, A.; Pour-Aboughadareh, A.; Jamshidi, B.; Janda, T.; Alaylı, A.; Nadaroğlu, H. Magnesium Oxide Nanoparticles: An Influential Element in Cowpea (Vigna unguiculata L. Walp) Tissue Culture. Agronomy 2023, 13, 1646. [Google Scholar] [CrossRef]
- Nalci, O.B.; Nadaroglu, H.; Pour, A.H.; Gungor, A.A.; Haliloglu, K. Effects of ZnO, CuO and γ-Fe3O4 nanoparticles on mature embryo culture of wheat (Triticum aestivum L.). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 269–277. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Aydin, M.; Çiçek, S.; Yiğider, E.; Demirel, S.; Piekutowska, M.; Szulc, P.; Niedbała, G. Machine Learning Analysis of the Impact of Silver Nitrate and Silver Nanoparticles on Wheat (Triticum aestivum L.): Callus Induction, Plant Regeneration, and DNA Methylation. Plants 2023, 12, 4151. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Metals in bone: Aluminum, boron, cadmium, chromium, lanthanum, lead, silicon, and strontium. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 515–531. [Google Scholar]
- Brooks, R.R. Geobotany and Biogeochemistry in Mineral Exploration; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Gupta, D.K.; Walther, C. Behaviour of Strontium in Plants and the Environment; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gungor, A.A.; Nadaroglu, H.; Gultekin, D.D. Synthesis and characterization of nano-strontium oxide (SrO) Using erzincan cimin grape (Vitis vinifera, Cimin). Chem. Sci. Int. J. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Alayli, A.; Ceker, S.; Ogutcu, H.; Agar, G. Biosynthesis of Silver nanoparticles and investigation of genotoxic effects and antimicrobial activity. Int. J. Nano Dimens. 2020, 11, 158–167. [Google Scholar]
- Nadaroglu, H.; Gungor, A.A.; Ince, S.; Babagil, A. Green synthesis and characterisation of platinum nanoparticles using quail egg yolk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 43–47. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Nezami, A.; Khazaei, H.R.; Boroumand, R.Z.; Hosseini, A. Effects of drought stress and defoliation on sunflower (Helianthus annuus) in controlled conditions. Desert 2008, 12, 99–104. [Google Scholar]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Tiryaki, D.; Aydın, İ.; Atıcı, Ö. Psychrotolerant bacteria isolated from the leaf apoplast of cold-adapted wild plants improve the cold resistance of bean (Phaseolus vulgaris L.) under low temperature. Cryobiology 2019, 86, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Apsana, G.; George, P.; Devanna, N.; Yuvasravana, R. Biomimetic synthesis and antibacterial properties of strontium oxide nanoparticles using Ocimum sanctum leaf extract. Asian J. Pharm. Clin. Res. 2018, 11, 384–389. [Google Scholar]
- Apsana, G.; George, P.; Devanna, N. Eco-Friendly Approach for the Efficient Biosynthesis of Mg3 (PO4) 2 Nanoparticles Using Plant Extract of Ocimum sanctum. Adv. Sci. Lett. 2018, 24, 5514–5518. [Google Scholar] [CrossRef]
- Alavi, M.A.; Morsali, A. Syntheses and characterization of Sr(OH)2 and SrCO3 nanostructures by ultrasonic method. Ultrason. Sonochem. 2010, 17, 132–138. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.-R.; Lee, K.J.; Stott, N.E.; Kim, D. Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology 2008, 19, 415604. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Latta, D.E.; McLean, J.E.; Britt, D.W.; Boyanov, M.I.; Anderson, A.J. Fate of CuO and ZnO nano-and microparticles in the plant environment. Environ. Sci. Technol. 2013, 47, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Shen, Y.; Ma, C.; Zhang, X.; Cao, W.; Rui, Y. Effects of TiO2 nanoparticles on wheat (Triticum aestivum L.) seedlings cultivated under super-elevated and normal CO2 conditions. PLoS ONE 2017, 12, e0178088. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Rafique, R.; Arshad, M.; Khokhar, M.; Qazi, I.; Hamza, A.; Virk, N. Growth response of wheat to titania nanoparticles application. NUST J. Eng. Sci. 2014, 7, 42–46. [Google Scholar]
- McManus, P.; Hortin, J.; Anderson, A.J.; Jacobson, A.R.; Britt, D.W.; Stewart, J.; McLean, J.E. Rhizosphere interactions between copper oxide nanoparticles and wheat root exudates in a sand matrix: Influences on copper bioavailability and uptake. Environ. Toxicol. Chem. 2018, 37, 2619–2632. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Ebadi, M.; Baharara, J. Effects of titanium dioxide nanoparticles (TiO2) on germination and seedling growth of Vitex plants (Vitex agnus-castus L.). J. BioSci. Biotech. 2019, 8, 141–149. [Google Scholar]
- Zengin, F.K.; Munzuroglu, O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol. Cracoviensia Ser. Bot. 2005, 47, 157–164. [Google Scholar]
- Demir, N. Determination of Some Bioactivities and Chemical Composition of Tulip (Tulipa armena) Plant and Investigation of Usability as Homeopathic Drugs. Int. J. Innov. Res. Rev. 2018, 2, 21–23. [Google Scholar]
- Ali, S.; Rizwan, M.; Hussain, A.; ur Rehman, M.Z.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Yuce, M.; Taspinar, M.S.; Aydin, M.; Agar, G. Response of NAC transcription factor genes against chromium stress in sunflower (Helianthus annuus L.). Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 479–487. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Chen, F.; Luo, X.; Musante, C.; White, J.C.; Zhao, X.; Wang, Z.; Xing, B. New insight into the mechanism of graphene oxide-enhanced phytotoxicity of arsenic species. J. Hazard. Mater. 2021, 410, 124959. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef]
- Tozlu, E.; Mohammadi, P.; Kotan, M.S.; Nadaroglu, H.; Kotan, R. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary, the causal agent of white mould disease in red cabbage, by some bacteria. Plant Prot. Sci. 2016, 52, 188–198. [Google Scholar] [CrossRef]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Park, T.J.; Kallerhoff, J.; Arshad, M. Dose-dependent physiological responses of Triticum aestivum L. to soil applied TiO2 nanoparticles: Alterations in chlorophyll content, H2O2 production, and genotoxicity. Agric. Ecosyst. Environ. 2018, 255, 95–101. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Selim, S.; AbdElgawad, H. NiO-nanoparticles induce reduced phytotoxic hazards in wheat (Triticum aestivum L.) grown under future climate CO2. Chemosphere 2019, 220, 1047–1057. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Wang, C.; Yin, S.-S.; Li, X.-L.; Hu, W.-J.; Simon, M.; Shen, Z.-J.; Xiao, Q.; Chu, C.-C. Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. J. Hazard. Mater. 2015, 297, 173–182. [Google Scholar]
- Da Costa, M.; Sharma, P. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- García-Gómez, C.; García, S.; Obrador, A.F.; González, D.; Babín, M.; Fernández, M.D. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotoxicol. Environ. Saf. 2018, 160, 222–230. [Google Scholar] [CrossRef]
- Büyük, İ.; Soydam Aydin, S.; Aras, E. Molecular responses of plants to stress conditions. Turk. Bull. Hyg. Exp. Biol. 2012, 69, 97–110. [Google Scholar]
- Büyük, M.; İnci, M.; Tan, A.; Tümay, M. Improved instantaneous power theory based current harmonic extraction for unbalanced electrical grid conditions. Electr. Power Syst. Res. 2019, 177, 106014. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: A life cycle field study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef]
- Jhanzab, H.M.; Razzaq, A.; Bibi, Y.; Yasmeen, F.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. Proteomic analysis of the effect of inorganic and organic chemicals on silver nanoparticles in wheat. Int. J. Mol. Sci. 2019, 20, 825. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaysım, M.G.; Kumlay, A.M.; Haliloglu, K.; Türkoğlu, A.; Piekutowska, M.; Nadaroğlu, H.; Alayli, A.; Niedbała, G. Physiological and Antioxidative Effects of Strontium Oxide Nanoparticles on Wheat. Agronomy 2024, 14, 770. https://doi.org/10.3390/agronomy14040770
Kaysım MG, Kumlay AM, Haliloglu K, Türkoğlu A, Piekutowska M, Nadaroğlu H, Alayli A, Niedbała G. Physiological and Antioxidative Effects of Strontium Oxide Nanoparticles on Wheat. Agronomy. 2024; 14(4):770. https://doi.org/10.3390/agronomy14040770
Chicago/Turabian StyleKaysım, Mustafa Güven, Ahmet Metin Kumlay, Kamil Haliloglu, Aras Türkoğlu, Magdalena Piekutowska, Hayrunnisa Nadaroğlu, Azize Alayli, and Gniewko Niedbała. 2024. "Physiological and Antioxidative Effects of Strontium Oxide Nanoparticles on Wheat" Agronomy 14, no. 4: 770. https://doi.org/10.3390/agronomy14040770
APA StyleKaysım, M. G., Kumlay, A. M., Haliloglu, K., Türkoğlu, A., Piekutowska, M., Nadaroğlu, H., Alayli, A., & Niedbała, G. (2024). Physiological and Antioxidative Effects of Strontium Oxide Nanoparticles on Wheat. Agronomy, 14(4), 770. https://doi.org/10.3390/agronomy14040770







