Abstract
The occurrence of root-knot nematode disease has seriously constrained the development of the Chinese herbal medicine industry. China is one of the largest producers of Polygonatum sibiricum and Peucedanum praeruptorum in the world, but the unidentified root-knot nematodes have become important pests of these two Chinese herbal medicines in China. Both morphological characteristics and molecular identification were used to identify the nematodes. The identification results showed that Meloidogyne incognita and M. arenaria were the causal species of root-knot nematode infection in P. sibiricum, and M. hapla was the causal species of the infection in P. praeruptorum. Through investigation, this is the first report of M. incognita and M. arenaria infecting P. sibiricum, and M. hapla infecting P. praeruptorum, in China. The two Chinese herbs are being severely damaged by various root-knot nematodes, and this damage should be taken seriously.
1. Introduction
Traditional Chinese medicine has great development potential and vitality, but the large-scale planting of various Chinese herbal plants has also led to frequent diseases, including viruses, bacteria, fungi, and nematodes [1,2,3]. Among them, root-knot nematodes (Meloidogyne spp.) are major limiting factors for the production of Chinese herbal plants in China and globally [4,5,6]. These parasites can affect various types of plants and cause losses of more than USD 157 billion worldwide every year [7,8,9]. In addition, root-knot nematodes can also co-infect plants with other soil-borne pathogens, further inhibiting plant growth and exacerbating losses [10].
Several effective strategies for controlling root-knot nematodes include crop rotation, soil solarization, and the use of nematicides [11,12]. However, accurately identifying the species of RKN is a precondition and foundation for developing effective prevention and control strategies. Traditional identification methods of root-knot nematode species mainly include female perineal pattern, morphometrics, and isozyme analysis [13,14]. However, traditional methods have shortcomings, such as highly conserved inter-species with similar morphologies and high intraspecific variability [15,16]. With the advancement of technology, molecular characterization methods are gradually being used to distinguish root-knot nematode species due to their advantages in terms of specificity and sensitivity [17]. The commonly used molecular method is PCR (polymerase chain reaction) amplifications based on ribosomal DNA (rDNA) and the intergenic spacer region (IGS) sequence [16,18]. Polygonatum sibiricum and Peucedanum praeruptorum have high medicinal values, and all the roots have a drug effect [19,20]. In order to more effectively control the occurrence of root-knot nematode diseases and avoid losses, it is necessary to understand the species of root-knot nematodes on these two Chinese medicinal herbs. In this study, we identified the pathogens that cause root-knot nematode disease in P. sibiricum and P. praeruptorum by combining morphological characteristics with molecular biology. The identification result can provide a basis for subsequent management measures.
2. Materials and Methods
2.1. Root-Knot Nematode Isolation and Morphological Characteristics
In September 2023, we randomly collected infected samples from P. sibiricum and P. praeruptorum in Mengzi City of southwest China (23°01′–23°34′ N, 103°13′–103°49′ E) and packed the sample plants and rhizosphere soil into sealed plastic bags and stored them at 4 °C in a refrigerator.
Referring to the method of Yang et al. [21], we isolated approximately 20 female adults and egg masses from each plant sample. The females from each medicinal plant are used for observation and photography, and their morphological values were measured according to the method of Xie Hui [22]. In addition, the picked egg masses from the corresponding female were incubated in a Petri dish containing distilled water at 28 °C for 2 days to obtain second-stage juveniles (J2s). According to the method of Zhang et al. [17], the J2 were fixed on glass slides and measured and photographed using an inverted microscope.
2.2. DNA Extraction and Molecular Identification
DNA was extracted from 10 individual females from each medicinal plant, as described by Adam et al. [18] and Williams et al. [23], with slight modifications. Briefly, a female was put into an Eppendorf PCR tube containing 5 µL of sterile water and pierced with a sterile bamboo stick, and 5 µL of lysis buffer (20 µL of 10 × PCR buffer, 16 µL of MgCl2, 1 µL of proteinase K, and 63 µL of sterilized ddH2O) was added to each tube. Centrifuge was performed briefly, and then the tube was placed in liquid nitrogen for 20 min; then, these tubes were incubated at 56 °C for 80 min, followed by 95 °C for 5 min, consecutively. PCR amplification was performed using the sequence characterized amplified region (SCAR)-specific primers listed in Table 1. PCR reactions were performed in a final volume of 25 µL of solution, which contained 2.5 µL of 10 × PCR buffer (Mg2+, Plus), 2 µL of dNTPs (Mixture), 1 μL of each primer, 2 μL of isolated DNA, 0.5 µL of 5 U/µL Taq enzyme, and 16 μL of distilled water. The PCR amplification program includes an initial denaturation at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 30 s, annealing temperature for 30 s, and elongation at 72 °C for 1 min, followed by a final extension at 72 °C for 10 min. The annealing temperatures were 62 °C for primers MiF/MiR, 61 °C for primers Far/Rar, and 50 °C for primers JMV1/JMV hapla. The PCR products were analyzed by 1% agarose gel electrophoresis and observed under UV illumination. In addition, the PCR products were sequenced by Tsingke (Kunming), and the sequencing results were analyzed by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?, last accessed on 28 January 2024) in GenBank.

Table 1.
Species-specific primers used for molecular identification of root-knot nematodes.
2.3. Pathogenicity Analysis
In order to verify the pathogenicity of nematode isolates on P. sibiricum and P. praeruptorum plants, the P. sibiricum and P. praeruptorum seedlings were planted in plastic pots containing 1000 cm3 sterilized soil, respectively. Subsequently, purified 1500 M. incognita and M. arenaria J2s were inoculated into the P. sibiricum seedlings, and M. hapla J2s were inoculated into the P. praeruptorum seedlings, using a sterilized micropipette, respectively. These plants were kept at a temperature of 20–25 °C in a greenhouse, and root-knot nematode disease was investigated 60 days later.
3. Results
3.1. Isolation and Identification of Root-Knot Nematode Species Infecting P. sibiricum and P. praeruptorum
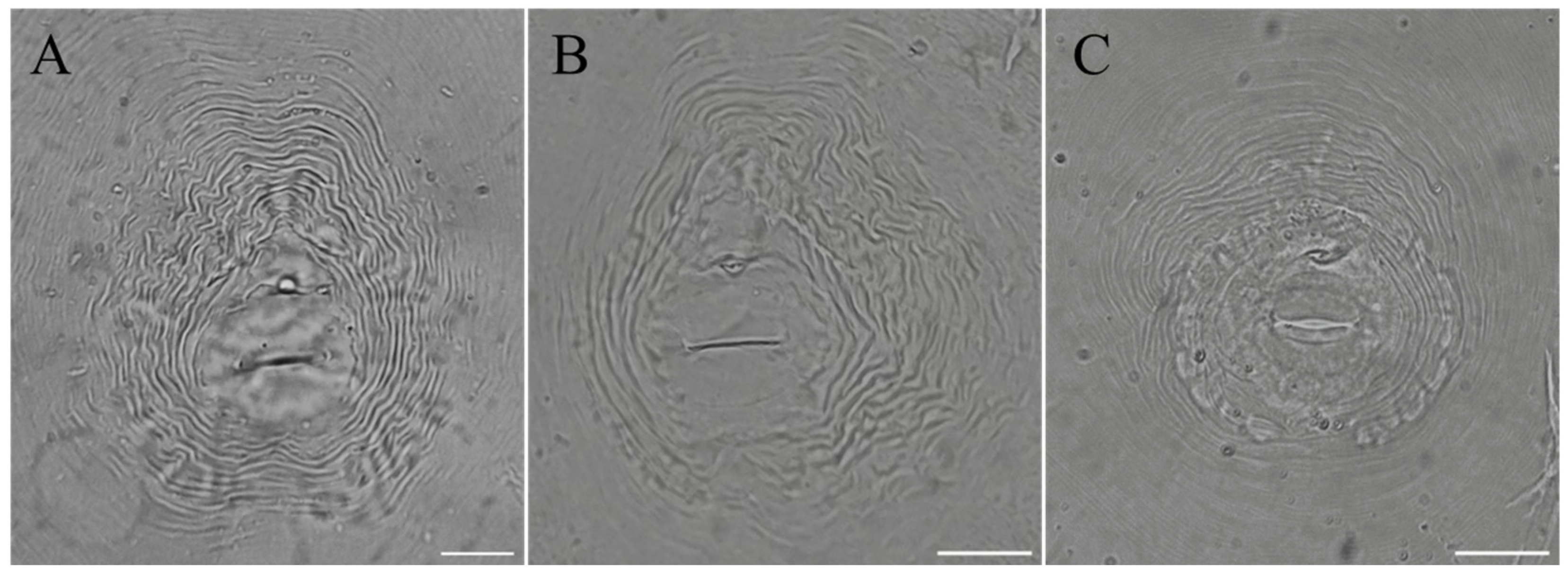
The root-knot nematode species isolated from P. sibiricum and P. praeruptorum were identified by using their morphology and PCR amplification with species-specific primers. The perineal pattern morphology of female adults is shown in Figure 1. Most of the perineum morphology of female adults isolated from Polygonatum sibiricum (Population I) showed an obvious high and squared dorsal arch, without obvious lateral lines (Figure 1A). In addition, some perineal pattern (Population II) showed a low dorsal arch, and the lateral field was marked by bifurcated and broken striae (Figure 1B). The female adults isolated from Peucedanum praeruptorum (Population III) showed round hexagons or flat ovals, with dots in the tail end area (Figure 1C).
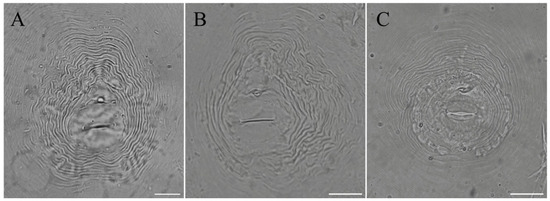
Figure 1.
The perineal patterns of females. (A,B) Perineal patterns of females extracted from infected Polygonatum sibiricum; (C) perineal pattern of females extracted from infected Peucedanum praeruptorum. The scale bars in the image are 160 pixels.
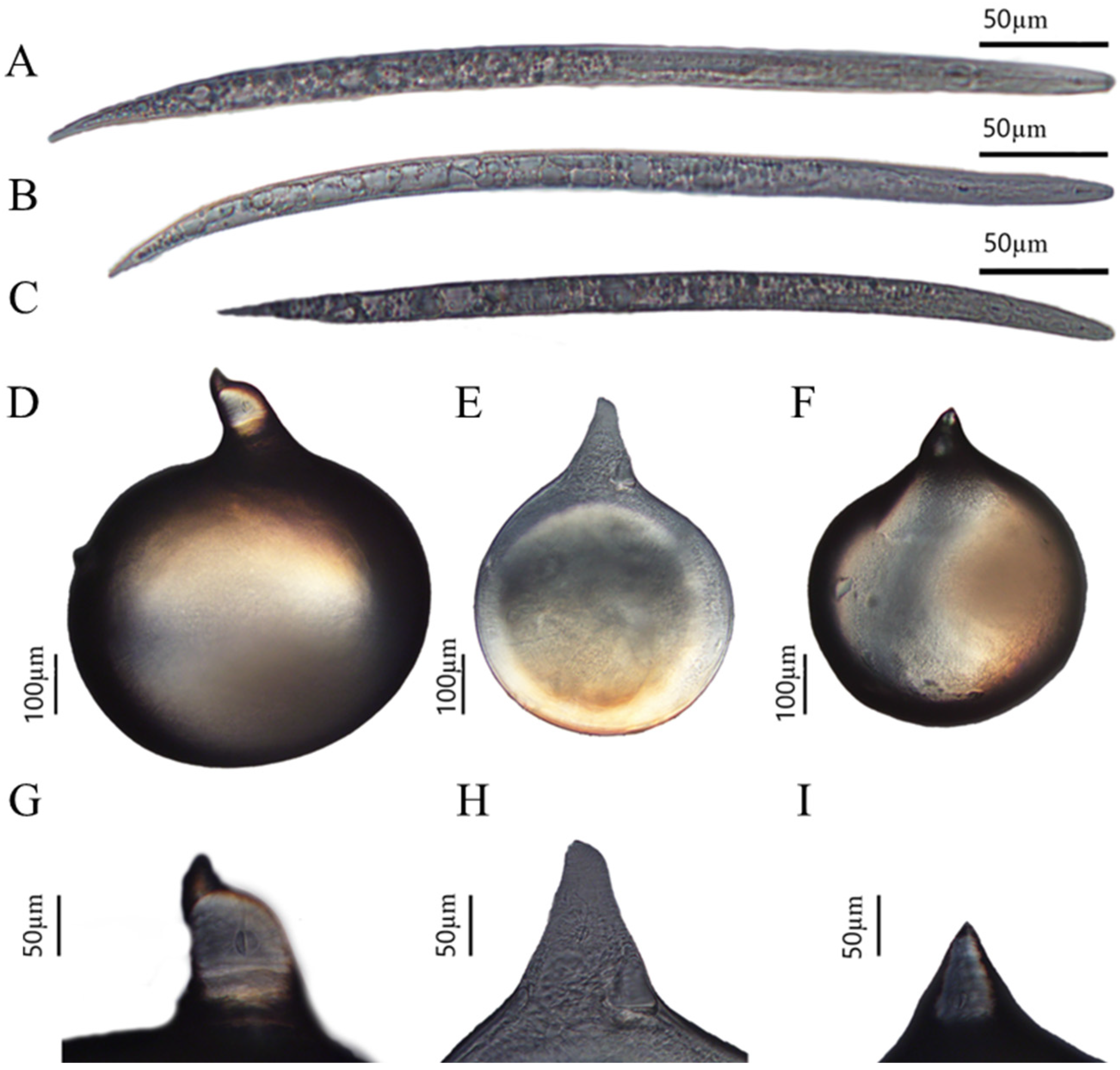
Based on the above three perineum morphologies, morphological observation and measurement were performed on females and second-stage juveniles from corresponding female egg masses. The bodies of all three female species were pear shaped or spherical, with a protruding neck of varying sizes, and no posterior protrusions (Figure 2D–F). The stylet of the females was slender and slightly curved toward the dorsally, tapering toward the tip (Figure 2G–I). The second-stage juveniles had a slender body with conical ends which was vermiform. The stylet was slender, meaning it was narrow and sharply pointed, with a straight cone (Figure 2A–C). However, there were significant differences in the measurements, such as body length and width between females and second-stage juveniles corresponding to the three types of perineum morphologies (Table 2 and Table 3). These morphological characteristics from root-knot nematode species on P. sibiricum are consistent with M. incognita and M. arenaria, and root-knot nematode species on P. praeruptorum are consistent with M. hapla, as described by Hunt and Handoo [7].
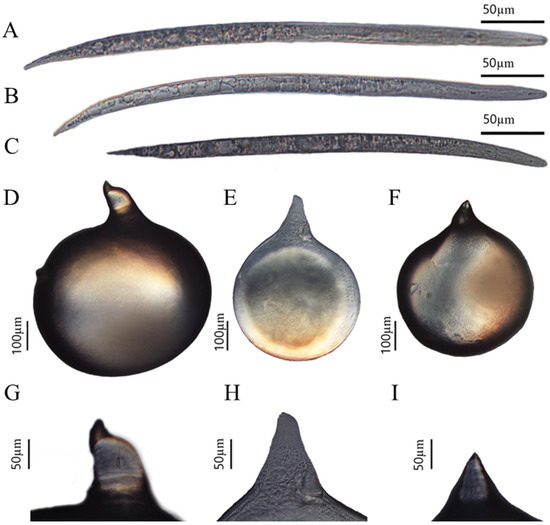
Figure 2.
Representative morphological characteristics of Meloidognye spp, including whole second-stage juvenile (A–C), whole female (D–F), and female neck (G–I). (A,D,G) Representative morphological characteristics of Population I from Polygonatum sibiricum; (B,E,H) representative morphological characteristics of Population II from Polygonatum sibiricum; (C,F,I) representative morphological characteristics of Population III from Peucedanum praeruptorum.

Table 2.
Measurements of females of Meloidognye spp. in Polygonatum sibiricum and Peucedanum praeruptorum root-knot.

Table 3.
Measurements of second-stage juveniles (J2s) from corresponding female population egg masses.
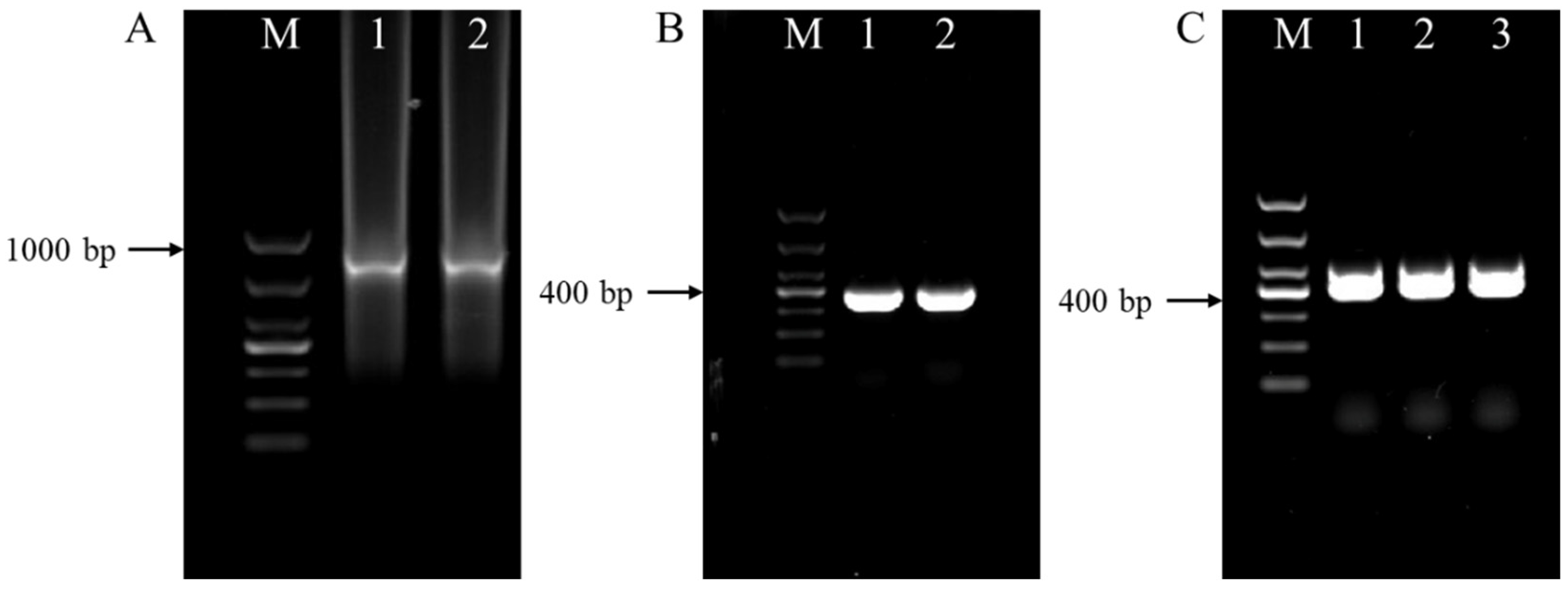
Additionally, PCR amplification was performed for all the individual female samples from P. sibiricum analyzed using species-specific primers MiF/MiR and Far/Rar, which produced 999 and 420 bp products for M. incognita and M. arenaria, respectively (Figure 3A,B). M. hapla-specific primers (JMV1/JMVhapla.) amplified the female samples of P. praeruptorum, producing a 440 bp amplification product of the same size as M. hapla (Figure 3C). These sequences of the amplified product were submitted to GenBank for a BLAST search, and the sequences (GenBank accession no. PP259100, PP236047, and PP259099) revealed the highest similarity (≥99.74%) to sequences of M. incognita, M. arenaria, and M. hapla, respectively.
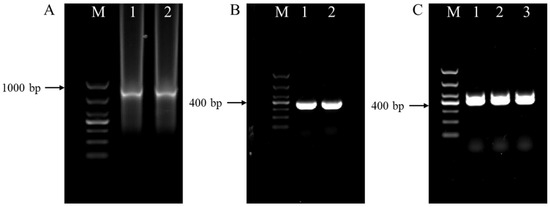
Figure 3.
PCR products obtained from genomic DNA of representative populations of root-knot nematodes isolated from infested Polygonatum sibiricum and Peucedanum praeruptorum roots. (A) Amplification product (999 bp) with the specific primer pair MiF/MiR; (B) amplification product (420 bp) using the specific primer pair Far/Rar; (C) amplification product (440 bp) using the specific primer pair JMV1/JMVhapla; M = 1 kb DNA marker.
3.2. Pathogenicity Analysis
Finally, to ensure the reliability and veracity of the conclusion, the isolated nematode populations were re-inoculated into P. sibiricum and P. praeruptorum seedlings, respectively. After 60 days, the plants were removed from the pot and the soil was gently removed from the roots, and a large number of galls were found on each root system (Figure 4).

Figure 4.
Symptoms observed on Polygonatum sibiricum and Peucedanum praeruptorum caused by Meloidognye spp. (A) Polygonatum sibiricum-inoculated M. incognita; (B) Polygonatum sibiricum-inoculated M. arenaria; (C) Peucedanum praeruptorum-inoculated M. hapla.
4. Discussion and Conclusions
Traditional Chinese medicine is considered one of the most important therapeutic techniques, and it has been widely used in China [24]. P. sibiricum and P. praeruptorum are both used in traditional Chinese medicine, so they are widely cultivated [19,20]. Large-scale planting results in decreasing yields and a corresponding increase in soil-borne diseases [25]. As an important soil-borne disease, infection with root-knot nematodes can destroy the entire root system and hinder the absorption of minerals and water by plant roots, affecting the yield and quality of Chinese medicinal herbs [26]. The accurate identification of RKN species is crucial for developing effective control strategies [10]. In this study, the results of morphological characteristics analysis and PCR amplification determined that the root-knot nematodes species on P. sibiricum were M. arenaria and M. incognita, and the root-knot nematodes species on P. praeruptorum was M. hapla. Characterization of the P. sibiricum and P. praeruptorum root-knot nematode species provided a reference for subsequent disease management strategies.
Author Contributions
X.W. designed the experiments and contributed to data analysis and writing. J.W. conducted the research and investigation. S.D. and X.Y. assisted in identification experiments and data analysis. Y.W. provided resources and proposed revision suggestions for the manuscript. X.H. provided resources and revised the manuscript. W.W. designed the experiments and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by China Agriculture Research System (CARS-21), Major Science and Technology Project of Kunming (2021JH002), Yunnan Science and Technology Special Mission (202204BI090003), and the National Natural Science Foundation of China (32160618).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, R.; Chen, C.; Wang, J.; Lu, D.; Gong, X. First Report of Epicoccum sorghinum Causing Leaf Spot on Paris polyphylla in China. Plant Dis. 2019, 103, 1426. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Q.; Cui, X.; Chen, R.; Li, X.; Qiu, B.; Ge, F. A Transcriptome Analysis Uncovers Panax notoginseng Resistance to Fusarium solani Induced by Methyl Jasmonate. Genes Genom. 2019, 41, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, X.; Anane, R.F.; Chen, Z.; Li, S.; Wen, G.; Cai, H.; Zhao, M. First Report of Lily Virus X Infecting Paris polyphylla Var. yunnanensis in Yunnnan, China. Plant Dis. 2023, 108, 537. [Google Scholar]
- Li, W.; Li, H.; Liu, Y.; Ni, C.; Xu, X.; Xu, Z. First Report of Northern Root-Knot Nematode Meloidogyne hapla on Codonopsis pilosula in China. Plant Dis. 2020, 104, 2295. [Google Scholar] [CrossRef]
- Wu, W.; Xu, S.; Gao, Z.; Zhu, S.; Zhu, Y.; Wang, Y.; He, X. Pathogen Identification of Gentiana macrophylla Root-Knot Nematode Disease in Yulong, China. J. Nematol. 2021, 53, e2021-56. [Google Scholar] [CrossRef]
- Wu, W.; Ye, K.; Zhou, S.; Guo, L.; Zhu, S.; Zhu, Y.; Wang, Y.; He, X. Characterization of a Root-Knot Nematode Infecting Aconitum carmichaelii in Southwest China. Plant Dis. 2023, 107, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.J.; Handoo, Z.A. Taxonomy, Identification and Principal Species. In Root-Knot Nematodes; CABI: Wallingford, UK, 2009; pp. 55–97. [Google Scholar]
- Anwar, S.; McKenry, M. Incidence and Population Density of Plant-Parasitic Nematodes Infecting Vegetable Crops and Associated Yield Losses in Punjab, Pakistan. Pak. J. Zool. 2012, 44, 327–333. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Sun, Y.; Chen, Y.; Pei, Y.; Feng, T.; Che, H. Occurrence of Root-Knot Nematode (Meloidogyne spp.) on Peppers in Hainan, China, and M. enterolobii and M. incognita Resistance of Common Cultivars. Plant Dis. 2023, 107, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.R.; Koenning, S.R. Developing Sustainable Systems for Nematode Management. Annu. Rev. Phytopathol. 1998, 36, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a Major Threat to Tomato Production: Current Status and Future Prospects for Its Management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.M.; Sasser, J. Identification of Meloidogyne Species on the Basis of Differential Host Test and Perineal-Pattern Morphology. Adv. Treatise Meloidogyne 1985, 2, 69–77. [Google Scholar]
- Eisenback, J.; Hirschmann, H.; Sasser, J.; Triantaphyllou, A. A Guide to the Four Most Common Species of Root-Knot Nematodes (Meloidogyne spp.), with a Pictorial Key; State University, Depto. of Plant Pathology: Washington, DC, USA, 1981. [Google Scholar]
- Baidoo, R.; Joseph, S.; Mengistu, T.M.; Brito, J.A.; McSorley, R.; Stamps, R.H.; Crow, W.T. Mitochondrial Haplotype-Based Identification of Root-Knot Nematodes (Meloidogyne spp.) on Cut Foliage Crops in Florida. J. Nematol. 2016, 48, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Blok, V.C.; Powers, T.O. Biochemical and Molecular Identification. In Root-Knot Nematodes; CABI: Wallingford, UK, 2009; pp. 98–118. [Google Scholar]
- Zhang, P.; Shao, H.; You, C.; Feng, Y.; Xie, Z. Characterization of Root-Knot Nematodes Infecting Mulberry in Southern China. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.M.; Phillips, M.S.; Blok, V.C. Molecular Diagnostic Key for Identification of Single Juveniles of Seven Common and Economically Important Species of Root-knot Nematode (Meloidogyne spp.). Plant Pathol. 2007, 56, 190–197. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Han, C.; Nie, S. Advances in Polygonatum sibiricum Polysaccharides: Extraction, Purification, Structure, Biosynthesis, and Bioactivity. Front. Nutr. 2022, 9, 1074671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Doh, E.-J.; Lee, G. Chemotaxonomic Classification of Peucedanum japonicum and Its Chemical Correlation with Peucedanum praeruptorum, Angelica decursiva, and Saposhnikovia divaricata by Liquid Chromatography Combined with Chemometrics. Molecules 2022, 27, 1675. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.; Dong, H.; Zhang, W.; Hu, X. Pathogen identification of Eupatorium adenophorum root-knot nematode disease in Yunnan Province. J. Plant Prot. 2020, 47, 657–665. [Google Scholar]
- Xie, H. Taxonomy of Plant Nematodes, 2nd ed.; Higher Education Press (HEP): Beijing, China, 2005; Available online: https://academic.hep.com.cn/skld/CN/book/978-7-04-017701-5-00 (accessed on 7 March 2024).
- Williams, B.D.; Schrank, B.; Huynh, C.; Shownkeen, R.; Waterston, R.H. A Genetic Mapping System in Caenorhabditis elegans Based on Polymorphic Sequence-Tagged Sites. Genetics 1992, 131, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum Polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mu, X.; Liang, Z.; Geng, X. Pilot study of obstruction effect of continuous cropping on Aconitum carmichael. Acta Bot. Boreali-Occident. Sin. 2007, 27, 2112–2115. [Google Scholar]
- Abad, P.; Favery, B.; Rosso, M.-N.; Castagnone-Sereno, P. Root-Knot Nematode Parasitism and Host Response: Molecular Basis of a Sophisticated Interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).