Abstract
With the increasing concerns of human health and the ecological environment, tobacco stalks from the cigarette industry have been recognized as hazardous wastes requiring cautious treatment. However, there is still a lack of a simple and efficient route for full utilization of tobacco stalks. Herein, we attempted to convert tobacco stalk waste to value-added carbohydrates, bio-oil, and biochar through sequential hydrothermal and pyrolysis treatments. The results showed a high carbohydrate yield under the optimized condition using a microwave reaction system. The subsequent pyrolysis of residual solids at high temperatures could produce unexpected high-level aromatic chemicals including phenols and even benzenes—difficult to form without the facilitation of catalysts or salts. The obtained by-product biochar showed rapid absorption of tetracycline in 5 min and gradual introparticle diffusion from 30 to 240 min. The isotherm result had the characteristics of the Langmuir model, displaying homogeneous active sites on the biochar surface. Probably due to the hydrothermal pretreatment before pyrolysis, the obtained biochar exhibited a high adsorption capacity toward tetracycline without introducing the activation chemicals. These results illustrate that the proposed strategy may pave the way for dealing with tobacco wastes in the agricultural field.
1. Introduction
Tobacco (Nicotiana tabacum) is one of the most widely cultivated non-edible crops worldwide and has been used to produce cigarettes for a long time [1]. It is well known that the huge economic value of tobacco means it occupies an important position in the field of global agriculture [2]. As the largest producer and consumer in the world, China manufactures a large amount of tobacco, with approximately 1 million tons of related waste produced every year [3]. Among these, tobacco stalks are one of the most important wastes resulting from producing cigarette from flue-cured tobacco and could be collected centrally for further application [4]. Moreover, tobacco stalks commonly contain some bioactive compounds such as nicotine [5]. It is, thus, imperative to develop an effective method for utilizing these raw materials completely and economically, so as to avoid unreasonable disposal that may pose a serious risk to both human health and environment [6].
During the past decades, many efforts have been devoted to making use of biomass wastes efficiently. Microwave-assisted hydrothermal treatment is an environmental-friendly method of fractionating the biomass components to obtain high-value compounds and has attracted much attention owing to the advantages of being relatively low-cost and operationally simple and having no need to employ organic solvents [7,8]. Although the main product—carbohydrates—can be used for bioethanol fermentation, the residual solid should be also carefully treated for valorization [9].
Pyrolysis is one of the most promising techniques for the conversion of biomass into chemicals and biochar [10]. Previous studies have proved that biochar materials could play critical roles as absorbents for removing contaminants from aqueous solutions. However, there remain at least two challenging issues: firstly, it is very difficult to produce aromatic compounds (benzenes and phenols) with high added value [11]; secondly, biochar obtained from the direct pyrolysis of biomass wastes may only provide limited adsorption capacity [12].
Herein, we attempted to convert tobacco stalk waste to value-added carbohydrates, bio-oil, and biochar through sequential hydrothermal and pyrolysis treatments. Response surface methodology was initially employed to investigate the effects of heating temperature and holding time on hydrothermal extraction efficiency in fresh water. Bio-oil was then obtained under different pyrolysis temperature conditions from 300 to 700 °C, and the corresponding components were analyzed using a gas chromatography mass spectrometer. The biochar product was finally characterized and applied to remove a pollutant from water. It is, thus, anticipated that this research may pave the way for improving the utilization of tobacco biomass resources in an environmentally sustainable way.
2. Materials and Methods
2.1. Chemicals and Materials
The tobacco stalks were kindly provided from Shandong tobacco company (Qingdao, China). These stalks were then dried at 105 °C overnight, to eliminate water possibly introduced during transportation, and subsequently ground through a 60-mesh sieve to give powder samples, which were stored in a desiccator before use. Tetracycline hydrochloride was obtained from Aladdin Reagent Co., Ltd. (Shanghai, China) with a purity of 96%. The certified reference solution of linear alkanes (from C9 to C30) was purchased from Sigma-Aldrich (Saint Louis, MI, USA). Other chemicals were at least analytical-grade reagents obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and employed without further purification.
2.2. Experimental Procedures
Microwave-assisted treatment of the stalk powder was carried out using a CEM-Mars 240/50 microwave oven (Singapore). A total of 0.6 g of sample was weighted into 10 mL water for each 75 mL Teflon vessel reactor, which was sealed and heated to the temperature of 90–180 °C under dynamic power of 800 W. By holding at the desired temperature for 0–40 min, the reactors in the instrument were cooled down to room temperature using flowing air. After centrifugation, the supernatant was separated to determine the carbohydrate amount immediately, while the residual solid was collected for further treatment after washing with distilled water three times and drying at 105 °C overnight. Based on the Box–Benhnken response surface methodology in Design-Expert software version 11, the experimental design consisted of 13 tests, as shown in Table S1, in order to evaluate the effects of heating temperature (A) and holding time (B) on the obtained carbohydrate amount.
The dried residual solid was then pyrolyzed with a heating rate of 10 °C/min to the desired temperature (300 °C, 500 °C, and 700 °C) and held for another 1 h in a tubular furnace. During the experiments, nitrogen gas was flushed through the reaction tube at a rate of 1 L/min to maintain the anaerobic condition. The volatile compounds were collected by using an ice-bath-cooled, gas-washed bottle filled with 100 mL dichloromethane solution, which was then reduced to 10 mL using a rotary evaporator and passed through a 0.2 μm filter prior to analysis. The obtained biochar was washed with distilled water three times, dried at 105 °C for 24 h, ground through a 100-mesh sieve, and stored in a desiccator at 25 °C before usage.
In order to further validate the applicability of biochar, batch experiments were carried out to study its adsorption of the contaminant tetracycline in aqueous solution. In brief, 50 mg of biochar was added into each 200 mL amber glass bottle with 100 mL of tetracycline solution. The mixture was vigorously shaken at 25 °C and 150 rpm, while the initial pH was adjusted to 7 with 0.1 M HCl and NaOH. At the predetermined time points, the sample solutions were taken from the bottles and filtered through the 0.2 μm membranes. The adsorption capacity (Qt) at any time (t) can be calculated using the following equation:
where C0 (mg/L) is the initial tetracycline concentration and Ct (mg/L) is the tetracycline concentration at any time (t). V (L) represents the volume of the tetracycline solution and M (g) is the amount of the adsorbents. The adsorption capacity (Qe) at equilibrium time can be calculated as follow:
where Ce (mg/L) is the equilibrium concentration of tetracycline. The pseudo-first order, pseudo-second order, Chrastil’s diffusion, and intraparticle diffusion models were then used to fit the kinetic experimental data as follows:
where K1 (1/min) is the pseudo-first order rate constant, K2 (g/mg/min) is the pseudo-second order rate constant, KC (L/g/min) is the Chrastil model rate constant, A0 (g/L) is the adsorbent amount, x is a heterogeneous structural diffusion resistance constant, Kid (mg/g/min0.5) is the intraparticle diffusion rate constant, and Cd (mg/g) is the intercept. The isotherm models (Langmuir, Freundlich, and Sips) were used to study the adsorption behavior of obtained biochar by using the following equations:
where KL (L/mg) is the Langmuir constant, Qm (mg/g) is the maximum adsorption capacity, KF (mg(1−n)Ln/g) is the Freundlich constant, 1/n is the model exponent, KS (L/mg) is the Sips constant, and y is the heterogeneity factor.
Qt = (C0 − Ct)V/M
Qe = (C0 − Ce)V/M
Qt = Qe(1 − e−K1t),
Qt = (Qe2K2t)/(1 + K2Qet),
Qt = Qe(1 − e−KcA0t)x,
Qt = Kidt0.5 + Cd
Qe = (QmKLCe)/(1 + KLCe),
Qe = KFCe(1/n),
Qe = (QmKSCe(1/y))/(1 + KSCe(1/y))
2.3. Analytical Methods
The carbohydrate amount (mg) was calculated as the product of solution volume (10 mL) and the corresponding volume determined carbohydrate concentration (mg/mL) based on the anthrone method with glucose standard solutions [8]. The tetracycline concentration was determined based on the calibration curve (Figure S1) using a SPECORD 200 UV–visible spectrometer of Analytik Jena (Jena, Germany) at the wavelength of 360 nm.
The collected volatile compounds were analyzed using an Agilent 7890A gas chromatography coupled with a 5975C mass spectrometer (Santa Clara, CA, USA) in the splitless mode with the electronic ionization of 70 eV and the injection volume of 1 μL. Separation was carried out on a DB-5MS fused silica capillary column with a 1.0 mL/min helium carrier gas. The temperature program started from 50 °C for 5 min, then increased to 280 °C at 5 °C/min, and was finally held at 280 °C for another 15 min. The obtained results were further analyzed to identify each compound using Agilent MassHunter Workstation 11.0 software on the basis of the characteristic mass spectrum and reference retention index summarized in the NIST14 and Wiley databases.
Scanning electron microscopy (SEM) was employed to examine the surface morphology using a Zeiss Merlin instrument (Oberkochen, Germany) with an electron high tension of 3.0 kV and athe working distance of 7.1 mm. Fourier transform infrared spectroscopy with KBr pellets was performed using a Thermo Fisher Nicolet iS5 instrument (Waltham, MA, USA) in the range of 4000–400 cm−1 with a resolution of 4 cm−1 and 32 cycles. The specific surface area was determined using a Micromeritics ASAP 2460 surface area instrument (Norcross, GA, USA) based on nitrogen sorption analysis.
3. Results and Discussion
3.1. Pretreatment of Tobacco Stalks for Extracting Carbohydrates
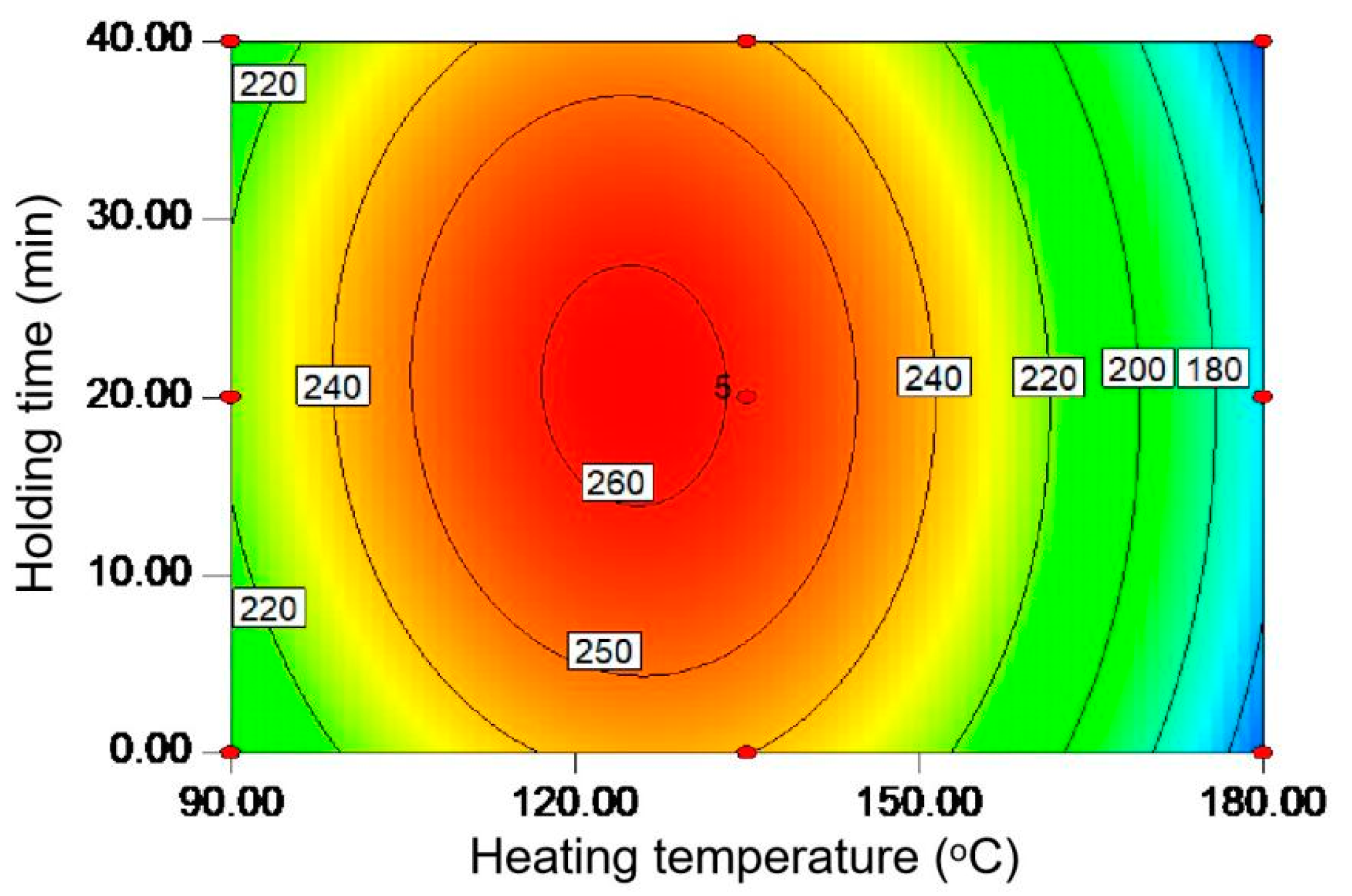
It has been established that the microwave-assisted hydrothermal method is effective in fractionating the biomass components to obtain value-added carbohydrates due to its high energy efficiency based on the dipole rotation of the polar solvent and ionic conduction of the dissolved ion [13]. Moreover, the operating parameters including heating temperature (A) and holding time (B) were found to be important for carbohydrate extraction [7]; thus, they were optimized here by using the response surface methodology. In Figure 1, the contour diagram showed the effects of these two variables on the carbohydrate yield (mg/g) from the tobacco stalk powder. It can be seen that the contour diagram is elliptical, confirming the presence of an obvious maximum value. Specifically, the carbohydrate yield increased from 90 to 125 °C and then decreased when reaching 180 °C. The above phenomenon may be attributed to the low viscosity and surface tension that occurs when increasing the temperature, which is conducive to the decomposition of hemicellulose [14]. However, further increasing the temperature may lead to the breakage of polysaccharide chains and the formation of smaller molecules [15]. Similarly, the extraction efficiency can also increase with heating to 20 min and then decrease by prolonging the time to 40 min. At the beginning of the reaction, the structure of biomass is relatively stable. The extension of reaction time could facilitate the solvent penetration and the hemicellulose decomposition, but the protracted reaction time at high temperatures may destroy the produced carbohydrate [16]. It is known that the extraction of carbohydrates from biomass can be mainly considered as the depolymerization process of hemicellulose, resulting in the formation of oligosaccharides and then monosaccharides [17]. The high temperature applied here could enhance energy transfer into the biomass, which is beneficial for accelerating the release of intracellular substances into the solvent medium, but it is also possible to further degrade monosaccharides into by-products [13]. The low p-value and the high R2 in Table S2 confirm the model fits well to the experimental data [18]. The polynomial regression equation can be then written as:
Y = 258.9 − 28.4A + 0.6B − 2.8AB − 64.9A2 − 18.2B2.
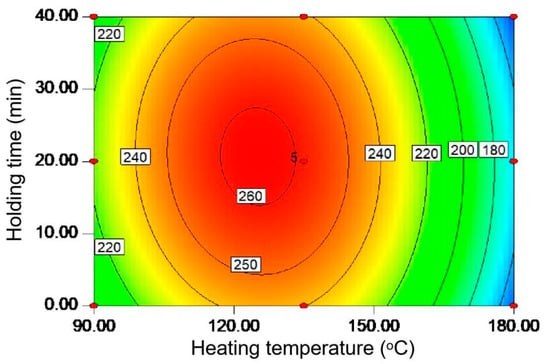
Figure 1.
The contour diagram for optimized carbohydrate yield (mg/g) from tobacco stalks under different heating temperatures (°C) and holding times (min).
From this equation, the theoretically optimal condition was found to be 125.1 °C and 20.7 min to realize the carbohydrate yield (Y) of 262.1 mg/g. It is, thus, believed that the high extraction efficiency of the proposed protocol for the carbohydrate should be beneficial to industrial production such as via bioethanol fermentation.
3.2. Pyrolysis of Residual Solid for Producing Bio-Oil and Biochar
After extracting carbohydrates from tobacco stalks, the remaining residual solid was considered to be a good raw material for producing aromatics via the pyrolysis process instead of using petroleum and coal [9]. The obtained volatile compounds from pyrolysis were analyzed using gas chromatography coupled with a mass spectrometer. By using the reference mixture solution of linear alkanes [19], the actual retention index (I) of each identified compound in this work could be calculated as follows [20]:
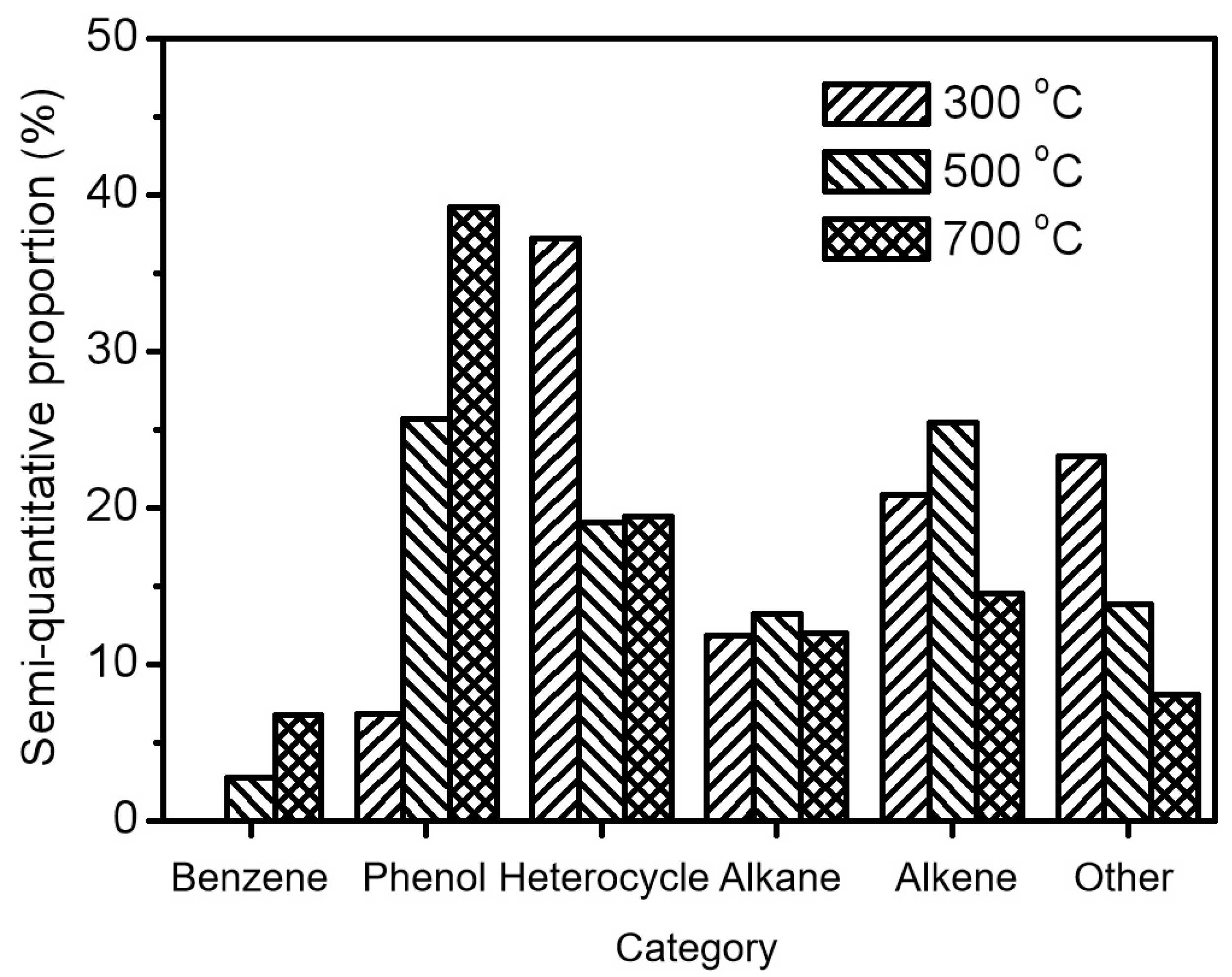
where tz < ti < tz+1, ti is the retention time of each identified compound, and tz and tz+1 are the retention times of linear alkanes with z and z + 1 carbon atoms, respectively. These identified compounds generated at 300 °C, 500 °C, and 700 °C (Tables S3–S8) were then classified into six categories including benzene, phenol, heterocycle, alkane, alkene, and other. Moreover, the semi-quantitative proportion, which means the ratio of the peak area of a specific compound to the total peak area of all identified compounds in the chromatography, was employed to further study the different abundance of products at pyrolysis temperatures of 300 °C, 500 °C, and 700 °C. It is worth mentioning that the semi-quantitative method is commonly and widely used in many previous investigations to evaluate the level of pyrolyzed products due to the complexity of the produced compounds and the difficulty of acquiring the corresponding commercial standards [21,22].
I = 100z + 100(ti − tz)/(tz+1 − tz)
As can be seen in Figure 2, the most abundant products are heterocycle compounds from the pyrolysis of the residual solid at 300 °C, accounting for 37.2% of all identified products. In particular, nicotine was found to account for 18.8% of all identified products, as shown in Table S5. It is well known that nicotine is the main alkaloid in tobacco responsible for addiction and is commonly transferred by heating [5]. The result illustrates that the pyrolyzed products of tobacco stalks should still be attributed to the volatilization of tobacco components instead of the formation of new compounds through chemical bond cleavage. Actually, the semi-quantitative proportion of heterocycle products, as well as alkane, alkene, and other products, decreased with the increase in pyrolysis temperature, as displayed in Figure 2. On the contrary, the semi-quantitative proportion of phenol products increased significantly from 6.8% at 300 °C to 25.6% (500 °C) and 39.2% (700 °C). It is known that phenols are rarely reported products that are scarcely generated in the pyrolysis of lignocellulose and are mainly derived from the breakage of lignin [23]. Moreover, 6.8% of identified products from the pyrolysis of the residual solid at 700 °C are benzene compounds, which are not detected at all for pyrolysis at the low temperature of 300 °C. It is worth mentioning that benzene products can only be generated from biomass through the assistance of catalysts like noble metals or chloride elements, instead of direct pyrolysis [24,25]. In our recent study, the chloride element in seawater was introduced to promote the formation of 73.9% aromatic compounds (37.5% benzenes and 36.4% phenols) in all identified products [10]. However, it is surprising to note that a large amount of aromatic compounds (phenols and benzenes) could be obtained here by combining hydrothermal pretreatment and high-temperature pyrolysis. Due to the absence of catalysts or salts, this strategy is considered to have wide applicability to produce value-added aromatics in the agricultural field by using biomass instead of petroleum/coal [9,26]. The obtained products could then be separated via commonly used distillation technology for further utilization [27].
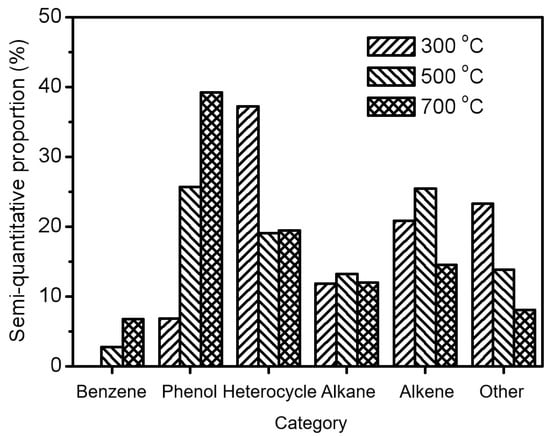
Figure 2.
The semi-quantitative proportions of different product types from the pyrolysis of the residual solid at 300 °C, 500 °C, and 700 °C.
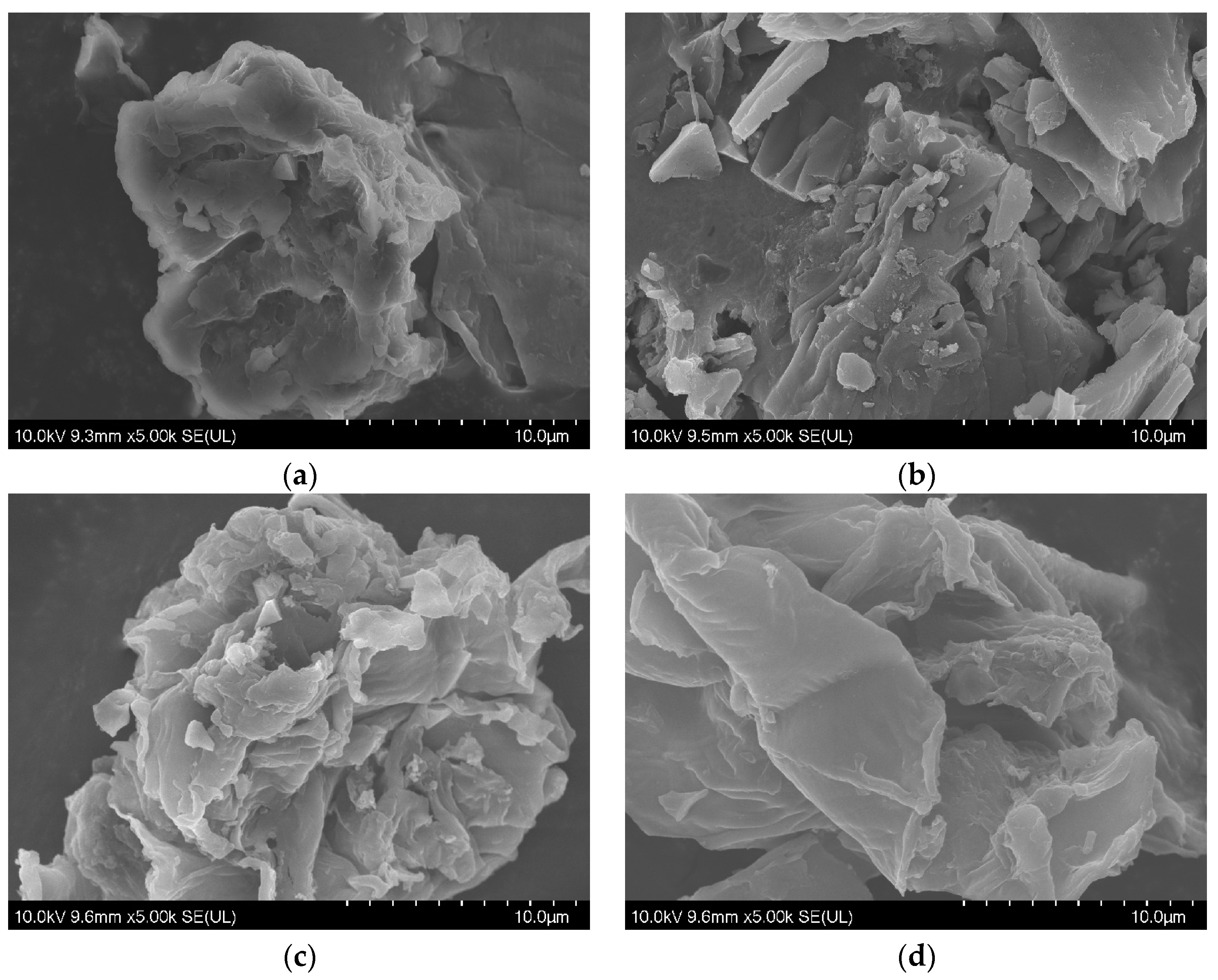
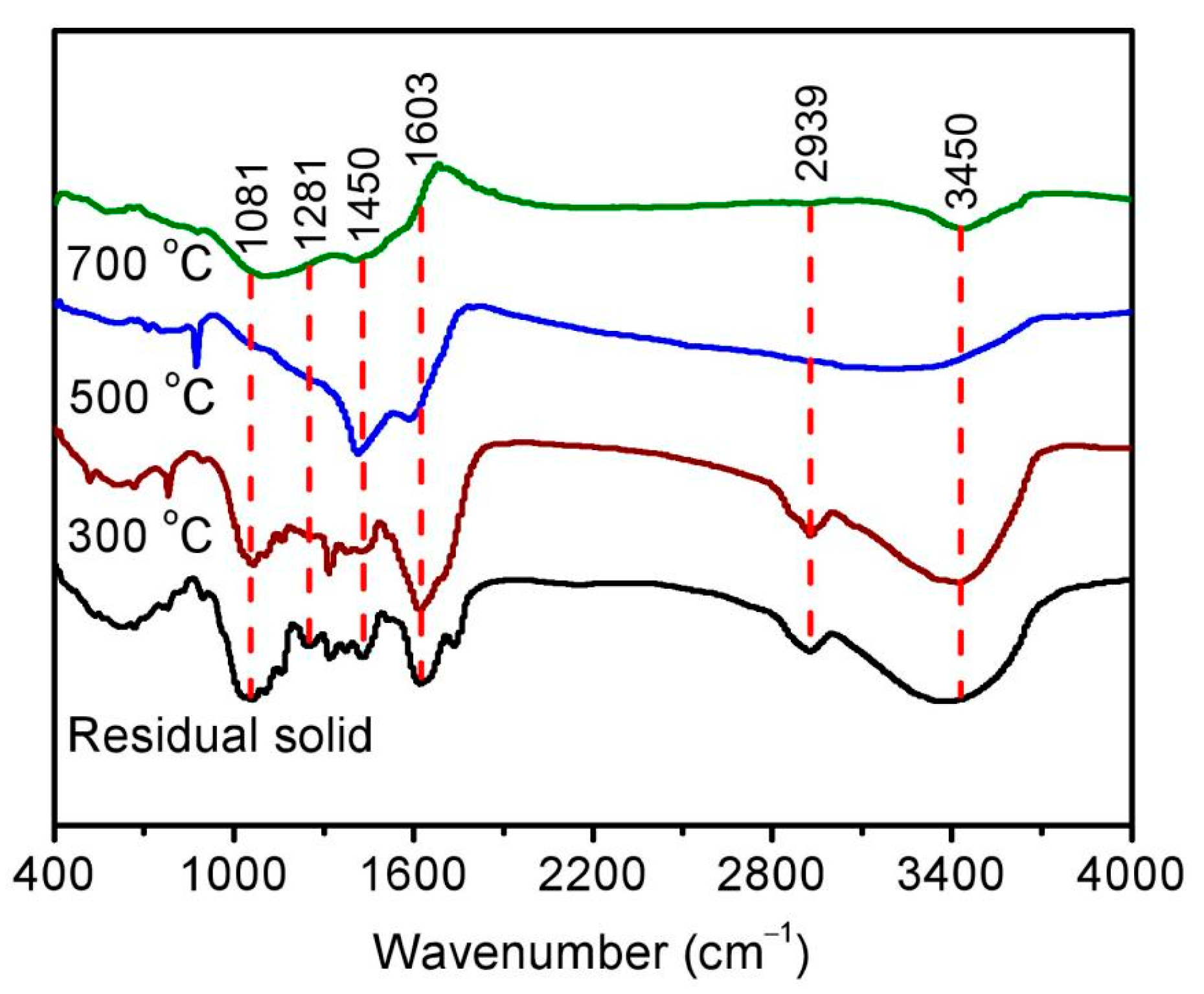
After the pyrolysis treatment, biochar could be also obtained as an important product in addition to bio-oil. The corresponding morphology was then studied using a scanning electron microscope, showing a relatively flat and smooth surface of residual solid in Figure 3a. Following pyrolysis at high temperature, some irregular cracks and voids can be found on the surface of biochar in Figure 3b–d, resulting in a rough surface and loose structure. The variation in functional groups was further investigated using a Fourier transform infrared spectrometer, as shown in Figure 4. Adsorptive peaks were identified at 3450 cm−1, 2939 cm−1, 1603 cm−1, 1450 cm−1, 1281 cm−1, and 1081 cm−1, corresponding to the vibrations of O–H [28], aliphatic C-H [29], C=O [30], phenolic O–H [31], C–O [24], and C–O–C [32]. The enhancement in these peaks for the residual solid could be attributed to the removal of hemicellulose and increase in functional groups [14], while the gradual reduction in these peaks with increasing pyrolysis temperature for biochar should be the result of the volatilization of oxygen/hydrogen and graphitization of the surface [28].
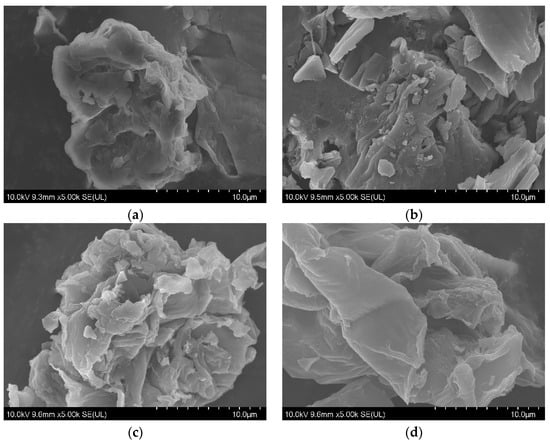
Figure 3.
The scanning electron microscopy images (5000× magnification) of the residual solid (a) and biochar obtained at temperature conditions of 300 °C (b), 500 °C (c), and 700 °C (d).
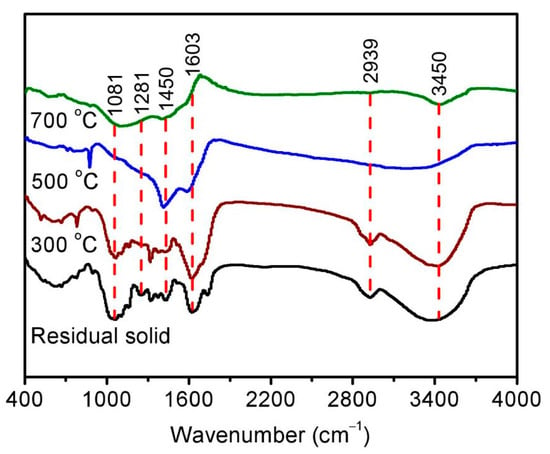
Figure 4.
The Fourier transform infrared spectrum of the residual solid (black) and biochar obtained at temperature conditions of 300 °C (brown), 500 °C (blue), and 700 °C (green).
3.3. Application of Obtained Biochar for Removing Pollutant
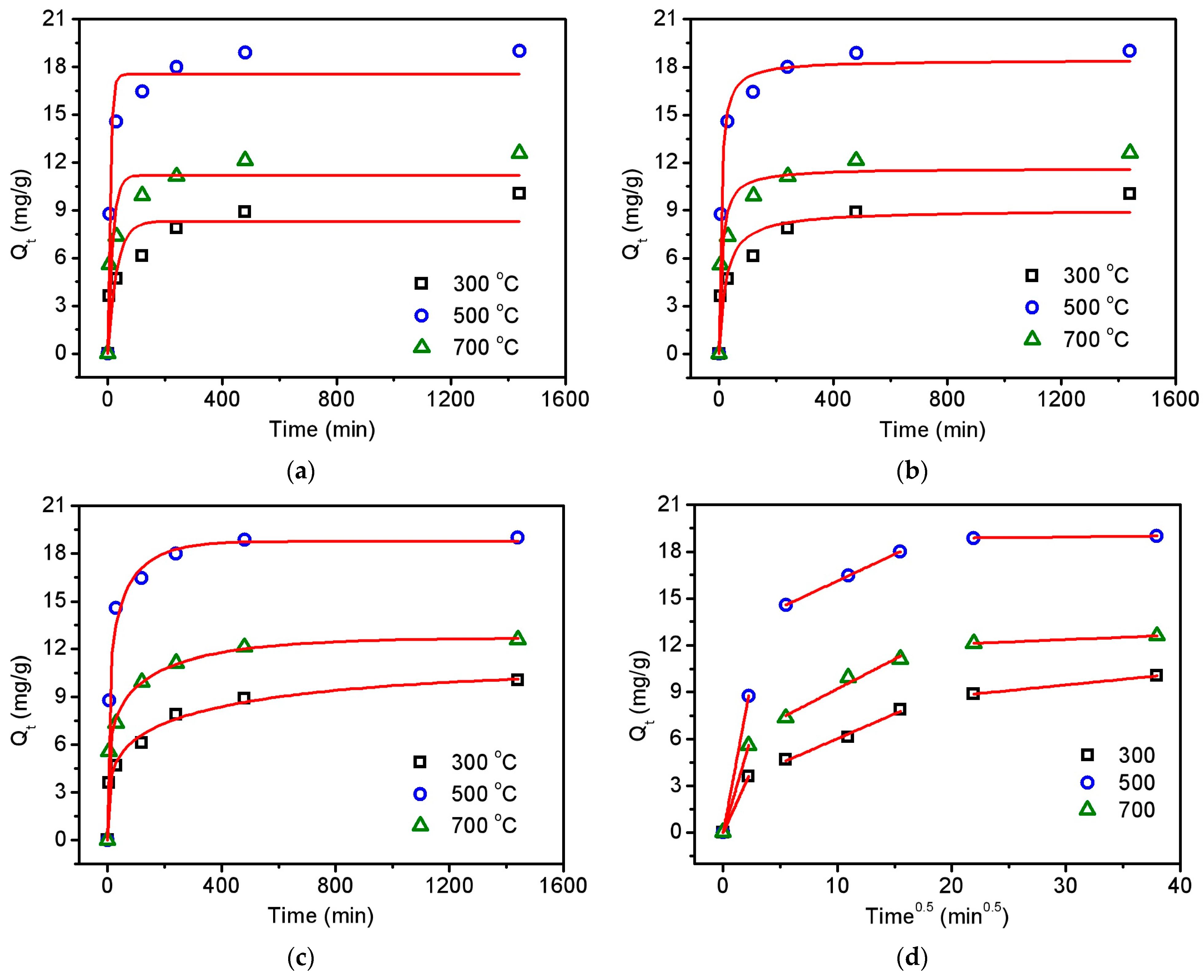
As an emerging carbon material from agricultural waste, biochar has the various advantages of being environmental-friendly and cost-efficient [33]. Thus, in the proof-of-concept experiment, biochar was employed to remove the contaminant antibiotic tetracycline from water by using due to its ubiquitous occurrence in the environment and potential hazard to human health [34,35]. It is worth mentioning that this adsorption technology is widely considered to be a highly effective method for environmental remediation based on its features of low cost and negligible secondary pollution [36,37]. The results of tetracycline adsorption on the biochar can be found in Table S9. In the preliminary experiment, the residual solid from hydrothermal treatment showed no adsorption effect on tetracycline, as can be seen in Figure S2. After the pyrolysis process, the obtained biochar from pyrolyzing the residual solid at 300 °C, 500 °C, and 700 °C can rapidly remove the tetracycline under an environmentally relevant condition (pH = 7), as displayed in Figure 5. It is found in Figure 5a,b that the experimental data could be fitted better using a pseudo-second-order model than using a pseudo-first-order model, due to the higher R2 values (0.869–0.988) of the pseudo-second-order model than those (0.775–0.950) of the pseudo-first-order model, as shown in Table S10. This result demonstrates that the adsorption reaction may involve initial physical adsorption to form a monolayer of tetracycline on the biochar surface and subsequent chemical adsorption through stable binding [31].
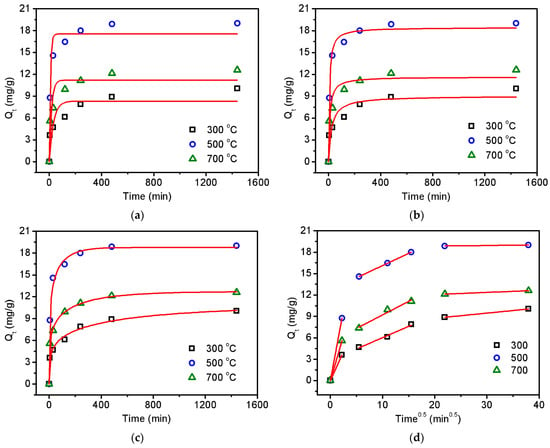
Figure 5.
Adsorption kinetics of tetracycline on the obtained biochar using pseudo-first-order (a), pseudo-second-order (b), Chrastil’s diffusion (c), and introparticle diffusion (d) models. Initial pH—7; initial tetracycline—30 mg/L; adsorbent dose—0.5 g/L; temperature—25 °C.
In order to gain deeper insight into the adsorption mechanism, two diffusion kinetic models were also employed in this work. It is shown in Figure 5c that the experimental data fit very well (R2 values of 0.989–0.998 in Table S11) with the Chrastil’s diffusion model, which is usually applied for describing the adsorption kinetics in a diffusion-controlled system [38]. The heterogeneous structural diffusion resistance constants in Table S11 ranged from 0.204 to 0.226, which are very low values (<0.5), indicating the strong limitation of the diffusion resistance on the adsorption rate. The adsorption process was then divided into three parts, as can be seen in Figure 5d, by considering the introparticle diffusion model [39]. The first and sharpest part could be attributed to the rapid diffusion of tetracycline from the aqueous solution to the biochar surface in the initial 5 min. The second linear part with lower slopes, representing the slow adsorption rate, should also correspond to the gradual introduction of tetracycline into the pore sites, dominated by introparticle diffusion from 30 to 240 min [40]. The third linear part represents the adsorption equilibrium from 480 to 1440 min.
It is worth noting that the biochar obtained at 300 °C showed the lowest adsorption capacity towards tetracycline. This can be easily understood since the pyrolysis experiment results in Figure 2 and Figure 4 confirm the predominant volatilization of tobacco components instead of chemical bond cleavage and surface graphitization. The result was also supported by the lowest specific surface area of 3.3 m2/g for the biochar obtained at 300 °C. Very interestingly, the biochar obtained at 500 °C exhibited better performance than that obtained at 700 °C in adsorbing tetracycline, albeit their specific surface areas are comparable to each other (10.0 m2/g for 500 °C and 10.7 m2/g for 700 °C). This indicates that the improvement in adsorption efficiency may not only depend on the increased graphitization degree at high temperatures but also on the preservation of functional groups.
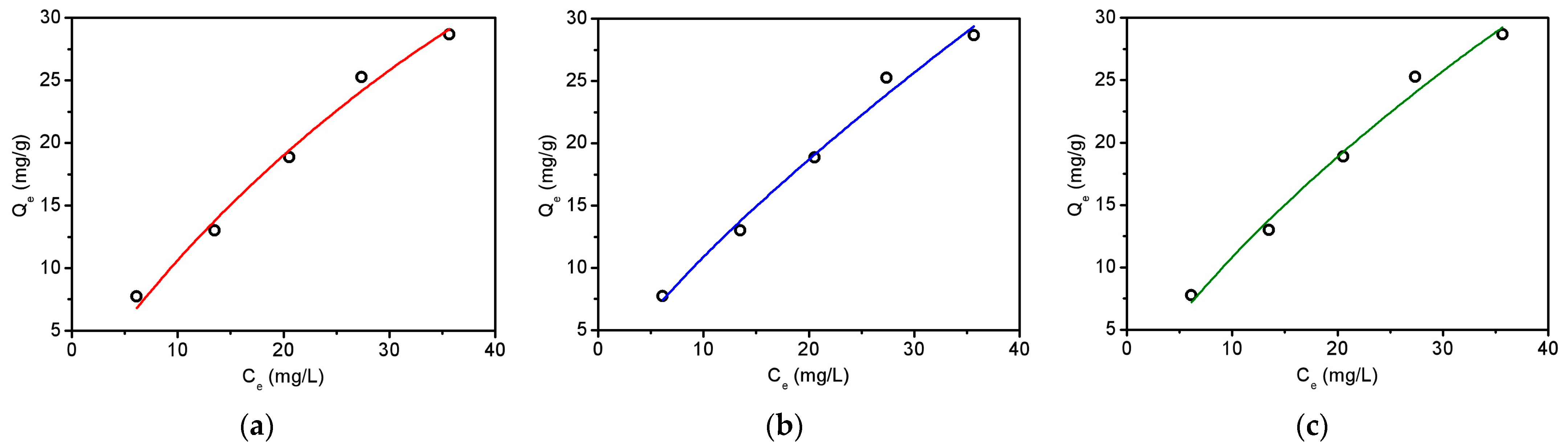
The nonlinear isotherm models were then used to study the adsorption mechanism. It is found in Figure 6a,b that both the Langmuir and Freundlich models could simulate the experimental data well. Moreover, the value of “n” in the Freundlich equation was calculated to be 1.3 (Table S12), which is between 1 and 10, suggesting favorable tetracycline adsorption using biochar [32]. The three-parameter Sips model was further employed to understand the adsorption mechanism [38]. As a combination form of Langmuir and Freundlich expressions, the Sips model fit well to the experimental data with an R2 value of 0.980, as displayed in Table S12. The obtained heterogeneity factor of 1.1 (Table S12), which is close to 1, illustrates that the isotherm should have more characteristics of the Langmuir model, showing the homogeneous active sites on biochar surface. The maximum adsorption capacity was then calculated to be 90.1 mg tetracycline/g biochar using the Langmuir model and is compared with some previously reported biochar materials in Table S13. It was found that the maximum adsorption capacity of biochar obtained in this work is relatively higher than that of biochar derived from the direct pyrolysis of sludge, rice straw, swine manure, spent coffee ground, and peanut shells [41,42,43]. This may be attributed to the hydrothermal pretreatment of tobacco stalks before pyrolysis. It is noticeable that some activation methods using alkaline or ferric could further increase the adsorption capacity. However, the introduction of these chemicals would not only increase the costs but also possibly corrode the reactor. Consequently, the two steps treatment protocol in this work should be very promising for making biochar materials for adsorption.
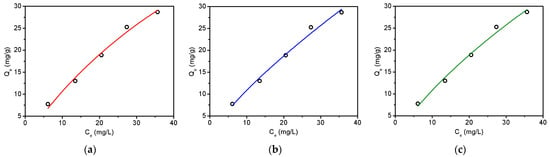
Figure 6.
Adsorption isotherms of tetracycline on the obtained biochar (pyrolysis at 500 °C) using Langmuir (a), Freundlich (b), and Sips (c) models. Initial pH—7; initial tetracycline—10–50 mg/L; adsorbent dose—0.5 g/L; temperature—25 °C.
4. Conclusions
In this work, we attempted to fully utilize the wasted stalks of flue-cured tobacco, which could be centrally collected and processed to avoid the potential threat to both human health and the ecological environment. By using response surface methodology, a high carbohydrate yield of 262.1 mg/g tobacco stalks was successfully achieved using fresh water in the microwave-assisted hydrothermal treatment. The residual solid was further pyrolyzed at different temperatures to produce bio-oil and biochar. It is very interesting to find that a large amount of aromatic compounds such as phenols and benzenes could be obtained at high temperatures without catalysts or salts. The result demonstrated that the combination of hydrothermal treatment and high temperature pyrolysis could promote the transformation of tobacco biomass to value-added petrochemicals. The changes in morphology and functional groups were studied using different characterization methods. The obtained biochar was employed to remove tetracycline from water. The kinetic and isotherm simulations confirmed the favorable adsorption of tetracycline using biochar, involving rapidly external mass transfer and slowly introparticle diffusion processes. It is, thus, anticipated that this research may pave the way for improving the utilization of tobacco biomass resources in an environmentally sustainable way.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14040801/s1. Figure S1: The calibration curve of absorbance at 360 nm versus tetracycline concentration [TC] measured using an UV–visible spectrometer; Figure S2: The variation of tetracycline concentration [TC] with time using the residual solid (microwave-assisted, hydrothermal-treated tobacco stalks) as an adsorbent; Table S1: The effects of heating temperature (A) and holding time (B) on the carbohydrate yield (mg/g) after extraction from tobacco stalk powder samples; Table S2: Analysis of variance and regression coefficients of the response surface model; Table S3: The identified benzene products from the pyrolysis of the residual solid under different temperature conditions; Table S4: The identified phenol products from the pyrolysis of the residual solid under different temperature conditions; Table S5: The identified heterocycle products from the pyrolysis of the residual solid under different temperature conditions; Table S6: The identified alkane products from the pyrolysis of the residual solid under different temperature conditions; Table S7: The identified alkene products from the pyrolysis of the residual solid the different temperature conditions; Table S8: The identified other products from the pyrolysis of the residual solid under different temperature conditions; Table S9: The tetracycline adsorption (Qt) at a predetermined time point on the obtained biochar at different pyrolysis temperatures; Table S10: Kinetic parameters of tetracycline adsorption on the biochar obtained at different pyrolysis temperatures using pseudo-first-order and pseudo-second-order models; Table S11: Kinetic parameters of tetracycline adsorption on the biochar obtained at different pyrolysis temperatures using intraparticle diffusion and Chrastil’s diffusion models; Table S12: Isotherm parameters of tetracycline adsorption on the biochar obtained at different pyrolysis temperatures using Langmuir, Freundlich, and Sips models; Table S13. Comparison of the maximum adsorption capacity and other parameters of some previously reported biochar from different biomasses for the removal of tetracycline [12,41,42,43].
Author Contributions
Conceptualization, Y.L. and D.S.; methodology, Y.L. and G.Y.; formal analysis, Y.L. and G.Y.; investigation, F.K. and D.S.; writing—original draft preparation, Y.L. and G.Y.; writing—review and editing, R.L. and D.S.; supervision, F.K. and D.S.; funding acquisition, R.L. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Agricultural Science and Technology Innovation Program (ASTIPTRIC06), and Shandong Engineering Technology Research Laboratory of Suzhou Biomedical Engineering and Technology of Chinese Academy of Sciences (YCSKY202109058C1), and the Taishan Scholar Program of Shandong Province (tsqn202103132).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hatsukami, D.K.; Carroll, D.M. Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev. Med. 2020, 140, 106099. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, R.; Zappe, A.L.; de Oliveira, P.F.; Machado, E.L.; Lawisch-Rodriguez, A.D.A.; Rodriguez-Lopez, D.A. Carbon footprint of agricultural production and processing of tobacco (Nicotiana tabacum) in southern Brazil. Environ. Technol. Innov. 2020, 18, 100625. [Google Scholar] [CrossRef]
- Ye, J.B.; Zheng, S.S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.J.; Zheng, J.Q.; Mao, D.B.; Yang, X.P. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- He, J.T.; Yao, N.; Sun, Z.Y.; Li, F.; Cai, H.Q.; Jin, L.F.; Tang, Y.Q. Improved biogas production from tobacco processing waste via biochar-assisted thermophilic anaerobic digestion. Ind. Crops Prod. 2023, 202, 117038. [Google Scholar] [CrossRef]
- Banožić, M.; Babic, J.; Jokic, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Faber, T.; Kumar, A.; Mackenbah, J.P.; Millett, C.; Basu, S.; Sheikh, A.; Been, J.V. Effect of tobacco control policies on perinatal and child health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e420–e437. [Google Scholar] [CrossRef]
- Luo, Y.P.; Fan, J.J.; Budarin, V.L.; Hu, C.W.; Clark, J.H. Microwave-assisted hydrothermal selective dissolution and utilisation of hemicellulose in Phyllostachys heterocycla cv. pubescens. Green Chem. 2017, 19, 4889–4899. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Bispo, L.D.O.; Colla, L.M.; Costa, J.A.V.; Canan, C.; Colla, E. Protein and carbohydrate extraction from S. platensis biomass by ultrasound and mechanical agitation. Food Res. Int. 2017, 99, 1028–1035. [Google Scholar] [CrossRef]
- Wang, S.R.; Dai, G.X.; Yang, H.P.; Luo, Z.Y. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Lin, Y.N.; Wang, C.; Yu, G.F.; Wang, H.Y.; Liang, R.N.; Kong, F.Y.; Song, D.A. Transformation of tobacco biomass into value-added carbohydrate, aromatics, and biochar. Biomass Convers. Bior. 2022, 14, 1–9, in press. [Google Scholar] [CrossRef]
- Meng, Q.L.; Yan, J.; Wu, R.Z.; Liu, H.Z.; Sun, Y.; Wu, N.N.; Xiang, J.F.; Zheng, L.R.; Zhang, J.; Han, B.X. Sustainable production of benzene from lignin. Nat. Commun. 2021, 12, 4534. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ma, A.; Chen, Y.; Zhang, M.; Zhang, Y.; Zhou, N.; Fan, S.; Wang, Y. The removal of tetracycline from aqueous solutions using peanut shell biochars prepared at different pyrolysis temperatures. Sustainability 2023, 15, 874. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhang, W.W.; Lei, F.H.; Yang, S.J.; Jiang, J.X. Coproduction of xylooligosaccharides and fermentable sugars from sugarcane bagasse by seawater hydrothermal pretreatment. Bioresour. Technol. 2020, 309, 123385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.J.; Clark, J.; Shen, P.L.; Li, Y.Q.; Zhang, C.S. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Guo, X.H.; Fu, Y.J.; Miao, F.W.; Yu, Q.S.; Liu, N.; Zhang, F.S. Efficient separation of functional xylooligosaccharide, cellulose and lignin from poplar via thermal acetic acid/sodium acetate hydrolysis and subsequent kraft pulping. Ind. Crops Prod. 2020, 153, 112575. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.C.; Budarin, V.L.; Fan, J.J.; Remon, J.; Li, T.Z.; Hu, C.W.; Clark, J.H. Sodium chloride-assisted depolymerization of xylo-oligomers to xylose. ACS Sustain. Chem. Eng. 2018, 6, 4098–4104. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019, 289, 351–359. [Google Scholar] [CrossRef]
- Melo, T.O.; Marques, F.A.; Hansel, F.A. Pyrolysis-gas chromatography-mass spectrometry Kováts retention index of pyrolysis products of lignocellulosic materials. J. Anal. Appl. Pyrolysis 2017, 126, 332–336. [Google Scholar] [CrossRef]
- Ettre, L.S. Retention index expressions. Chromatographia 2003, 58, 491–494. [Google Scholar] [CrossRef]
- Zhao, N.; Li, B.X. The effect of sodium chloride on the pyrolysis of rice husk. Appl. Energy 2016, 178, 346–352. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.F.; Dong, D.H.; Gao, G.G.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Mortaza, G.; Hu, X. Co-pyrolysis of cellulose/lignin and sawdust: Influence of secondary condensation of the volatiles on characteristics of biochar. Energy 2021, 226, 120442. [Google Scholar] [CrossRef]
- Lee, T.; Jung, S.; Lin, K.Y.A.; Tsang, Y.F.; Kwon, E.E. Mitigation of harmful chemical formation from pyrolysis of tobacco waste using CO2. J. Hazard. Mater. 2021, 401, 123416. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Zhang, S.P.; Chen, W.B.; Cai, Q.J. Pyrolysis of tobacco wastes for bio-oil with aroma compounds. J. Anal. Appl. Pyrolysis 2018, 136, 248–254. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, X.B.; Ma, Z.H.; Duan, C.; Liu, S.W.; Yu, H.L.; Li, X.P.; Cai, L.P.; Shi, S.Q.; Ni, Y.H. Breaking the lignin conversion bottleneck for multiple products: Co-production of aryl monomers and carbon nanospheres using one-step catalyst-free depolymerization. Fuel 2021, 285, 119211. [Google Scholar] [CrossRef]
- Guo, G.N.; Liu, X.; Li, R.; Li, Q.; Yu, H.B.; Li, M.J. Characterization of tobacco stalk lignin using nuclear magnetic resonance spectrometry and its pyrolysis behavior at different temperatures. J. Anal. Appl. Pyrolysis 2019, 142, 104665. [Google Scholar] [CrossRef]
- Guo, X.J.; Wang, S.R.; Guo, Z.G.; Liu, Q.; Luo, Z.Y.; Cen, K.F. Pyrolysis characteristics of bio-oil fractions separated by molecular distillation. Appl. Energy 2010, 87, 2892–2898. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, J.T.; Zeng, Q.B.; Liang, Z.X.; Ye, X.X.; Lv, Y.C.; Liu, M.H. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef]
- Chabi, N.; Baghdadi, M.; Sani, A.H.; Golzary, A.; Hosseinzadeh, M. Removal of tetracycline with aluminum boride carbide and boehmite particles decorated biochar derived from algae. Bioresour. Technol. 2020, 316, 123950. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total Environ. 2021, 751, 141755. [Google Scholar] [CrossRef]
- Xiang, W.; Wan, Y.S.; Zhang, X.Y.; Tan, Z.Z.; Xia, T.T.; Zheng, Y.L.; Gao, B. Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Zhang, X.B.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Song, D.A.; Cheng, H.Y.; Liu, R.P.; Qiang, Z.M.; He, H.; Liu, H.J.; Qu, J.H. Enhanced oxidation of tetracycline by permanganate via the alkali-induced alteration of the highest occupied molecular orbital and the electrostatic potential. Ind. Eng. Chem. Res. 2017, 56, 4703–4708. [Google Scholar] [CrossRef]
- Jiang, X.H.; Jefferson, W.A.; Song, D.A.; Cheng, H.Y.; Li, F.M.; Qiang, Z.M.; Zhang, A.Q.; Liu, H.J.; Qu, J.H. Regioselective oxidation of tetracycline by permanganate through alternating susceptible moiety and increasing electron donating ability. J. Environ. Sci. 2020, 87, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Yazidi, A.; Atrous, M.; Soetaredjo, F.E.; Sellaoui, L.; Ismadji, S.; Erto, A.; Bonilla-Petriciolet, A.; Dotto, G.L.; Lamine, A.B. Adsorption of amoxicillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: Experimental study and modeling analysis. Chem. Eng. J. 2020, 379, 122320. [Google Scholar] [CrossRef]
- Song, D.A.; Jiang, X.H.; Wang, D.B.; Fang, S.; Zhou, H.X.; Kong, F.Y. From the effective herbicide to the environmental contaminant: A review of recent studies on quinclorac. Environ. Exp. Bot. 2022, 193, 104706. [Google Scholar] [CrossRef]
- Velinov, N.; Mitrović, J.; Kostić, M.; Radović, M.; Petrović, M.; Bojić, D.; Bojić, A. Wood residue reuse for a synthesis of lignocellulosic biosorbent: Characterization and application for simultaneous removal of copper (II), Reactive Blue 19 and cyprodinil from water. Wood Sci. Technol. 2019, 53, 619–647. [Google Scholar] [CrossRef]
- Kostić, M.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Petrović, M.; Mitrović, J.; Bojić, A. Ultrasound-assisted synthesis of a new material based on MgCoAl-LDH: Characterization and optimization of sorption for progressive treatment of water. Environ. Technol. Innov. 2022, 26, 102358. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, Y.; Ding, L.; Yu, J.; Zhou, Q.; Kong, Y.L.; Ma, J.Y. Novel sodium bicarbonate activation of cassava ethanol sludge derived biochar for removing tetracycline from aqueous solution: Performance assessment and mechanism insight. Bioresour. Technol. 2021, 330, 124949. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Nguyen, T.B.; Huang, C.P.; Chen, C.W.; Bui, X.T.; Dong, C.D. Alkaline modified biochar derived from spent coffee ground for removal of tetracycline from aqueous solutions. J. Water Process Eng. 2021, 40, 101908. [Google Scholar] [CrossRef]
- Wang, H.; Fang, C.; Wang, Q.; Chu, Y.; Song, Y.; Chen, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv. 2018, 8, 16260–16268. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, G.; Yu, H.; Zhang, Z. Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal. Bioresour. Technol. 2016, 211, 566–573. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).