Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experiment 1—Starvation vs. Moderate Nutrient Availability in Semi-Hydroponic System
2.3. Experiment 2—High vs. Moderate Nutrient Availability in a Semi-Hydroponic System
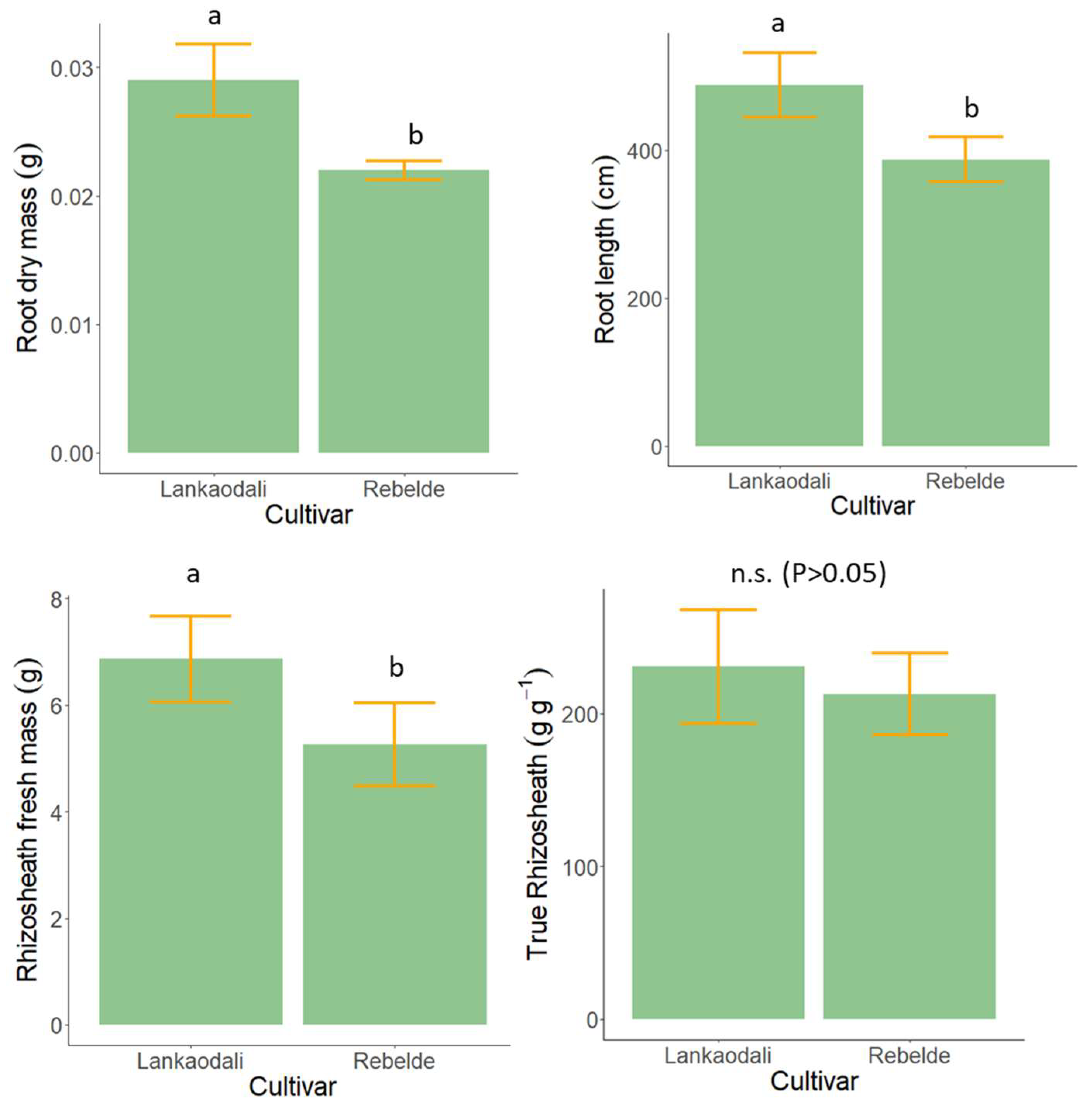
2.4. Experiment 3—Rhizosheath Formation and Root Architecture in Response to Drought Stress
2.4.1. Water Supply Treatments
2.4.2. Seedling Growth Conditions
2.4.3. Measurements
- RhizFM = RRhizFM – RFM g plant−1;
- RhizDM = RRhizDM − RDM g plant−1;
- True rhizosheath: RhizDM/RDM g g−1.
2.5. Statistical Analysis
3. Results
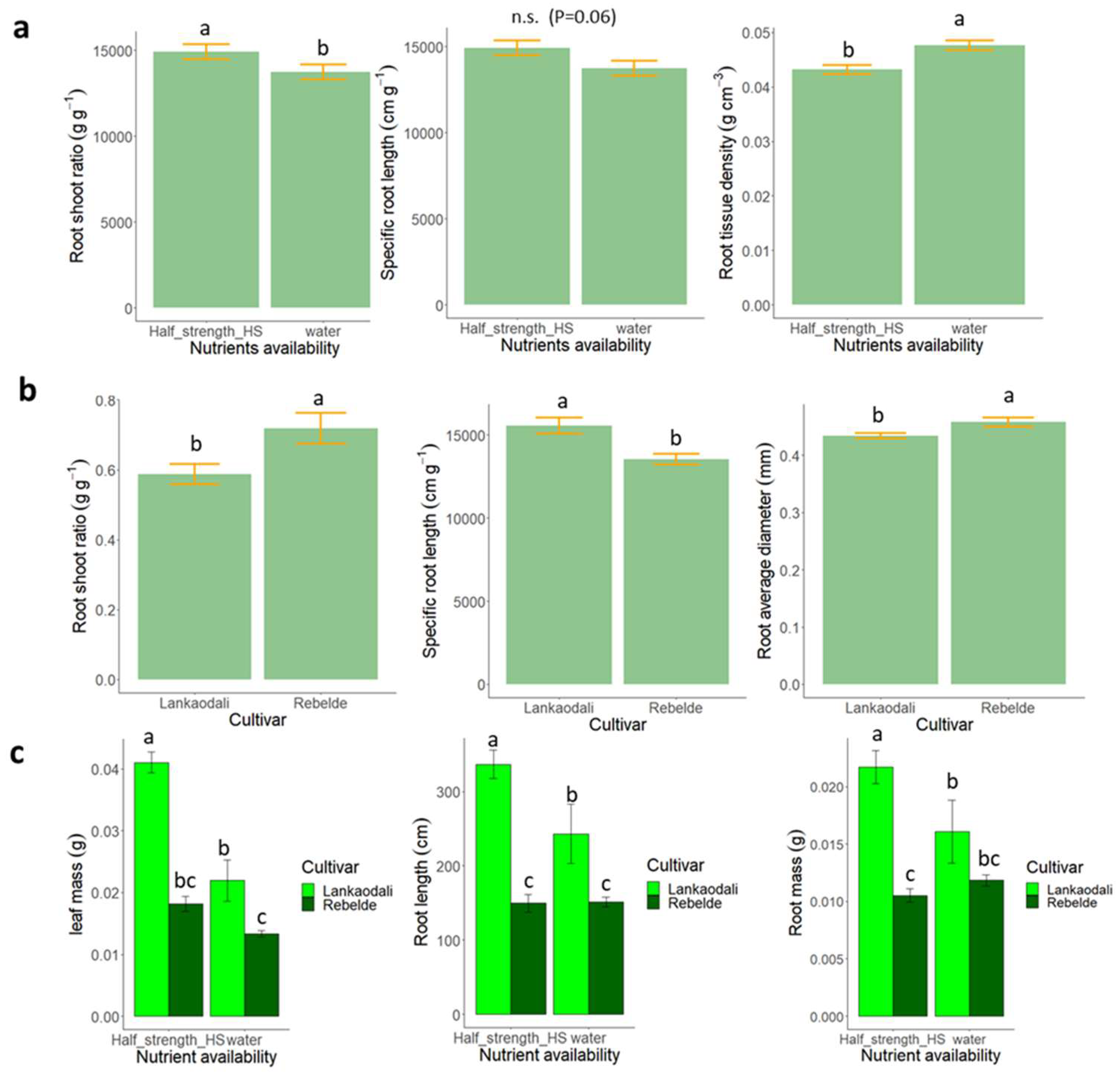
3.1. Experiment 1—Starvation vs. Moderate Nutrient Availability in Semi-Hydroponic System
3.2. Experiment 2—High vs. Moderate Nutrient Availability in Semi-Hydroponic System
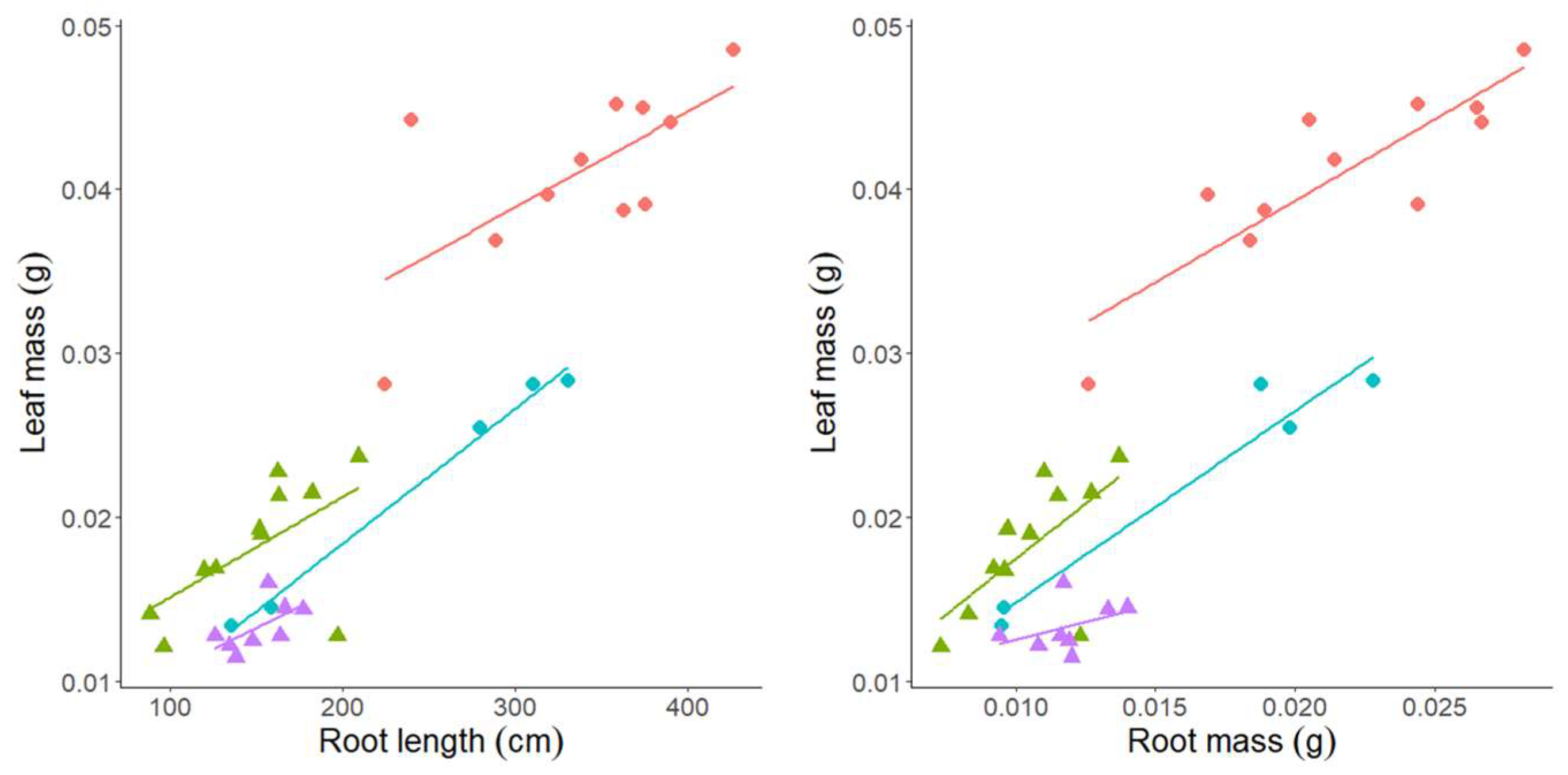
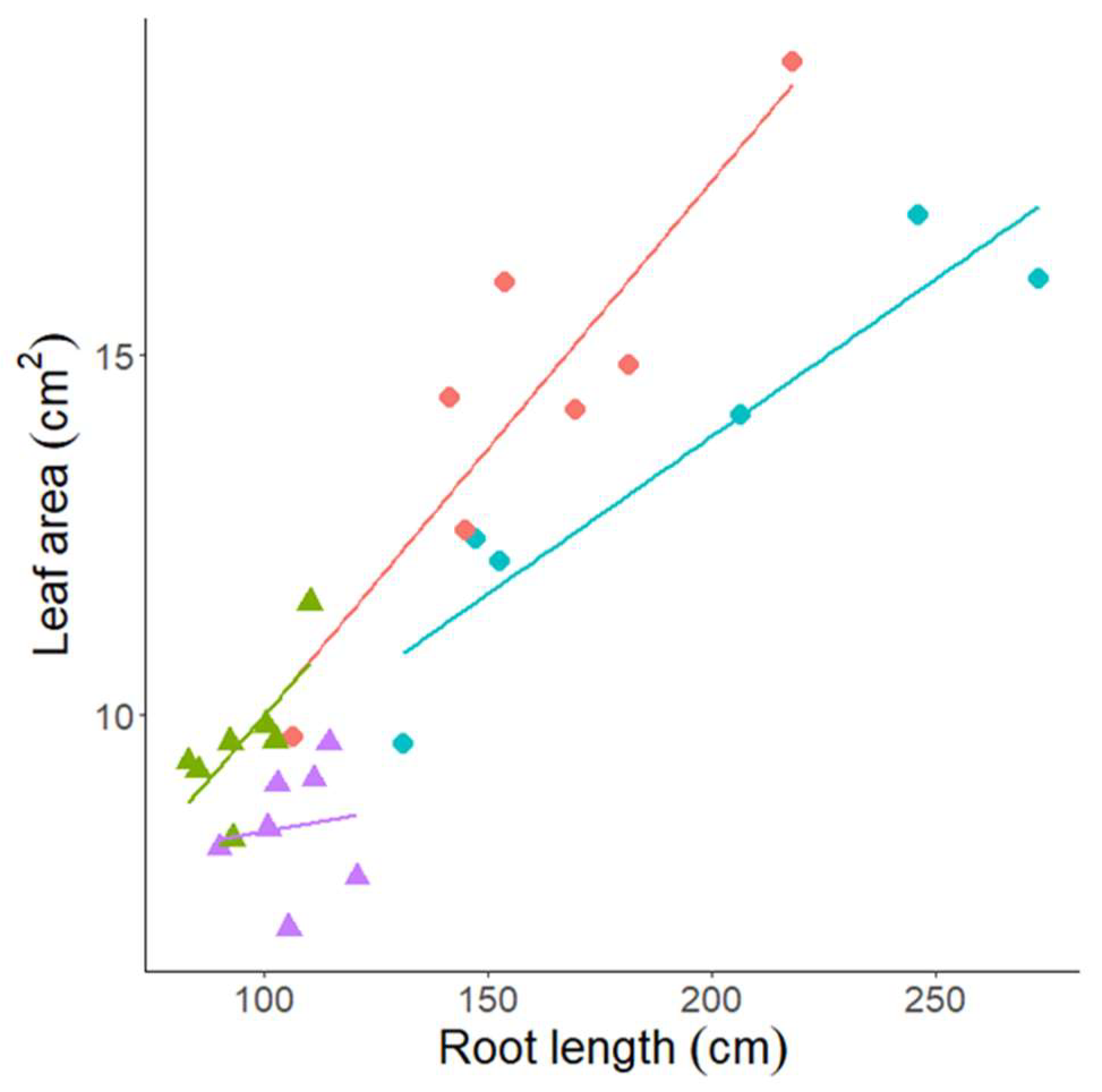
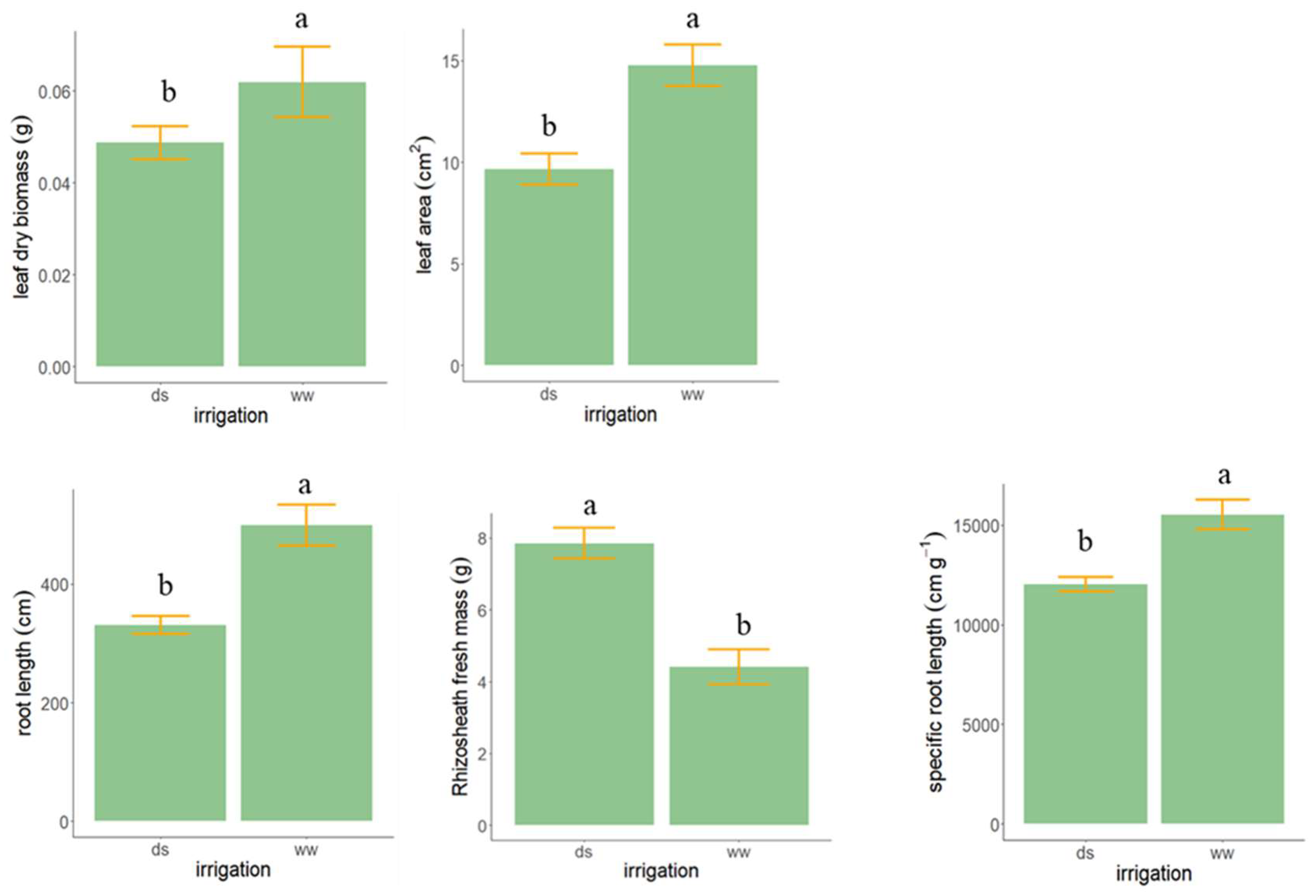
3.3. Experiment 3—Rhizosheath Formation and Root Architecture in Response to Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bentley, A.R. Breeding Wheat for the Future: Opportunities and Challenges. In Sustainable Agricultural Innovations for Resilient Agri-Food Systems; The Indian Ecological Society: Punjab, India, 2022; p. 7. [Google Scholar]
- Dadrasi, A.; Chaichi, M.; Nehbandani, A.; Soltani, E.; Nemati, A.; Salmani, F.; Heydari, M.; Yousefi, A.R. Global insight into understanding wheat yield and production through Agro-Ecological Zoning. Sci. Rep. 2023, 13, 15898. [Google Scholar] [CrossRef] [PubMed]
- Pequeno, D.N.; Hernandez-Ochoa, I.M.; Reynolds, M.; Sonder, K.; MoleroMilan, A.; Robertson, R.D.; Lopes, M.S.; Xiong, W.; Kropff, M.; Asseng, S. Climate impact and adaptation to heat and drought stress of regional and global wheat production. Environ. Res. Lett. 2021, 16, 054070. [Google Scholar] [CrossRef]
- Wasson, A.P.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Richards, R.A.; Watt, M. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J. Exp. Bot. 2014, 65, 6231–6249. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; Devoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Howe, G.N.; Graham, J.M. Impact of subsoil water use on wheat yield. Aust. J. Agric. Res. 2007, 58, 303–315. [Google Scholar] [CrossRef]
- Motzo, R.; Attene, G.; Deidda, M. Genotypic variation in durum wheat root systems at different stages of development in a Mediterranean environment. Euphytica 1992, 66, 197–206. [Google Scholar] [CrossRef]
- Středa, T.; Dostál, V.; Horáková, V.; Chloupek, O. Effective use of water by wheat varieties with different root system sizes in rain-fed experiments in Central Europe. Agric. Water Manag. 2012, 104, 203–209. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Stangoulis, J. Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiol. Plant. 2019, 41, 109. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2002, 21, 335–351. [Google Scholar] [CrossRef]
- Ouyang, W.; Yin, X.; Yang, J.; Struik, P.C. Comparisons with wheat reveal root anatomical and histochemical constraints of rice under water-deficit stress. Plant Soil 2020, 452, 547–568. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Dodd, I.C.; Waseem, M.; Weifeng, X. Rhizosheath: An adaptive root trait to improve plant tolerance to phosphorus and water deficits? Plant Cell Environ. 2022, 45, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.E. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annu. Rev. Plant Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Brown, L.K.; Ramsay, L.; White, P.J.; Newton, A.C.; Bengough, A.G.; Russell, J.; Thomas, W.T.B. Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytol. 2014, 203, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ndour, P.M.S.; Heulin, T.; Achouak, W.; Laplaze, L.; Cournac, L. The rhizosheath: From desert plants adaptation to crop breeding. Plant Soil 2020, 456, 1–13. [Google Scholar] [CrossRef]
- Volkens, G. Die Flora Der Aegyptisch-Arabischen Wtiste Auf Grundlage Anatomisch-Physiologischer Forschungen: Dargestelit Von Dr. Georg Volkens… Gerbr" Uder Boratraeger; Eggers, Ed.; Kessinger Publishing: Whitefish, MT, USA, 1887. [Google Scholar]
- Brown, L.K.; George, T.S.; Neugebauer, K.; White, P.J. The rhizosheath–A potential trait for future agricultural sustainability occurs in orders throughout the angiosperms. Plant Soil 2017, 418, 115–128. [Google Scholar] [CrossRef]
- Young, I.M. Variation in moisture contents between bulk soil and the rhizosheath of wheat (Triticum aestivum L. cv. Wembley). New Phytol. 1995, 130, 135–139. [Google Scholar] [CrossRef]
- Cheraghi, M.; Mousavi, S.M.; Zarebanadkouki, M. Functions of rhizosheath on facilitating the uptake of water and nutrients under drought stress: A review. Plant Soil 2023, 1–25. [Google Scholar]
- Basirat, M.; Mousavi, S.M.; Abbaszadeh, S.; Ebrahimi, M.; Zarebanadkouki, M. The rhizosheath: A potential root trait helping plants to tolerate drought stress. Plant Soil 2019, 445, 565–575. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Siddique, K.H.; Simpson, R.J. Unwrapping the rhizosheath. Plant Soil 2017, 418, 129–139. [Google Scholar] [CrossRef]
- James, R.A.; Weligama, C.; Verbyla, K.; Ryan, P.R.; Rebetzke, G.J.; Rattey, A.; Richardson, A.E.; Delhaize, E. Rhizosheaths on wheat grown in acid soils: Phosphorus acquisition efficiency and genetic control. J. Exp. Bot. 2016, 67, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.; Fusi, M.; Ramond, J.B.; Van Goethem, M.W.; Seferji, K.; Maggs-Kölling, G.; Cowan, D.A.; Daffonchio, D. The plant rhizosheath–root niche is an edaphic “mini-oasis” in hyperarid deserts with enhanced microbial competition. ISME Commun. 2022, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Gioia, T.; Galinski, A.; Lenz, H.; Müller, C.; Lentz, J.; Heinz, K.; Briese, C.; Putz, A.; Fiorani, F.; Watt, M.; et al. GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Funct. Plant Biol. 2016, 44, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Vitale, P.; Fania, F.; Esposito, S.; Pecorella, I.; Pecchioni, N.; Palombieri, S.; Sestili, F.; Lafiandra, D.; Taranto, F.; De Vita, P. QTL Analysis of five morpho-physiological traits in bread wheat using two mapping populations derived from common parents. Genes 2021, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; D’Agostino, N.; Taranto, F.; Sonnante, G.; Sestili, F.; Lafiandra, D.; De Vita, P. Whole-exome sequencing of selected bread wheat recombinant inbred lines as a useful resource for allele mining and bulked segregant analysis. Front. Genet. 2022, 13, 1058471. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.A.; Rebetzke, G.J.; Richards, R.A. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2013, 112, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. Decimal code for growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- van Rossum, B.J. Genotype by Environment Analysis Using statgenGxE. Available online: https://biometris.github.io/statgenGxE/index.html (accessed on 1 December 2023).
- Schmidt, P.; Hartung, J.; Bennewitz, J.; Piepho, H.P. Heritability in plant breeding on a genotype-difference basis. Genetics 2019, 212, 991–1008. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Lian, L.; de Los Campos, G. FW: An R package for Finlay–Wilkinson regression that incorporates genomic/pedigree information and covariance structures between environments. G3 Genes Genomes Genet. 2016, 6, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A.; Lukacs, Z. Seedling vigour in wheat-sources of variation for genetic and agronomic improvement. Aust. J. Agric. Res. 2002, 53, 41–50. [Google Scholar] [CrossRef]
- Zhao, Z.; Rebetzke, G.J.; Zheng, B.; Chapman, S.C.; Wang, E. Modelling impact of early vigour on wheat yield in dryland regions. J. Exp. Bot. 2019, 70, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Richards, R.A.; Condon, A.G.; Liu, D.C.; Rebetzke, G.J. Recurrent selection for wider seedling leaves increases early biomass and leaf area in wheat (Triticum aestivum L.). J. Exp. Bot. 2015, 66, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Mullan, D.J.; Reynolds, M.P. Quantifying genetic effects of ground cover on soil water evaporation using digital imaging. Funct. Plant Biol. 2010, 37, 703–712. [Google Scholar] [CrossRef]
- Liao, M.; Palta, J.A.; Fillery, I.R. Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Watt, M.; Kirkegaard, J.A.; Rebetzke, G.J. A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Funct. Plant Biol. 2005, 32, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Rebetzke, G.J.; Zhang, H.; Ingvordsen, C.H.; Condon, A.G.; Rich, S.M.; Ellis, M.H. Genotypic variation and covariation in wheat seedling seminal root architecture and grain yield under field conditions. Theor. Appl. Genet. 2022, 135, 3247–3264. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Richards, R.A. Genetic improvement of early vigour in wheat. Aust. J. Agric. Res. 1999, 50, 291–302. [Google Scholar] [CrossRef]
- Su, Z.; Hao, C.; Wang, L.; Dong, Y.; Zhang, X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 122, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bai, Z.; Li, X.; Wang, P.; Wu, Q.; Yang, L.; Li, L.; Li, X. SNP identification and allelic-specific PCR markers development for TaGW2, a gene linked to wheat kernel weight. Theor. Appl. Genet. 2012, 125, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- López-Castañeda, C.; Richards, R.A.; Farquhar, G.D.; Williamson, R.E. Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Sci. 1996, 36, 1257–1266. [Google Scholar] [CrossRef]
- Abdel-Ghani, A.H.; Aref Al-Dalain, S.; Tarawneh, G.H.; Al-Taae, H.H.; Al-Tarawneh, B.H.; Owais, S.J.; Al-Ebissat, A.S.; Al-Mayta, R.; AL-Dala’een, J.A.; Duwayri, M.A. Kernel quality, germination rate and seedling performance of eight wheat varieties produced under three different irrigation regimes. Aust. J. Crop Sci. 2022, 16, 1135–1143. [Google Scholar] [CrossRef]
- Sanguineti, M.C.; Li, S.; Maccaferri, M.; Corneti, S.; Rotondo, F.; Chiari, T.; Tuberosa, R. Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann. Appl. Biol. 2007, 151, 291–305. [Google Scholar] [CrossRef]
- Kimurto, P.K.; Kinyua, M.G.; Birechl, R.; Korir, P.C.; Njoka, E.M.; Njau, P.N. Root and shoot characteristics as selection criteria for drought tolerance in bread wheat (Triticum aestivum L.) at seedling stage under tropical environment. Discov. Innov. 2005, 17, 74–84. [Google Scholar] [CrossRef]
- Hyles, J.; Bloomfield, M.T.; Hunt, J.R.; Trethowan, R.M.; Trevaskis, B. Phenology and related traits for wheat adaptation. Heredity 2020, 125, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Palta, J.A.; Rebetzke, G.J.; Milroy, S.P. Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth. Funct. Plant Biol. 2013, 41, 215–222. [Google Scholar]
- Su, J.Y.; Zheng, Q.; Li, H.W.; Li, B.; Jing, R.L.; Tong, Y.P.; Li, Z.S. Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Sci. 2009, 176, 824–836. [Google Scholar] [CrossRef]
- Kang, J.G.; van Iersel, M.W. Nutrient solution concentration affects shoot: Root ratio, leaf area ratio, and growth of subirrigated salvia (Salvia splendens). HortScience 2004, 39, 49–54. [Google Scholar] [CrossRef]
- Termaat, A.; Munns, R. Use of concentrated macronutrient solutions to separate osmotic from NaCl-specific effects on plant growth. Funct. Plant Biol. 1986, 13, 509–522. [Google Scholar] [CrossRef]
- Tucker, S.S.; Craine, J.M.; Nippert, J.B. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2011, 2, art48. [Google Scholar] [CrossRef]
- Delhaize, E.; Rathjen, T.M.; Cavanagh, C.R. The genetics of rhizosheath size in a multiparent mapping population of wheat. J. Exp. Bot. 2015, 66, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, S.M.; Warren, C.R.; Macdonald, C.; Trethowan, R.M.; Young, I.M. Soil-root interaction in the rhizosheath regulates the water uptake of wheat. Rhizosphere 2022, 21, 100462. [Google Scholar] [CrossRef]
- Bochicchio, R.; Labella, R.; Vitti, A.; Nuzzaci, M.; Logozzo, G.; Amato, M. Root morphology, allometric relations and rhizosheath of ancient and modern tetraploid wheats (Triticum durum Desf.) in response to inoculation with Trichoderma harzianum T-22. Plants 2022, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Bochicchio, R.; Labella, R.; Rossi, R.; Perniola, M.; Amato, M. Effects of Soil Water Shortage on Seedling Shoot and Root Growth of Saragolle Lucana Tetraploid Wheat (Triticum durum Desf.) Landrace. Plants 2022, 11, 3492. [Google Scholar] [CrossRef] [PubMed]
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Ackah, F.K.; Amoah, K.K.; Nyarko, M.A.; Andoh, D.A. Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Yang, F.; Dai, X.; Meng, S.; Hu, M.; Kou, L.; Fu, X. Relationships between root exudation and root morphological and architectural traits vary with growing season. Tree Physiol. 2023, 44, tpad118. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, J.P.; Guillemet, M.; Dubost, A.; Simon, L.; Ortet, P.; Barakat, M.; Heulin, T.; Achouak, W.; Haichar, F.E.Z. Plant nutrient resource use strategies shape active rhizosphere microbiota through root exudation. Front. Plant Sci. 2018, 9, 1662. [Google Scholar] [CrossRef]
- Guyonnet, J.P.; Cantarel, A.A.; Simon, L.; Haichar, F.E.Z. Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol. Evol. 2018, 8, 8573–8581. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Zhang, M.; Wang, J. Effect of Cynodon dactylon community on the conservation and reinforcement of riparian shallow soil in the Three Gorges Reservoir area. Ecol. Process. 2015, 4, 3. [Google Scholar] [CrossRef]
- Wang, G.Y.; Huang, Y.G.; Li, R.F.; Chang, J.M.; Fu, J.L. Influence of vetiver root on strength of expansive soil-experimental study. PLoS ONE 2020, 15, e0244818. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.E.D.O.; Ferreira Filho, A.W.P. Genetic control of wheat seedling root growth. Sci. Agric. 2005, 62, 325–330. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mwadzingeni, L.; Zengeni, R.; Mutema, M.; Chaplot, V. Variance components and heritability of traits related to root: Shoot biomass allocation and drought tolerance in wheat. Euphytica 2018, 214, 225. [Google Scholar] [CrossRef]
- Cane, M.A.; Maccaferri, M.; Nazemi, G.; Salvi, S.; Francia, R.; Colalongo, C.; Tuberosa, R. Association mapping for root architectural traits in durum wheat seedlings as related to agronomic performance. Mol. Breed. 2014, 34, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Fernando, K.M.; Mayes, S.; Sparkes, D.L. Identifying seedling root architectural traits associated with yield and yield components in wheat. Ann. Bot. 2017, 119, 1115–1129. [Google Scholar] [CrossRef]
- Bai, C.; Ge, Y.; Ashton, R.W.; Evans, J.; Milne, A.; Hawkesford, M.J.; Whalley, W.R.; Parry, M.A.J.; Melichar, J.; Feuerhelm, D.; et al. The relationships between seedling root screens, root growth in the field and grain yield for wheat. Plant Soil 2019, 440, 311–326. [Google Scholar] [CrossRef]

| 0 vs. 50% (v/v) Hoagland Solution | Fully Fixed Model | Fully Random Model | Finlay–Wilkinson Analysis | Heritability | ||
|---|---|---|---|---|---|---|
| Leaf Mass (g) | p-Value | % Variance Explained | Rank | GenMean | Sensitivity (p-Value) | Broad-Sense Heritability |
| trial | 8.391 × 10−9 | 21.18 | Lankaodali | 0.034 | 0.0002 | 0.81 |
| genotype | 3.699 × 10−12 | 46.4 | Rebelde | 0.016 | ||
| genotype:trial | 0.0002105 | 22.01 | ||||
| residuals | 10.4 | |||||
| RL (cm) | p-value | % Variance explained | Rank | GenMean | Sensitivity (p-value) | Broad-sense heritability |
| trial | 0.005188 | 0 | Lankaodali | 301.775 | 0.0188 | 0.90 |
| genotype | 2.16 × 10−9 | 66.23 | Rebelde | 150.385 | ||
| genotype:trial | 0.018813 | 12.77 | ||||
| residuals | 21.01 | |||||
| RM (g) | p-value | % Variance explained | Rank | Mean | Sensitivity (p-value) | Broad-sense heritability |
| trial | 0.05205 | 0 | Lankaodali | 0.020 | 0.0142 | 0.80 |
| genotype | 1.25 × 10−7 | 57.88 | Rebelde | 0.011 | ||
| genotype:trial | 0.01416 | 13.14 | ||||
| residuals | 28.98 | |||||
| SRL (cm g−1) | p-value | % Variance explained | Rank | Mean | Sensitivity (p-value) | Broad-sense heritability |
| trial | 0.048947 | 6.5 | Rebelde | 13,596.120 | 0.5563 | 0.93 |
| genotype | 0.001601 | 37.88 | Lankaodali | 15,536.780 | ||
| genotype:trial | 0.556314 | 0 | ||||
| residuals | 55.62 | |||||
| Average diameter (mm) | p-value | % Variance explained | Rank | Mean | Sensitivity (p-value) | Broad-sense heritability |
| trial | 0.61754 | 0 | Lankaodali | 0.433 | 0.5147 | 0.87 |
| genotype | 0.01528 | 23.74 | Rebelde | 0.458 | ||
| genotype:trial | 0.51472 | 0 | ||||
| residuals | 76.26 | |||||
| Root-to-shoot mass ratio (g g−1) | p-value | % Variance explained | Rank | Mean | Sensitivity (p-value) | Broad-sense heritability |
| trial | 1.078 × 10−8 | 66.28 | Rebelde | 0.704 | 0.1649 | 0.83 |
| genotype | 0.004868 | 10.48 | Lankaodali | 0.599 | ||
| genotype:trial | 0.164884 | 2.17 | ||||
| residuals | 21.07 | |||||
| Lankaodali | Leaf Mass | Root Mass | Root Length | Root Surface Area | Root Volume | Root Tissue Density | Root Average Diameter | Root–Shoot Mass Ratio | Specific Root Length |
|---|---|---|---|---|---|---|---|---|---|
| Leaf mass | 1 | 0.83 | 0.82 | 0.84 | 0.85 | −0.23 | 0.44 | −0.54 | −0.11 |
| Root mass | 1 | 0.91 | 0.95 | 0.96 | −0.03 | 0.5 | 0.01 | −0.34 | |
| Root length | 1 | 0.99 | 0.96 | −0.33 | 0.25 | −0.13 | 0.06 | ||
| Root surface area | 1 | 0.99 | −0.33 | 0.38 | −0.13 | −0.04 | |||
| Root volume | 1 | −0.31 | 0.49 | −0.12 | −0.13 | ||||
| Root tissue density | 1 | −0.06 | 0.47 | −0.71 | |||||
| Root average diameter | 1 | −0.07 | −0.64 | ||||||
| Root shoot mass ratio | 1 | −0.38 | |||||||
| Specific root length | 1 | ||||||||
| Rebelde | Leaf mass | Root mass | Root length | Root surface area | Root volume | Root tissue density | Root average diameter | Root–shoot mass ratio | Specific root length |
| Leaf mass | 1 | 0.21 | 0.45 | 0.42 | 0.31 | 0 | −0.41 | −0.74 | 0.56 |
| Root mass | 1 | 0.86 | 0.9 | 0.85 | 0.73 | −0.62 | 0.49 | 0.19 | |
| Root length | 1 | 0.97 | 0.79 | 0.57 | −0.85 | 0.19 | 0.65 | ||
| Root surface area | 1 | 0.91 | 0.48 | −0.71 | 0.25 | 0.54 | |||
| Root volume | 1 | 0.26 | −0.37 | 0.31 | 0.26 | ||||
| Root tissue density | 1 | −0.68 | 0.49 | 0.05 | |||||
| Root average diameter | 1 | −0.07 | −0.77 | ||||||
| Root shoot mass ratio | 1 | −0.34 | |||||||
| Specific root length | 1 |
| p-Values | |||||
|---|---|---|---|---|---|
| Cultivar | Nutrient Solution | Cultivarx Nutrient Solution | Sample Size | ||
| Shoot | Leaf length (cm) | n.s. | n.s. | n.s. | 27 |
| Coleptile length (cm) | <0.001 | 0.015 | n.s. | 27 | |
| Leaf mass (g) | <0.001 | n.s. | n.s. | 27 | |
| Leaf area (cm2) | <0.001 | n.s. | n.s. | 27 | |
| Root | Root mass (g) | <0.001 | n.s. | n.s. | 27 |
| Root lenght (cm) | <0.001 | n.s. | n.s. | 27 | |
| Primary root length (cm) | <0.001 | 0.002 | n.s. | 27 | |
| Lateral root length (cm) | <0.001 | n.s. | n.s. | 27 | |
| Root volume (cm3) | <0.001 | 0.032 | n.s. | 27 | |
| Average diameter (mm) | 0.049 | n.s. | n.s. | 27 | |
| Root tissue density (g cm−3) | 0.038 | n.s. | n.s. | 27 | |
| Specific root length (cm g−1) | n.s. | n.s. | n.s. | 27 | |
| Root-to-shoot ratio | Root-to-shoot mass ratio (g g−1) | <0.001 | <0.001 | 0.011 | 27 |
| Root surface area to leaf area (cm2 cm−2) | 0.004 | <0.001 | n.s. | 27 |
| Lankaodali | Rebelde | |||
|---|---|---|---|---|
| Mean | St. Dev. | Mean | St. Dev. | |
| Coleoptile length (cm) | 5.469 | 0.325 | 4.336 | 0.237 |
| Plant height (cm) | 21.385 | 2.347 | 20.486 | 1.542 |
| Leaf mass (g) | 0.027 | 0.005 | 0.018 | 0.002 |
| Leaf area (cm2) | 14.012 | 2.725 | 9.055 | 1.095 |
| Root length (cm) | 174.966 | 48.234 | 101.086 | 11.141 |
| Root dry mass (g) | 0.017 | 0.003 | 0.010 | 0.001 |
| Root average diameter (mm) | 0.575 | 0.028 | 0.556 | 0.018 |
| Specific root length (cm g−1) | 10,287.956 | 1252.641 | 10,219.814 | 1289.727 |
| Pimary root length (cm) | 27.615 | 1.319 | 25.243 | 1.476 |
| Number of seminal roots | 5.7 | 0.8 | 4.4 | 0.9 |
| Lateral root length (cm) | 147.351 | 47.842 | 75.844 | 10.204 |
| Root-to-shoot area ratio (cm2 cm−2) | 2.240 | 0.329 | 1.976 | 0.344 |
| Root tissue density (g cm−3) | 0.038 | 0.003 | 0.041 | 0.004 |
| Lankaodali | Leaf Mass | Leaf Area | Root Length | Root Mass | Lateral Length | Primary Root Length | Root Volume | Root Tissue Density | Root Average Diameter | Root–Shoot Mass Ratio | Root–Shoot Area Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf mass | 1 | 0.95 | 0.9 | 0.87 | 0.9 | 0.31 | 0.84 | −0.25 | −0.77 | −0.42 | 0.11 |
| Leaf area | 1 | 0.76 | 0.77 | 0.76 | 0.2 | 0.73 | −0.21 | −0.65 | −0.47 | −0.12 | |
| Root length | 1 | 0.94 | 1 | 0.31 | 0.96 | −0.42 | −0.78 | −0.08 | 0.53 | ||
| Root mass | 1 | 0.94 | 0.24 | 0.96 | −0.25 | −0.61 | 0.08 | 0.46 | |||
| Lateral length | 1 | 0.28 | 0.96 | −0.42 | −0.77 | −0.08 | 0.53 | ||||
| Primary root length | 1 | 0.29 | −0.21 | −0.31 | −0.22 | 0.15 | |||||
| Root volume | 1 | −0.51 | −0.58 | 0.07 | 0.58 | ||||||
| Root tissue density | 1 | 0.13 | 0.01 | −0.5 | |||||||
| Root average diameter | 1 | 0.46 | −0.19 | ||||||||
| Root–shoot mass ratio | 1 | 0.61 | |||||||||
| Root–shoot area ratio | 1 | ||||||||||
| Rebelde | Leaf mass | Leaf area | Root length | Root mass | lateral Length | Primary root length | Root volume | Root tissue density | Root average diameter | Root–shoot mass ratio | Root–shoot area ratio |
| Leaf mass | 1 | 0.54 | −0.19 | −0.04 | −0.14 | −0.49 | −0.24 | 0.26 | −0.12 | −0.66 | −0.54 |
| Leaf area | 1 | 0 | −0.2 | 0.03 | −0.19 | 0.05 | −0.34 | 0.1 | −0.52 | −0.71 | |
| Root length | 1 | 0.57 | 0.99 | 0.67 | 0.87 | −0.29 | −0.04 | 0.53 | 0.68 | ||
| Root mass | 1 | 0.55 | 0.49 | 0.7 | 0.48 | 0.37 | 0.77 | 0.55 | |||
| Lateral length | 1 | 0.59 | 0.83 | −0.27 | −0.11 | 0.48 | 0.65 | ||||
| Primary root length | 1 | 0.83 | −0.37 | 0.44 | 0.66 | 0.65 | |||||
| Root volume | 1 | −0.29 | 0.46 | 0.65 | 0.63 | ||||||
| Root tissue density | 1 | −0.1 | 0.22 | −0.03 | |||||||
| Root average diameter | 1 | 0.33 | 0.06 | ||||||||
| Root–shoot mass ratio | 1 | 0.76 | |||||||||
| Root–shoot area ratio | 1 |
| Fully Fixed Model | Fully Random Model | ||
|---|---|---|---|
| RL (cm) | p-value | % Variance explained | Broad-Sense Heritability |
| trial | 0.0005885 | 47.68 | 0.43 |
| genotype | 0.0184716 | 6.92 | |
| genotype:trial | 0.1350462 | 12.11 | |
| residuals | 33.29 | ||
| RM (g) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.18174 | 5.12 | 0.74 |
| genotype | 0.04326 | 21.33 | |
| genotype:trial | 0.34349 | 2.99 | |
| residuals | 70.56 | ||
| RSR (g g−1) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.04392 | 30.99 | 0.84 |
| genotype | 0.03172 | 31.67 | |
| genotype:trial | 0.40754 | 0 | |
| residuals | 37.34 | ||
| SRL (cm g−1) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.003978 | 51.67 | 0 |
| genotype | 0.877224 | 0 | |
| genotype:trial | 0.587084 | 0 | |
| residuals | 48.33 | ||
| RTD (g cm−3) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.003628 | 40.26 | 0 |
| genotype | 0.576157 | 0 | |
| genotype:trial | 0.048963 | 12.56 | |
| residuals | 47.18 | ||
| Fresh rhizosheath mass (g) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.0001638 | 76.21 | 0.85 |
| genotype | 0.0371157 | 11.06 | |
| genotype:trial | 0.7069003 | 0 | |
| residuals | 12.72 | ||
| Leaf area (cm2) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.000006768 | 62.43 | 0.95 |
| genotype | 0.00005954 | 30.53 | |
| genotype:trial | 0.1164 | 2.73 | |
| residuals | 4.31 | ||
| Shoot biomass (g) | p-value | % Variance explained | Broad-sense heritability |
| trial | 0.026183 | 12.84 | 0.74 |
| genotype | 0.001251 | 43.76 | |
| genotype:trial | 0.048981 | 24.46 | |
| residuals | 18.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, R.; Bochicchio, R.; Labella, R.; Amato, M.; De Vita, P. Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress. Agronomy 2024, 14, 775. https://doi.org/10.3390/agronomy14040775
Rossi R, Bochicchio R, Labella R, Amato M, De Vita P. Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress. Agronomy. 2024; 14(4):775. https://doi.org/10.3390/agronomy14040775
Chicago/Turabian StyleRossi, Roberta, Rocco Bochicchio, Rosanna Labella, Mariana Amato, and Pasquale De Vita. 2024. "Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress" Agronomy 14, no. 4: 775. https://doi.org/10.3390/agronomy14040775
APA StyleRossi, R., Bochicchio, R., Labella, R., Amato, M., & De Vita, P. (2024). Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress. Agronomy, 14(4), 775. https://doi.org/10.3390/agronomy14040775









