Creation of Bacterial Blight Resistant Rice by Targeting Homologous Sequences of Xa13 and Xa25 Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Vectors Construction and Rice Transformation
2.3. Rice Transformation
2.4. Genotype Analysis
2.5. Disease Assays
3. Results
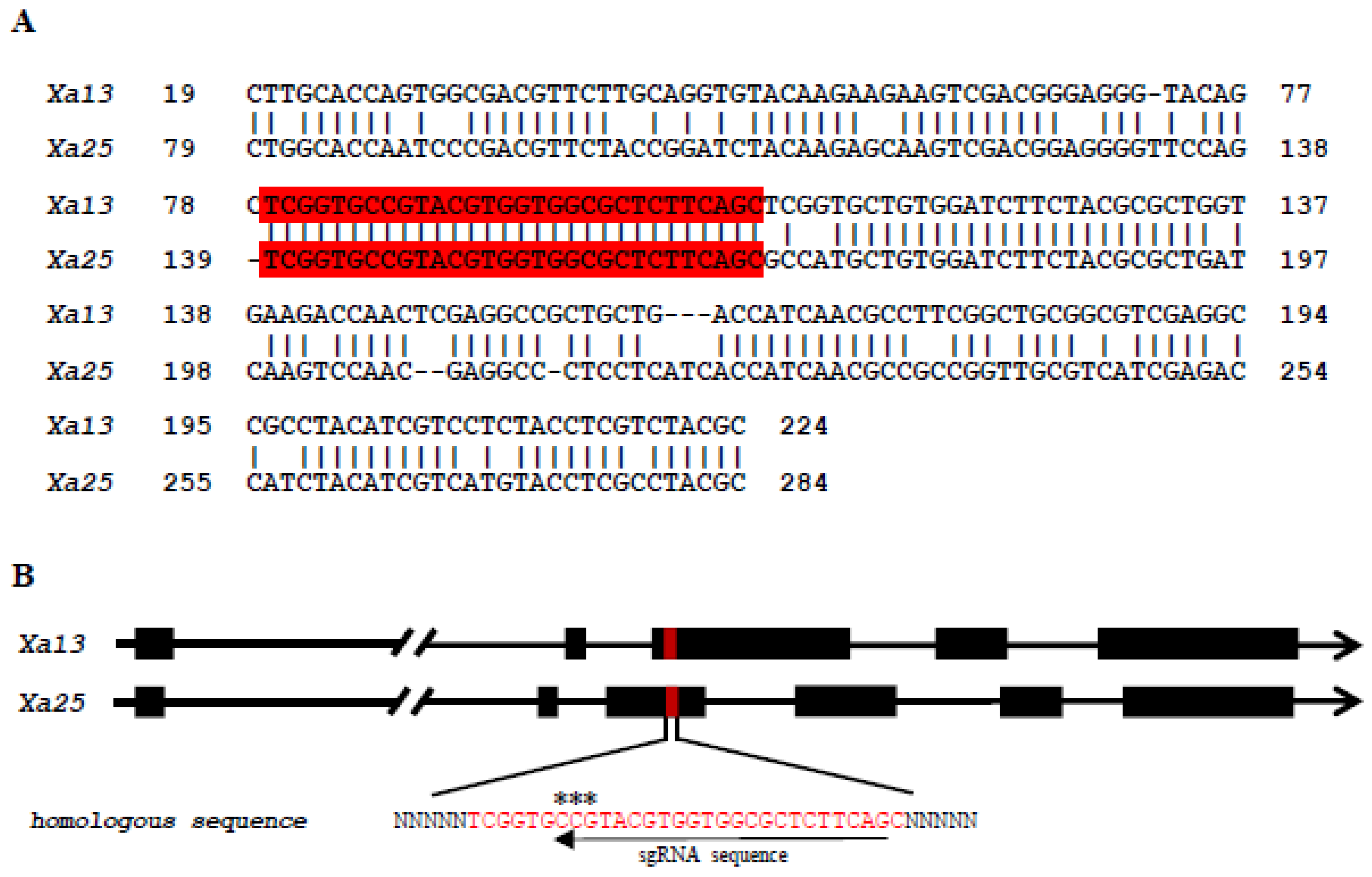
3.1. Selection of the Targeted Genes and sgRNA Recognition Site
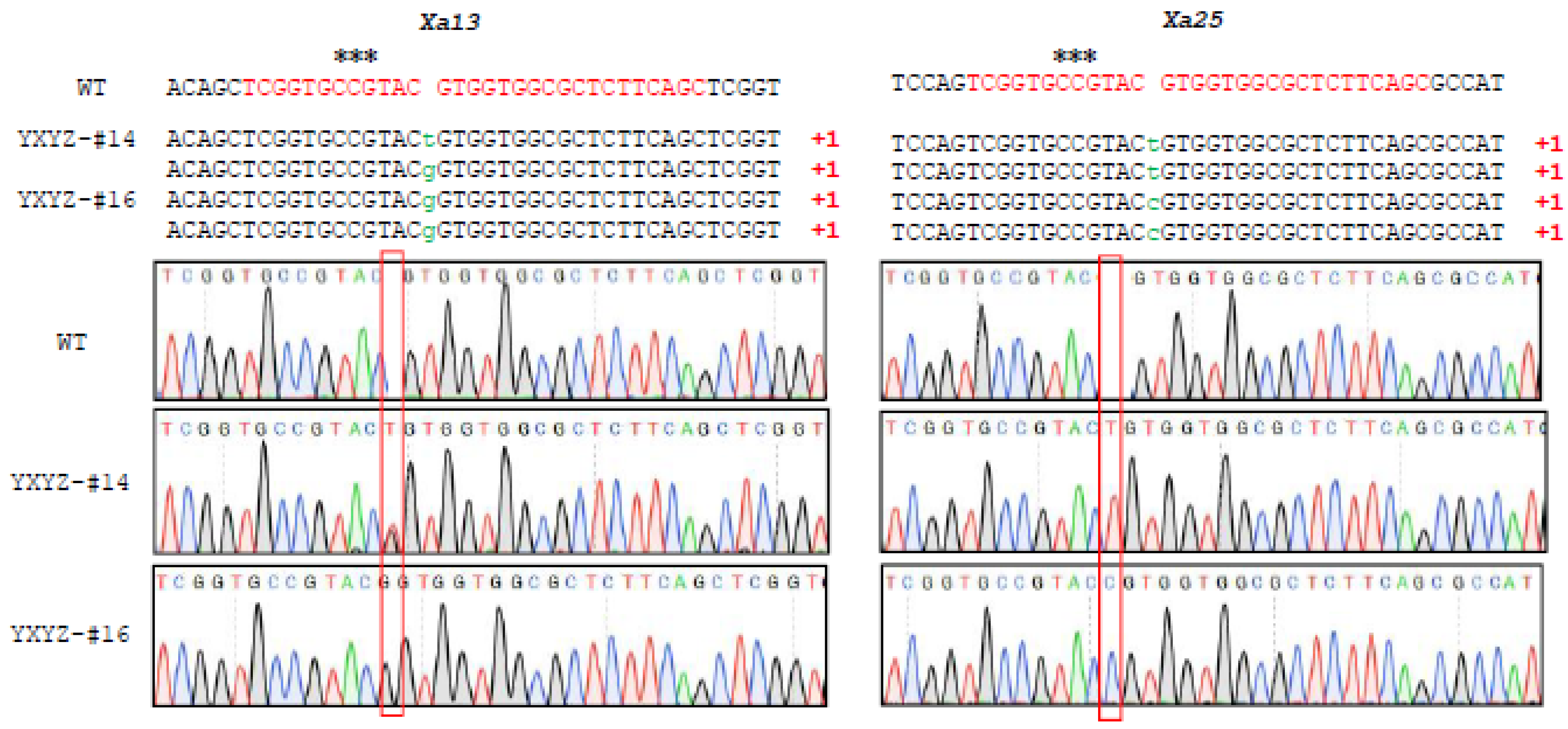
3.2. Efficient CRISPR/Cas9-Mediated Targeted Mutagenesis in T0 Transgenic Rice
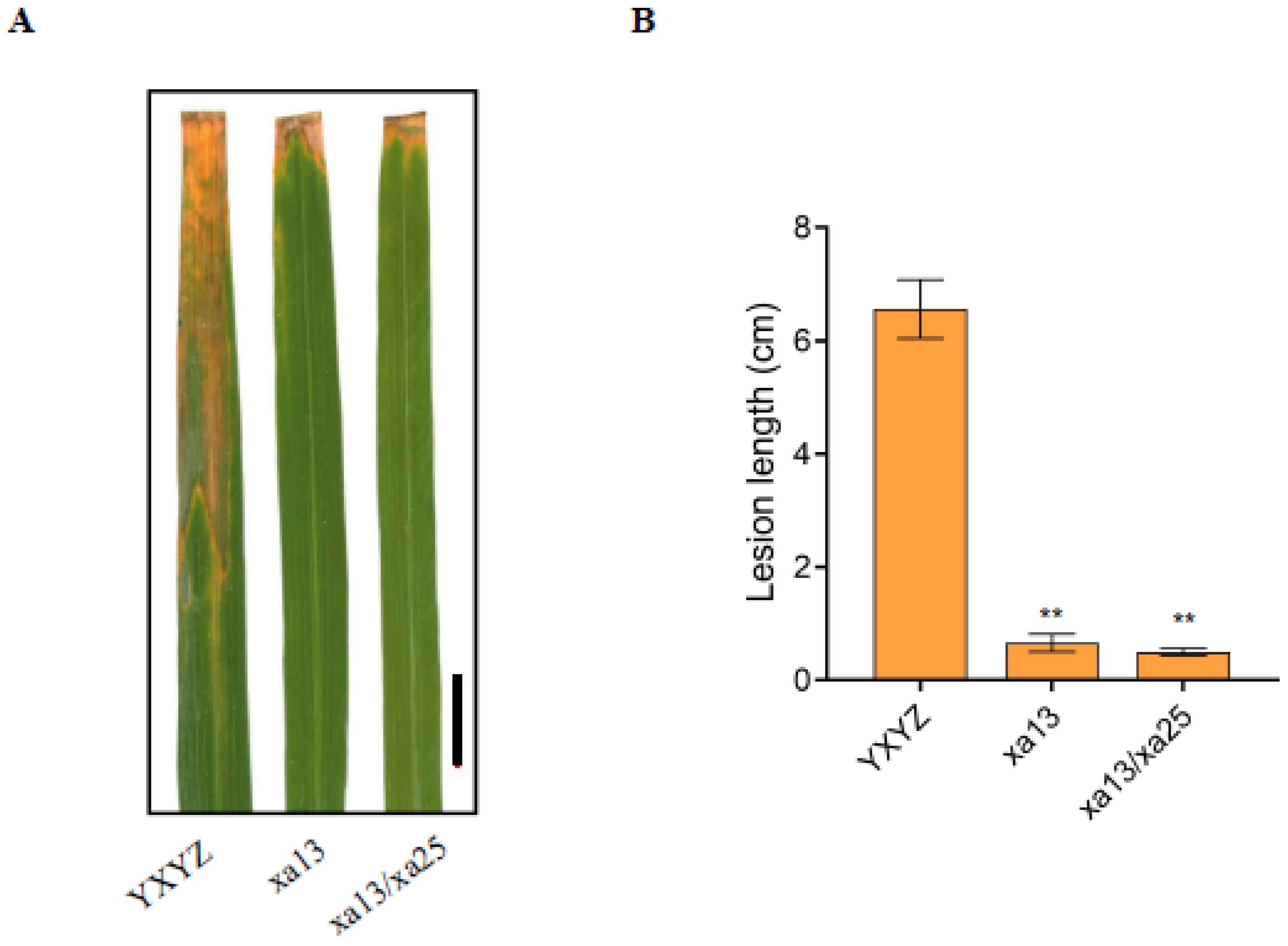
3.3. The Homozygous Lines Increased the Bacterial Blight Resistance of Rice
3.4. Putative Off-Target Analysis
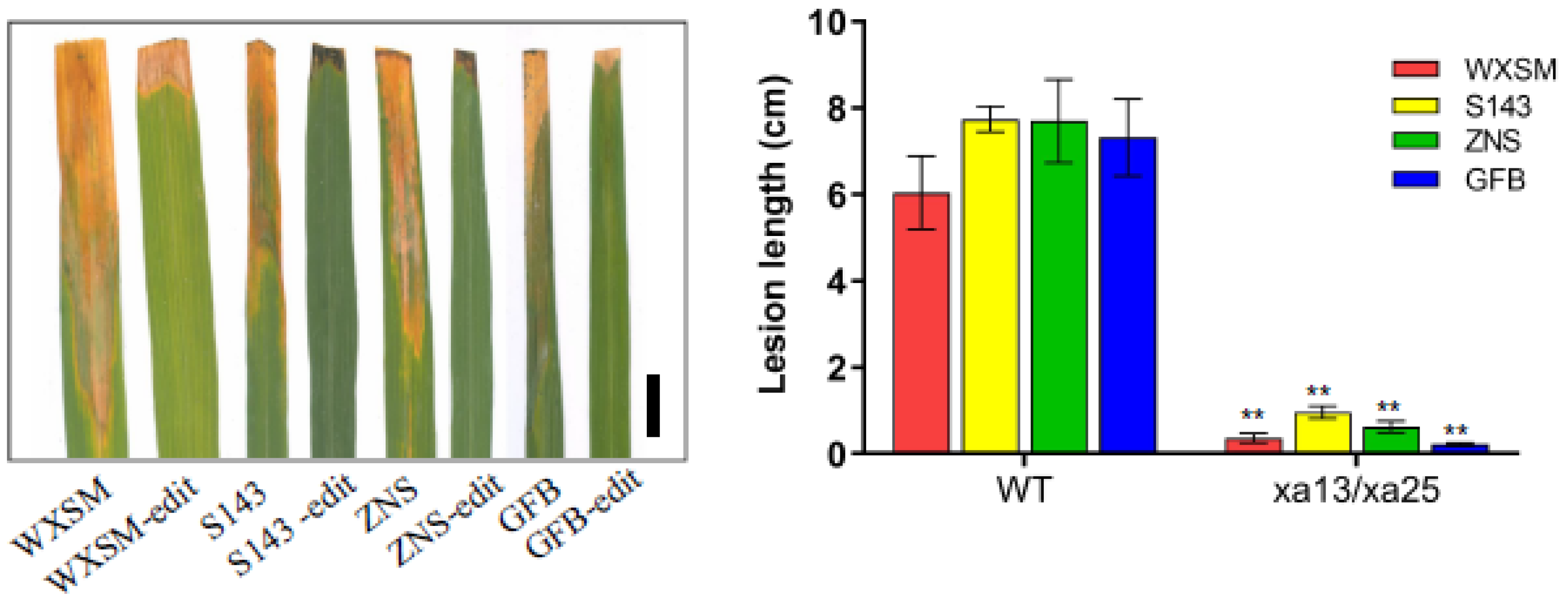
3.5. Xa13/xa25 Double Mutants Show Increased Resistance to Bacterial Blight Disease under the Background of Four High-Quality Varieties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mew, T.W. Current status and future prospects of research on bacterial blight of rice. Annu. Rev. Phytopathol. 1987, 25, 359–382. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Triplett, L.; Leach, J.E.; Wang, G. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu. Rev. Phytopathol. 2014, 52, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Coaker, G.; Zhou, J.; Dong, X. Plant immune mechanisms: From reductionistic to holistic points of view. Mol. Plant 2020, 13, 1358–1378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)—An Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas9 system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.-L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Mao, Y.; Xu, N.; Zhang, B.; Wei, P.; Yang, D.-L.; Wang, Z.; Zhang, Z.; Zheng, R.; Yang, L.; et al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 4632–4637. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sugio, A.; White, F.F. Os8N3 is a host disease susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA 2006, 103, 10503–10508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, M.; Zhou, Y.; Li, X.; Xiao, J.; Wang, S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011, 34, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Hutin, M.; Sabot, F.O.; Ghesquiere, A.; Koebnik, R.; Szurek, B. A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 2016, 84, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Ji, Z.; Liu, B.; Cheng, H.; Liu, H.; Liu, S.; Yang, B.; Chen, G. Xa1 allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun. 2020, 1, 100087. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Mei, L.; He, X.; Chen, L.; Liu, H.; Shen, S.; Ji, Z.; Zheng, X.; Zhang, Y.; et al. Xa7, a new executor R gene that confers durable and broad-spectrum resistance to bacterial blight disease in rice. Plant Commun. 2021, 2, 100143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xia, Z.; Zhou, Y.; Wan, J.; Li, D.; Chen, R.; Zhai, W.; Zhu, L. Testifying the rice bacterial blight resistance gene xa5 by genetic complementation and further analyzing xa5 (Xa5) in comparison with its homolog TFIIAg1. Mol. Genet. Genom. 2006, 275, 354–366. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Moon, H.; Park, C.-J. CRISPR/Cas9-targeted mutagenesis of Os8N3 in rice to confer resistance to Xanthomonas oryzae pv. oryzae. Rice 2019, 12, 67. [Google Scholar] [CrossRef]
- Iyer, A.S.; McCouch, S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant-Microbe Interact. 2004, 17, 1348–1354. [Google Scholar] [CrossRef]
- Chu, Z.; Yuan, M.; Yao, J.; Ge, X.; Yuan, B.; Xu, C.; Li, X.; Fu, B.; Li, Z.; Bennetzen, J.L.; et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006, 20, 1250–1255. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plantscontaining high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Ma, X.; Li, J.; Chen, J.; Liu, Y.G. DSDecode: A webbased tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, S.; Li, H.; Song, C.; Xie, W.; Zuo, S.; Zhou, X.; Zhou, C.; Ji, Z.; Zhou, H. Genome editing of a dominant resistance gene for broad-spectrum resistance to bacterial diseases in rice without growth penalty. Plant Biotechnol. J. 2024, 22, 529–531. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, Y.; Vu, N.T.Q.; Shen, S.; Xia, K.; Zhang, M. CRISPR/Cas9-mediated mutation of OsSWEET14 in rice cv. Zhonghua11 confers resistance to Xanthomonas oryzae pv. oryzae without yield penalty. BMC Plant Biol. 2020, 20, 313. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Wang, Y.; Liu, L.; Li, Y.; Yang, Y.; Liu, L.; Zou, L.; Chen, G. A varied AvrXa23-like TALE enables the bacterial blight pathogen to avoid being trapped by Xa23 resistance gene in rice. J. Adv. Res. 2022, 42, 263–272. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhu, J.K. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018, 60, 369–375. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]

| Rice Variety | No. of Transgenic Plants | No. of Plants with Mutations | No. of Plants with Single Gene Mutations | No. of Plants with xa13/xa25 Double Gene Mutations | |
|---|---|---|---|---|---|
| Mutations of xa13 (%) | Mutations of xa25 | ||||
| YXYZ | 55 | 32 | 29 | 26 | 18 |
| WXSM | 63 | 33 | 27 | 23 | 16 |
| S143 | 43 | 26 | 19 | 19 | 12 |
| ZNS | 34 | 20 | 13 | 15 | 7 |
| GFB | 61 | 35 | 29 | 24 | 16 |
| Total | 256 | 146 | 127 | 107 | 69 |
| Target | Name of Putative Off-Target Sites | Putative Off-Target Locus | Putative Off-Target Sequence * | No. of Mismatch Bases | No. of Plants Examined | No. of Indel Mutation |
|---|---|---|---|---|---|---|
| Cas9/sgRNA | OFF1 | ch02: 18455705 | GCTGAAGAGCGTCACCACGTACGG | 2 | 4 | 0 |
| OFF2 | ch05: 15644350 | GTCGAGGAGCGCCACCACGTGCGG | 4 | 4 | 0 | |
| OFF3 | ch09: 13253210 | GCTGAAGGCCGTCACCACGTCCGG | 4 | 4 | 0 | |
| OFF4 | ch08: 25248560 | GCTGAAGCACACCACCATGTACGG | 4 | 4 | 0 | |
| OFF5 | ch09:14373426 | GCTGAACAGCTCCCCCACGTCCGG | 4 | 4 | 0 | |
| OFF6 | Ch03: 7709411 | GCTGGAGAGCTCCACCACGGACGG | 4 | 4 | 0 | |
| OFF7 | Ch03: 13007484 | GCTCAGCAGCGCCACCGCGTACGG | 5 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Yang, X.; Luo, P.; Yan, J.; Cao, X.; Qian, H.; Zhu, X.; Fan, Y.; Mei, F.; Fan, M.; et al. Creation of Bacterial Blight Resistant Rice by Targeting Homologous Sequences of Xa13 and Xa25 Genes. Agronomy 2024, 14, 800. https://doi.org/10.3390/agronomy14040800
Zhu Y, Yang X, Luo P, Yan J, Cao X, Qian H, Zhu X, Fan Y, Mei F, Fan M, et al. Creation of Bacterial Blight Resistant Rice by Targeting Homologous Sequences of Xa13 and Xa25 Genes. Agronomy. 2024; 14(4):800. https://doi.org/10.3390/agronomy14040800
Chicago/Turabian StyleZhu, Yiwang, Xiaohuai Yang, Peirun Luo, Jingwan Yan, Xinglan Cao, Hongge Qian, Xiying Zhu, Yujin Fan, Fating Mei, Meiying Fan, and et al. 2024. "Creation of Bacterial Blight Resistant Rice by Targeting Homologous Sequences of Xa13 and Xa25 Genes" Agronomy 14, no. 4: 800. https://doi.org/10.3390/agronomy14040800





